1.1 Anatomy
The heart is vital for human life. It is made of specialised muscle and is responsible for pumping blood to all the organs and tissues via the arteries.
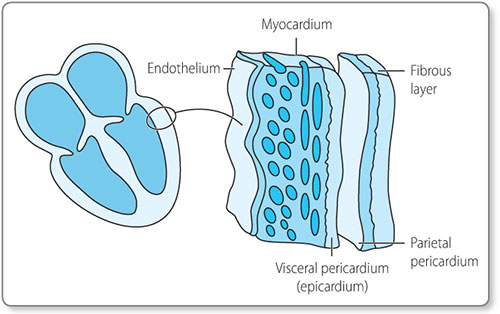
Embryologically, the heart is derived from the mesodermic cell layer during the third week of gestation. This group of cells, called the mesocardium, differentiates to form the various layers of the heart: the endothelium, myocardium, epicardium and pericardium (Figure 1.1).
The heart is situated in the middle of the thorax, slightly offset to the left. On examination the heart is felt and heard more strongly on the left due to the larger, stronger left ventricle.
Systemic and pulmonary circulations
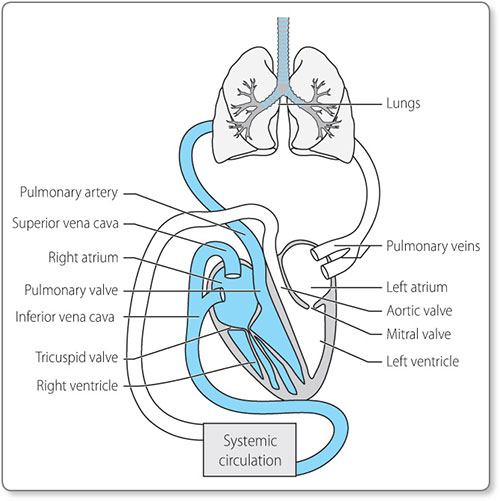
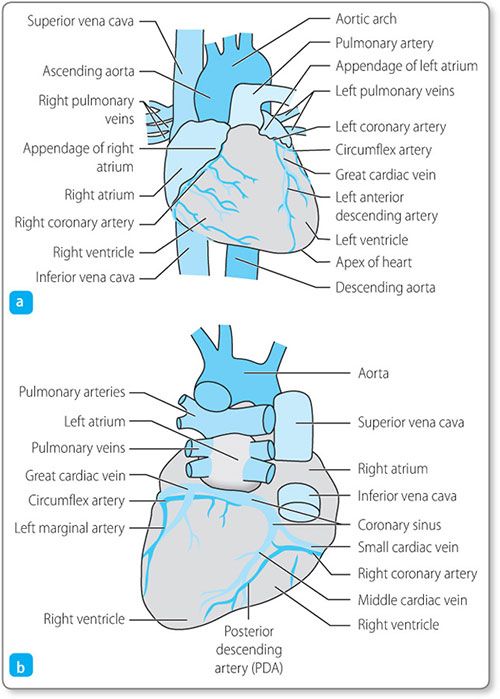
The human heart consists of four chambers: two receiving chambers, the atria, and two pumping chambers, the ventricles (Figure 1.2).
These form two separate circulatory routes, the pulmonary circulation delivering blood to and from the lungs, and the systemic circulation delivering blood to and from the rest of the body. The cardiac chambers are separated by valves, which ensure that there is no backflow of blood, and provide electrical insulation between the atria and the ventricles.
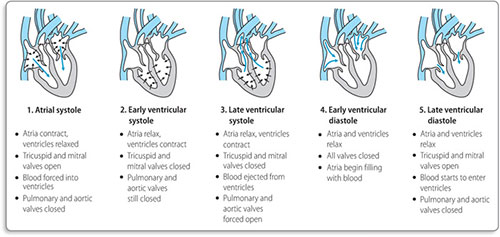
Deoxygenated blood from the body returns to the right atrium via the superior and inferior venae cavae. There is passive flow of blood from the right atrium into the right ventricle, followed by contraction of the right atrium to push more blood through the tricuspid valve into the right ventricle (Figure 1.3). The right ventricle then contracts, forcing the tricuspid valve to shut and ejecting blood though the pulmonary valve to the pulmonary arteries and on to the lungs.
At the lung capillary beds, gas exchange occurs and the blood becomes oxygenated. It then travels onwards via the pulmonary veins back to the heart, arriving at the left atrium.
At the left atrium there is passive flow of oxygenated blood into the left ventricle, followed by atrial contraction, which pushes more blood through the mitral valve into the left ventricle. The left ventricle is required to generate great pressure to deliver blood to the body, and consequently has greater muscle mass than the right ventricle. When the left ventricle contracts, the mitral valve closes and blood is propelled through the aortic valve into the aorta. From there oxygenated blood is distributed to the organs and tissues of the body, where oxygen delivery occurs at the capillary beds. The deoxygenated blood returns via the venous system to the venae cavae, and then to the right atrium.
Coronary circulation
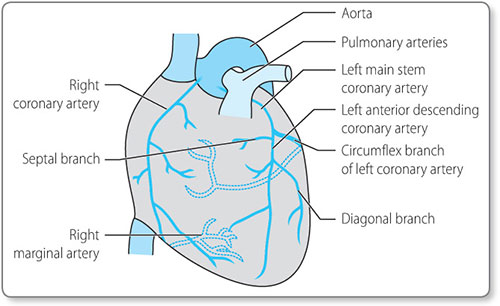
The heart, like any other muscle, requires a blood supply in order to provide it with the necessary supplies required for metabolism and contraction. The heart receives its oxygenated blood via the coronary arteries (Figure 1.4).
These vessels arise from the aorta just beyond the aortic valve (Figure 1.5).
In most people the right and left coronary arteries originate from the corresponding sides of the aorta. The right coronary artery supplies blood to the right ventricle and the inferior part of the left ventricle. It also gives branches to the right atrium, including the sinoatrial (SA) node and gives a branch to the atrioventricular (AV) node. The left main stem of the coronary artery divides into two large arteries. The left 6circumflex artery supplies the posterior part of the left ventricle. The left anterior descending artery supplies a large area of the left ventricle, including the anterior portion and apex, the septum (via septal branches) and the lateral aspect (via diagonal branches).
The anatomy of the conducting system
The left and right atria lie superiorly and are separated by the interatrial septum (Figure 1.6). The right atrium is derived from two embryological parts: the smooth walled venous (posterior) component is derived from the sinus venosus, and the muscular anterior part is derived from the rudimentary right atrium. Where the two fuse there is a vertical crest that extends from the superior to the inferior vena cava. This is known as the crista terminalis.
The SA node is located adjacent to the crista terminalis in the right atrium and lies inferior to the superior vena cava. This area of specialised cardiac cells forms the heart's own ‘pacemaker’.
Where the superior part of the crista terminalis meets the interatrial septum it gives rise to a specialised conduction pathway that conducts to the left atrium. This is known as Bachmann's bundle.
The atria are connected to their corresponding ventricles via the atrioventricular valves: the tricuspid valve lies between the right atrium and the right ventricle, and the mitral valve lies between the left atrium and the left ventricle.
The mitral and tricuspid valves and their associated annuli electrically insulate the ventricles from the atria. The only electrical connection between the atria and the ventricles is through the atrioventricular node (AV node), which lies in the low right atrial septum near the tricuspid valve.
The AV node has two separate electrical inputs. The fast pathway originates in the atrial septum and enters the node anteriorly. The slow pathway is a continuation of the lower margin of the crista terminalis and enters the node posteriorly.
The AV node communicates directly with a specialised narrow tract of conducting tissue called the bundle of His. This bundle is at first a single pathway that runs in the membranous interventricular septum, but it subsequently divides into the right and left bundle branches, which run to the apex of the heart.
The left bundle divides further into the anterior and posterior fascicles. From the bundle branches, conduction myofibres (Purkinje fibres) spread throughout the ventricular myofibres. The His–Purkinje network has specialised, rapid conducting properties as compared with general myocardial conduction.
1.2 Physiology
Cardiac action potential
The heart consists of specialised muscle cells called myocytes. Muscle cell contraction is associated with the electrical discharge of these cells, and is called depolarisation. These electrical discharges can be detected and recorded at the surface of the body.
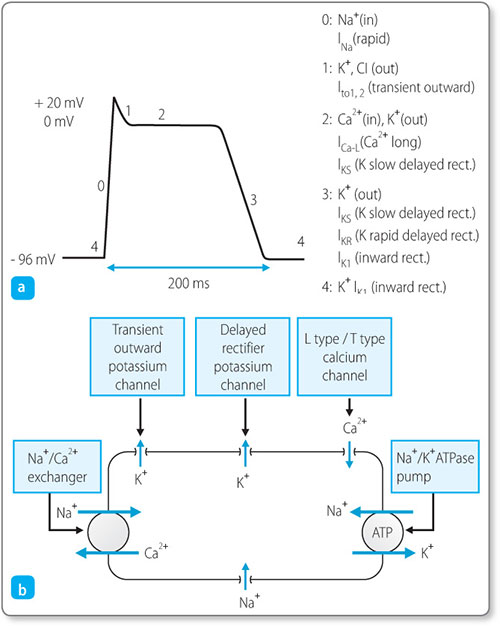
Cardiac cells have a resting membrane potential of –96 mV (Figure 1.7a). This means that there is a net negative charge inside the cell, maintained by membrane protein channels that control the movement of the key cations: sodium (Na+): calcium (Ca2+) and potassium (K+) (Figure 1.7b).
Figure 1.7: (a) the cardiac cell action potential and the sequence of membrane ion channel openings; (b) the movement of key cations (Na+, sodium; Ca2+, calcium, K+, potassium) in the cell.
When the channels are stimulated by a neighbouring muscle cell (or by depolarisation of the SA node) they reach a certain threshold, triggering a chain of events. Rapid sodium channels open and sodium ions flow into the cell, resulting in rapid depolarisation. Next, slow calcium channels open inside the sarcolemma and sarcoplasmic 9reticulum, increasing the calcium level in the cytosol, and at the same time the cell membrane becomes less permeable to potassium, slowing the outflow of potassium ions. The overall effect is of balancing the membrane potential at around 0 mV for a prolonged ‘plateau’ phase. By this mechanism cardiac cells are able to depolarise and contract for longer. Finally, potassium channels reopen and calcium channels close, more potassium leaves the cell, less calcium enters and the negative membrane potential is restored – this is called repolarisation.
During the repolarisation period it becomes impossible to cause the cell to depolarise again. This is the refractory period.
Cardiac electrical network
During development of the heart a small number of heart muscle cells develop a property called automaticity, whereby they repeatedly and rhythmically depolarise, or ‘fire’ action potentials. The area of heart muscle with the highest rate of automaticity is the SA node, the heart's natural pacemaker, which usually initiates myocardial depolarisation.
The rate at which the SA node depolarises is influenced by parasympathetic stimulation via the vagus nerve (reduces the rate) and sympathetic stimulation (increases the rate) via the sympathetic nerves and circulating catecholamines. The result is a ‘normal’ resting heart rate of around 60–100 beats/min.10
From the SA node the electrical impulse spreads to the right atrium through three intra-atrial pathways, while Bachmann's bundle carries the impulse to the left atrium.
Having activated the atria, the impulse enters the atrioventricular node; the brief delay that results allows time for the atrial contraction to finish, thus ensuring that sufficient blood is passed into the ventricles.
After the atrioventricular nodal delay, the impulse travels to the bundle of His, which then splits into the right bundle branch (RBB) (traversing the right ventricle) and a left bundle branch (LBB), which traverses the left ventricle and splits into the left posterior fascicle and a left anterior fascicle.
The posterior fascicle is a broad band of fibres that spreads over the posterior and inferior surfaces of the left ventricle. The anterior fascicle is a narrow band of fibres that spreads over the anterior and superior surfaces of the left ventricle.
Having traversed the bundle branches, the impulse finally passes into their terminal ramifications, the Purkinje fibres. These fibres traverse the thickness of the myocardium to activate the entire myocardial mass from the endocardial surface to the epicardial surface (i.e. inside to outside).
1.3 Cell physiology
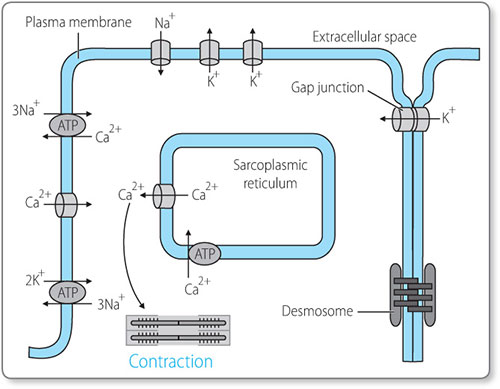
Within the cardiac cells there are hundreds of myofibrils, which are long, thin structures made of thin and thick filaments. The thin filaments are composed of a protein called actin, and the thick filaments are made of a protein called myosin.
When the cardiomyocyte (Figure 1.8) is stimulated by an action potential it causes the sarcoplasmic reticulum to release calcium into the cell. This calcium allows myosin heads to bind to actin filaments and pull them together, causing the myofibrils to shorten. This is how electrical activity (the action potential) causes the individual muscle cells to contract. The action potential passes to adjacent cells directly via special channels called gap junctions, and in this way the contraction spreads throughout the heart.11
1.4 Electrical activity and the ECG
The electrical activity within the heart can be detected by electrodes placed on the skin. The record of the heart's electrical activity is known as the electrocardiogram (ECG).
The ECG is recorded using adhesive, disposable electrodes attached to different sites on the patient, and the trace is drawn onto paper with standard-sized squares.
The ECG machine is usually calibrated such that a 1 mV signal results in a deflection of 1 cm (two large squares) on the ECG recording. The calibration signal should be included with every recording. The recording paper speed is normally 25 mm/s. If no electrical signal is detected, a flat line will be drawn; this is called the baseline.
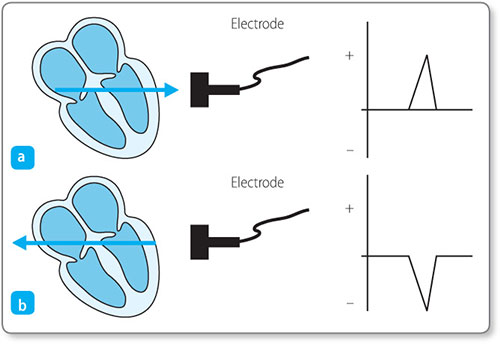
The direction of a deflection depends on two factors: the direction of spread of the electrical force and the location of the recording electrode.
Figure 1.9: Electrical impulses travelling toward and away from an electrode result in positive and negative deflections, respectively.
An electrical signal travelling towards an electrode is recorded as a positive deflection (above the baseline), and an electrical signal travelling away from an electrode is recorded as a negative deflection (below the baseline) (Figure 1.9).
Electrical activity is a vector quantity, i.e. it possesses both magnitude and direction. The size of a positive or negative deflection is determined by a combination of these two properties. For example, the ventricles have the greatest muscle mass, and therefore generate a larger electrical discharge (and consequently larger ECG complexes) than do the atria. However, the actual size of the complex recorded by the ECG will vary according to the location of electrode on the patient.
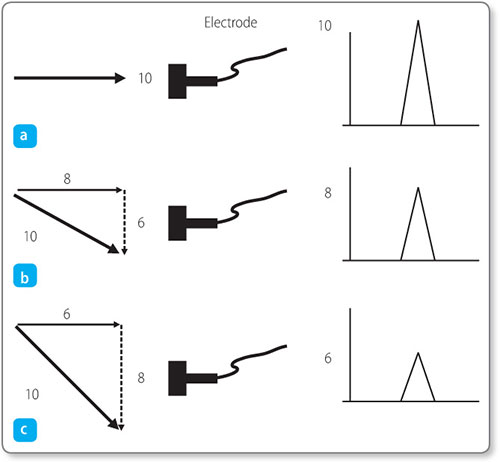
In the example shown in Figure 1.10a, the electrode is looking directly towards the direction of depolarisation. The vector component of electrical discharge towards the electrode is equal to its total magnitude and thus gives the maximum deflection on the ECG of 10 mV.
Figure 1.10: Vector summation according to the direction of depolarisation relative to the position of the electrode.
In Figures 1.10b and 1.10c the electrode is located such that the depolarisation is travelling in an oblique direction towards it. As a vector quantity, the electrical discharge can be considered as being composed of two components at mutual right angles. For these oblique cases, the component of depolarisation heading directly towards the electrode is less, so that a weaker voltage is recorded. The more oblique the angle, the lower the voltage.
The importance of electrode location and the propagation vector is discussed further in Chapter 2.
The recorded ECG provides information about the electrical activity occurring at different times in the cardiac cycle. As multiple electrodes are used to record the ECG, the electrical activity at different cardiac sites can be assessed, with each lead providing a different ‘window’ onto a different part of the heart.