INTRODUCTION
The study of the circulatory system started with the discovery of the microscope. The first element to be identified was the red blood cell (erythrocyte) in the late 1600s. A medical student Paul Ehrlich devised (in 1877) a triad stain, that detected white blood cells (leukocytes), and a new era in hematology dawned1,2.
What is Hematopoiesis?
Hematopoiesis is initiated by a multipotent stem cell that can expand by proliferation and also can differentiate into the various blood cells, thereby ensuring a continuous supply of mature blood cells. A staggering number of almost 200 billion red blood cells, 10 billion leukocytes, and 400 billion platelets are produced each day during the life-span of an individual2. Changing demands allow the bone marrow to meet those demands, e.g. the erythropoietic system undergoes serial adaptation to meet a progressively changing demand for oxygen in an embryo, fetus, and neonates. Finally a switch occurs from a fetal to an adult type of erythropoiesis, wherein fetal Hb is replaced by adult Hb especially after delivery, as the breathing neonate can extract O2 from lungs rather than from its mother's blood.
Site of Hematopoiesis
Hematopoiesis occurs extra embryonically in the yolk sac. However, a simultaneous additional hematopoiesis is observed in the periaortic tissues in mice and human primates2.
The hematopoietic stem cell is self-renewable. The pluripotent stem cell can proliferate and differentiate into different lineages of blood cells. Daughter cells can therefore, either retain the pluripotentiality of their parent cell, or can differentiate into progenitor cells that are destined to become more lineage committed hematopoietic cells that ultimately mature into erythrocytes or granulocytes or monocytes or lymphocytes or platelets4–8. There are 3 sequential phases of fetal hematopoiesis which, 2though overlapping, can broadly be subdivided into: (i) Mesoblastic, (ii) Hepatic, (iii) Myeloid phases : Mesoblastic in the yolk sac: weeks 2 through 5; Hepatic in the liver—weeks 5 through 24 and myeloid in the bone marrow—weeks 11 through adulthood9,10.
Mesoblastic Phase
Hematopoietic cells are first observed in the human embryo between day 16 to day 19 in the mesenchymal layer of the secondary yolk sac. By the 22nd day (3 weeks of gestation), blood islands are seen scattered throughout the mesodermal tissue6,7,11–13. Most blood islands are composed of hematopoietic cells surrounded by endothelial cells. Peripheral cells of the island form the walls of blood vessels; the centrally located cells become primitive blood cells, called hematocytoblasts. By 6 weeks, both intra-and extraembryonic vessels are macroscopically visible, and erythroblasts can be identified in the yolk sac vessels.
Red blood cells (RBCs) are the first to appear. Erythrocytes arise from large primitive megaloblastic erythropoiesis or from smaller definitive normoblastic erythropoiesis, and both arise from hematocytoblasts. Megaloblasts are large cells with abundant polychromatophilic cytoplasm and a nucleus in which chromatin is fine and widely dispersed. They give rise to red cells that are present in circulation 4 to 5 weeks after conception. Megaloblasts arise from intravascular sites and slowly get replaced by the normoblastic cells. Normoblastic erythropoiesis begins about the 6th gestational week and by the 10th week, accounts for over 90% of circulating RBCs.
By the 6th gestational week, the intravascular mesoblastic stage starts to decline and ceases by the end of the 3rd fetal month20.
Hepatic Phase
Blood formation starts in the liver by the 5th to 6th gestational week12 and continues for the first postnatal months. The pluripotent cells travel from the yolk sac to the liver via circulating blood. Erythropoiesis is predominant, and accounts for about 50% of the total nucleated blood cells during the 3rd to 5th gestational months5,6.
Hematopoiesis also occurs in the spleen and thymus during the third fetal month and a little later, in the lymph nodes. Apart from the stem cells, erythropoietic progenitors and granulocytic-monocytic progenitors are present, as revealed by studies done during 12 and 23 weeks of gestation. However, they are intrinsically different from the progenitors of adult origin.
Between 18 and 21 weeks of gestation, hematopoiesis decreases in the liver although it continues until term, unless the bone marrow is incapable of producing enough blood cells as in thalassemia.
Myeloid Phase
Myeloid period starts during 3rd to 6th fetal month and the bone marrow is the primary site of blood cell formation in the last trimester. Bone marrow progenitor cells, unlike adult progenitors, differentiate without added growth factors in vitro, or with the addition of either erythropoietin, IL-6, IL-9 or IL-11. This is because CD34+ fetal 3hematopoietic progenitor cells produce granulocytic-macrophage colony stimulating factor (GM-CSF) and IL-3 to form colonies13 (CFu = Colony forming units).
Bone marrow production of blood cells continues throughout postnatal life. Erythropoietic cells constitute about 31% of the total bone marrow cells at 12 weeks of gestation, fall to 20% and to even 10% thereafter14,18. Granulocytes represent 30 to 40% of cellular elements during the 10th and 24th gestational weeks, but during the last trimester of pregnancy, they increase rapidly and are higher than in adult blood1,15,16. Leucocyte production significantly begins in the myeloid period of hematopoiesis, with clavicular bone marrow being the first to produce leukocytes.
Thus, in the early embryo, red cells are large and most of them are nucleated and contain a large amount of hemoglobin. However, red cell count, Hb concentration and packed cell volume are very low. After delivery, the number of red cells, Hb concentration and volume of packed red cells increases. The mean size of cells, mean corpuscular volume, mean corpuscular hemoglobin as well as the proportion of circulating immature erythrocytes decrease. By 10th gestational week, the red cell count ranges from 500,000 to 1500,000 /mm3. At this time 5-10 percent of all the erythrocytes are nucleated and the reticulocyte count is approximately 80%. The hemoglobin concentration ranges from 6-9 g/dl and hematocrit ranges from 20-30%. By the 24th week Hb rises to approximately 14 g/dl and hematocrit to 40 percent and red blood cell count to 3,500,000/cmm. At the 24th week, only 0.3 to 0.5 percent of circulating cells are nucleated and reticulocyte count decreases to 10 percent or less. From this period until the termination of gestation (40th week), there is a slow rise in Hb, HCT and red blood cell count15–23.
|
The principle growth factor that regulates erythropoiesis in EPo (erythropoietin) a glycoprotein hormone. Epo maintains all the red cell production during fetal, neonatal, and adult life, by inhibiting the apoptotic death or PCD of erythroid progenitors and by stimulating the proliferation and their differentiation into normoblasts.
Development of Hematopoiesis
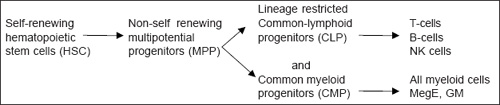
Hematopoietic stem cells (HSC) are slowly dividing clonogenic cells capable of self-renewal. They also have multilineage potential in non-hematopoietic systems (neural cells, hepatocytes, endothelium, muscle cells, etc.) which are down graded in multipotential progenitors (MPPs), common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs) and in hematopoietic systems (HSCs express myeloid genes and finally give rise to mature blood cells).
MPPs are not having the ability for self-renewal but highly proliferative, and prime for both lymphoid and myeloid cell differentiation.
CLPs give rise to T-, B-, and NK-cells, and not to myeloid cells, and contain clonogenic progenitors for T- and B- lymphocytes.
CMPs express only myeloid-related genes: megakaryocytic-erythrocytic (MegE) and granulocytic-monocytic (GM). Over 60% of single CMPs generate myeloid components MegE and GM.
Hence, co-expression of myeloerythroid genes exist in HSCs, MPPs, and CMPs; and co-expression of T-, B-, and NK-lymphoid genes in MPPs and CLPs (Fig. 1.1).
5Because erythropoietin (Epo) does not cross the placenta, the Epo concentrations measured in the fetus reflects fetal synthesis. Fetal Epo production is clearly regulated by requirements for tissue oxygenation; however, elevated Epo concentrations (up to 8000 mU/mL) have been reported in several pathologic states, such as fetal hypoxia, anemia (e.g. that caused by fetal Rhesus hemolytic disease), placental insufficiency, or infants of diabetic mothers.
The primary regulator in the control of erythroid cells maturation is erythropoietin (EPO). EPO is needed by pro-erythroblasts to differentiate. Withdrawal of EPO results in apoptosis or programmed cell death. During embryogenesis, EPO is present in amniotic fluid and extraembryonic celomic fluid. Fetal EPO production is regulated by requirements for tissue oxygenation. Circulating EPO concentration increases during fetal development from 4 mU/mL at 16 weeks of gestation to 40 mu/mL at term. After birth, its concentration decreases and reaches a nadir between the 4th and 6th week after birth, and attains adult values (15 mU/mL) by 10 to 12 weeks of age. In premature infants, anemia is more severe and persists longer, referred to as anemia of prematurity. This results from an inappropriately low EPO.
EPO is made primarily in the fetal liver, but in adults, in the kidney and secondarily in the liver. Renal EPO production accounts for less than 9% of total EPO mRNA expression until the 30th week of gestation, and increases to 27% by the 7th month of gestation, probably as a consequence of growth and maturation of the kidney, and the developmental increase in EPO-expressing interstitial cells.
Possibly the preterm neonate is comparatively more anemic than the term infant because in the former, the immature liver is primarily responsible for EPO production. Also, hypoxia is a better stimulator of EPO production by the renal cells as compared to the hepatic cells.
MPPs progress to either CLPs or CMPs, and priming of myeloid genes most likely precedes that of lymphoid genes.
Lymphopoiesis starts in fetal liver and lymph plexuses at the 7th week of gestation, in the thymus and gut-associated lymphoid tissue between the 7th and 10th week, and in the spleen and bone marrow between 10th and 12th week. Circulating lymphocytes are present in fetal blood between 7 and 8 weeks of gestation and by 12 weeks, the lymphocyte count could reach 10,000/mm3. During the latter half of intrauterine life, the number of lymphocytes diminish, gradually down to 3000/mm3 at term3, 11.
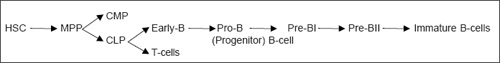
Development and Differentiation of B-cells
B-cells are present in multiple tissue sites in early fetal development. Pre-B-cells are present in 7 to 8 weeks gestational age fetal liver and 10 week gestational age fetal omentum. However, 18-20 week fetal tissues show B-cell development in marrow, liver, lung, kidneys and spleen. But, from midgestation to the entire life of an individual, the bone marrow (BM) is the main and only site of B-cell development. On attaining maturity, B-cells migrate into peripheral lymphoid tissues via circulation (spleen, lymph nodes, mucosa-associated lymphoid tissue).
Development of T-cells
Stem cells continuously go from the bone marrow (BM) to the thymus. In the BM, CLP differentiates into T-NK-DC (Dendritic Cells) progenitors. Either CLP or T-NK-DC progenitors migrate to the thymus where further development takes place throughout life (Fig. 1.3).
Myelopoiesis (Fig.1.1) Co-expression of myeloid cells (megakaryocytes, erythrocytes; granulocytes, monocytes) is detected in HSC, MPP, and CMP. Hematopoietic cell proliferation and differentiation are greatly controlled by growth factors and their receptors and transcription factors, e.g. Epo, on binding with its receptor, sends signals via the receptor's cytoplasmic domain to the committed progenitor cells for the development of both erythroid and megakaryocyte (MK) lineages.
Endogenous Epo binds the Epo receptor on 2 types of erythroid progenitor cells: the more primitive BFU-E and the more mature CFU-E. This leads to a stream of events resulting in proliferation and differentiation into mature red blood cells, and an inhibition of apoptosis (programmed cell death).
Thrombopoietin (Tpo) is a growth factor that binds to its receptor c-Mpl, on megakaryocytes (MK) and platelets.
It regulates the production of multipotent progenitor cells and platelets. It promotes MK mitosis and lineage-specific maturation events, and induces expression of von Willebrand Factor (vWF), platelet factor 4, glycorotein lb (receptor for vWF), and gpIIb/IIIa (receptor for fibrinogen) on platelets.
Granulocytes-monocytes (GM) GM-CSFR (granulocytes-monocyte colony stimulating factor receptor) on binding to GM-CSF sends out signals for growth and development and survival of polymorphonuclear leukocytes/neutrophils (PMN) monocytes-macrophages and Eo. Similarly, G-CSF and G-CSF receptor stimulates proliferation, survival and differentiation of myeloid progenitor cells towards neutrophils, i.e., for granulocyte differentiation, signals from the G-CSF receptor are essential.
Granulocyte-monocyte production occurs in the bone marrow. Its genes are expressed in HSC, MPPs, and CMPs. CMPs as mentioned before, express myeloid-related genes. The myeloid phase begins around 10 to 12 weeks of gestation and lasts for life.
In conclusion, the development of hematopoiesis is controlled by
- Growth factors and their receptors that are involved in the cell growth and differentiation
REFERENCES
- Wintrobe M. Milestone on the path of progress. In Wintrobe M (Ed). Blood, pure and eloquent. New York, Mchrew Hill, 1980; 1.
- Yoder. Marvin C Embryonic hematopoiesis in hematological problem of neonates. Christanson, Robert D WB Saunders & Co: Philadelphia, 2000; 1: 20.
- Orlic D, Bodine D. What defines a pluripotent hematopoietic stem cells (PHSC): Will the real PHSC, Please stand up. Blood 1994; 84: 3991.
- Ogana M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993; 81: 2844.
- Huyhn A, Dommergues M, Izac B et al. Characterisation of hematopoietic progenitors from human yolk sac and embryo. Blood 1995; 86: 4474.
- Tavassoli M. Embryonic foetal hemopoiesis - an overview. Blood Cells 1991; 17: 269–81.
- Zon L. Developmental biology of hematopoiesis. Blood 1995; 86: 2876.
- Till J, McCulloch E. A direct measurement of radiation sensitivity of normal mouse bone cell. Radiat Res 1961; 14: 213.
- Moore M, Owem J. Stem cell migration in developing myeloid and lymphoid system. Lancet 1967; 2: 658.
- Moore M, Metacaf D. Ontogeny of hematopoietic system—Yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Hematol 1970; 18: 270.
- Maximove AA. Relation of the blood cells to connective tissue and endothelium. Quoted. Frankin Oski JL, Naiman L. Hematologic problems in newborn. WB Saunders & Co: Philadelphia 1972; 1–31.
- Keleman E, Calvo W, Fliedner TM. Atlas of human hematopoietic development. New York Springer, 1979.
- C Dame, SE Tuul. The switch from fetal to adult erythropoiesis. Neonatal Hematology 2000; 27: 507–25.
- Onarbord P, Tavian M, Humeau L et al. Early ontogeny of the human marrow from long bones—An immunohistochemical study of hematopoiesis and its microenvironment. Blood 1996; 87: 4109–19.
- Mugrage ESR, Adresen MI. Value of red blood cells of average infants and children. Am J Dis Child 1936; 51: 775.
- Jevert CT. The occurrence and significance of the nucleated erythrocytes in the fetal vessels of placenta. Am J Obstet Gynaecol 1937; 37: 184.
- Walker JL, Turnbull EPN. Hemoglobin and red cell in the human foetus III. Foetal and adult hemoglobin. Arch Dis Child 1955; 30: 111.
- Thomas DB, Yoffey JM. Human foetal hematopoiesis I cellular composition of foetal blood. Br J Hematol 1962; 8: 290.
- Thomas DB, Yoffen JM. Human foetal hematopoiesis II Hepatic hematopoiesis in the human foetus. Br J Hematol 1964; 10: 193.
- Gilmour JR. Normal hematopoiesis in intrauterine and neonatal life. J Pathol Bacteriol 1941; 52: 25.
- Zaizov R, Matothy. , Red cell values on the first postnatal day as a function of gestational age. Am J Hematol 1976; 1: 276.
- Gilmount JR. Normal hemopoiesis in intrauterine and neonatal life. J Pathol Bacteriol 1941; 52: 25.
- Oski FA, Naiman JL, Stockman JA, Persm HA. Normal blood values in newborn period in hematological problems in the newborn. 1–31.
- Zaizov R, Mamoth Y. Am J Hematol 1976; 1: 276.
- Tucker W, Libien. , Fates of human B-cell precursors. Blood 2000; 96: 9–23.
- IL-7 from Bench to Clinics. Blood 2002; 99: 3892.
- Kaushansky K. Thrombopoietin : the primary regulator of platelet production. Blood 1995; 86: 419–31.