PART One
Introduction
INTRODUCTION
Reproduction is the creation of a new individual or individuals from previously existing individuals. The union of sperm and egg is the essential step in the process of reproduction.
In the historical milestones, the factors involved in reproduction remained a mystery till the earlier part of seventeenth century. Leeuwenhock of Holland for the first time in 1677, with his home-made microscope described fairly accurately the anatomy of the sperm. Thereafter in the 18th century, there was insignificant progress in the knowledge of biology. In 1822, Karl Dernt von Baer “Father of Modern Embryology” documented observation on eggs and their developmental stages. It was Oscar Hertwig in Germany who first demonstrated in 1875 by his observation on sea urchins that the union of sperm and egg was the essential step for reproduction. However, in humans, the remote site of these events and the secluded place of origin of the participants (gametes) made fertilization a difficult subject for study. Most of the knowledge about human reproduction was achieved through experiments in animal species. Scope of study directly in humans has only been possible following the advent of Assisted Reproduction. The understanding of the process of gametogenesis, their maturation, sperm-egg interaction, fertilization and implantation is one of the many major benefits that have been derived through clinical application of the Assisted Reproductive Technologies. Some of the information about the basics of human reproduction gathered so far through these technologies backed up by studies on animal models will be discussed in this chapter.
CLASSIFICATION
Fundamental requirements for sexual reproduction can be classified under three broad groups:
- Development of morphologically and functionally competent gametes (oocytes and spermatozoa).
- Existence of anatomically and physiologically normal male and female reproductive organs.
- Delicately balanced intricate interactive events leading to release of gametes (male and female), their transport, fusion and fertilization, cleavage and implantation.
DEVELOPMENT OF MORPHOLOGICALLY AND FUNCTIONALLY COMPETENT GAMETES
Gametes
The female gamete (oocyte) resides within the graffian follicle in the ovary and the male gamete (spermatocyte) within the seminiferous tubules lined by Sertoli cells in the testes. Ovaries and testes are known as female and male gonads, respectively.
Embryogenesis of Gonads and Gametes
Initially fetal gonad remains in an undifferentiated form (neither female nor male). In the third week of embryonic development, the intraembryonic mesoderm covered by celomic epithelium differentiates into three distinct parts: cervical, thoracic and caudal. The urogenital system of the embryo develops in the caudal part of the mesoderm covered by celomic epithelium. Three paired ridges develop in this part of mesoderm due to celomic condensation (Figure 1.1).
Figure 1.1: Undifferentiated embryonic ridges which will ultimately differentiate into Gonads (Gonadal ridge); male and female reproductive organs (wolffian or mesonephric and mullerian or paramesonephric ridges)
The most medial one is the gonadal or genital ridge. Lateral to gonadal ridge is the mesonephric ridge and the most lateral one is the paramesonephric ridge. Pronephros at the cranial end, mesonephros the midsegment and metanephros at the caudal end form the mesonephric ridge. Pronephros disappears in the course of embryonic development; mesonephros gives rise to wolffian system and metanephros is the precursor of the cortical part of kidney. Paramesonephric duct is the embryologic progenitor of mullerian system.
The gonad originating from gonadal ridge is composed of primitive germ cells, celomic surface epithelial cells and an inner core of medullary mesenchymal tissue. Apart from germ cell, the origin of somatic cells in the gonad (granulosa-theca cells in the ovary and Sertoli-Leydig cells in the testes) is controversial. One view suggests that gonad is formed by invasion of germinal epithelium which gives rise to gonadal tissue. The other view suggests that gonadal tissues develop from mesonephros.1 The germ cells develop within the primitive ectoderm but the specific cell of origin has not been identified. Germ cells have been detected 3rd week after fertilization in the primitive endoderm at the caudal end and in the dorsal wall of the adjacent yolk sac. They have also been found in the splanchnic mesoderm of the hindgut.2 Though originating in these areas, the germ cells can only survive in the gonadal ridges. Therefore, they have to migrate by the process of displacement because of growth of embryo and also by active ameboid movement, along the dorsal mesentery of the genital ridges. The germ cells migrate from the yolk sac through the hindgut to their gonadal sites between 4th and 6th week of gestation. The factors involved in the process of migration are perhaps mediated through chemotactic activity and adhesive peptides.
The germ cells are the precursors of ova or sperm. These germ cells multiply by the process of mitosis during migration. Before the gonads have differentiated either as testes or as ovaries (approximately 6th gestational weeks) the number of germ cells has reached a total of 10,000.3
The differentiation of the gonad either to a testes or to an ovary will be completed between 6th and 9th gestational weeks. This will depend on the sex chromosome complement of the developing embryo. If the chromosome complement is XY, testes will form and if it is XX the gonad will be an ovary. The fetal testes produces two types of hormones while ovary produces only one. Hormones produced by testes are 3testosterone and antimullerian hormone (AMH) while ovaries produce only estrogen. The male phenotype is dependant on the products of the fetal testes while the female phenotype is primarily the result of the absence of testes and consequently the testicular product.4 Traditionally, it has been suggested that development of testes is an ‘active’ process, whereas development of ovary is a ‘passive’ procedure. This means that presence of ‘Y’ chromosome (SRY gene on Y-chromosome) will lead to testicular development while the absence of ‘Y’ chromosome will lead to development of ovary. However, any process of differentiation will require gene expression and therefore, ovarian development too is also under the influence of yet some unidentified gene or genetic expression.
At 7 weeks gestation, Sertoli cells appear (either from celomic epithelial or from mesenchymal cells) and by aggregation, they form the seminiferous tubules. The primordial germ cells are embedded within the seminiferous tubules. The Sertoli cells produce ABP (androgen binding protein), necessary for transport of androgens produced by Leydig cells outside the lumen to within the lumen of seminiferous tubules necessary for spermatogenesis. Sertoli cells also produce inhibin.
Leydig cells are the steroidogenic cells of the testes. They develop from the mesenchymal cells at about 8 weeks of gestation.
The differentiation of the wolffian system begins with testosterone production. All androgen sensitive organs do not require prior conversion of testosterone to dihydrotestosterone (DHT). In the process of masculine differentiation, development of epididymis, vas deferens and seminal vesicles are dependent on testosterone while, development of penis, urethra and prostate are under the influence of DHT. In the female, the regression of wolffian system is due to lack of locally produced androgens and antimullerian hormone (AMH).
Maturation and Fertilizing Competence of the Gametes
Oocyte Maturation
Immediately following ovarian differentiation as female gonad at 6 to 8 weeks of gestation, the germ cells start rapid mitotic multiplication reaching to 6–7 million oogonia by 16 to 20 weeks.5 From this period, the germ cell content of the ovary will continue to decrease by a process known as physiological atresia (apoptosis), till the age of menopause around the age of 50 years or earlier when the store of oocytes will be finally exhausted.
By mitosis, the germ cells will give rise to oogonia. The oogonia are transformed to oocytes as they enter the first meiotic division and arrest in prophase. The process begins at 11 to 12 weeks, continues throughout pregnancy and is completed at birth. The arrest of meiosis at the end of prophase continues till puberty or thereafter till the oocyte comes out of the follicle by the process known as ovulation. The arrest at prophase is maintained by inhibiting substances secreted by granulosa cells (oocyte maturation inhibitory factor—OMIF). The resumption of meiosis of the oocyte prior to ovulation is probably induced by LH induced small amount of intrafollicular progesterone and various intrafollicular growth factors. After first meiotic division the oocyte becomes haploid and mature but still not fertilizable until the second meiotic division occurs. This happens when the spermatozoa enter into the oocyte and the second polar body is extruded. At each of these meiotic divisions, a polar body is extruded (first and second polar body) which removes the excess genetic material.
There is continuous loss of germ cells during the process of mitosis and meiosis. Those germ cells, which do not acquire granulosa cell covering to form graafian follicle, undergo degeneration. Apart from these, during the second half of pregnancy, there is continuous follicular growth and atresia. This consequently reduces the number of follicles at birth. Excessive germ cell loss may also be due to abnormal maternal chromosomal pattern as in 45XO.
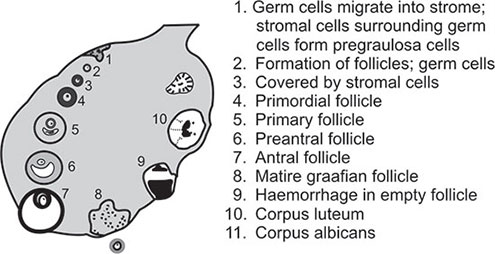
Follicle Formation
The pattern and control of follicle formation and growth varies in different phases of a woman's life.
Early intrauterine period:
Around 20 weeks of fetal life, follicle formation begins. Initially primordial follicles form. Primordial follicles 4consist of an oocyte arrested at prophase of meiosis-I enveloped by a single layer of spindle-shaped pregranulosa cells surrounded by a basement membrane. Eventually, all oocytes are covered in this manner. This process of primordial follicular development continues until all oocytes in the diplotene stage can be found in follicles shortly after birth. By change of cell type, pregranulosa cells assume the shape of typical granulosa cells and a primary follicle is formed. By further differentiation of granulosa cells a preantral follicle is formed.
At this stage, surrounding mesenchymal cells (future stroma of ovary) are compressed to form thin thecal layer. Appearance of Call-Exner body (coalescence of granulosa cells to form an antrum) will lead to formation of antral follicles. Antral follicles are found by the end of pregnancy but not in large numbers. During last stage of pregnancy theca cells are found surrounding the follicles.6
The process of follicle formation, variable degree of ripening and atresia starts from intrauterine fetal life. In the first half of pregnancy, follicle formation and growth are autonomous and not gonadotropin dependant. Estrogen production is insignificant at this stage. Unlike the male, gonadal steroids are not essential for development of female internal genital organs. The development of the mullerian duct into fallopian tubes, the uterus and the upper third of vagina is totally independent of the ovary and is due to the absence of testes.
Late intrauterine period:
In the second half of pregnancy some of the follicles become gonadotropin dependant.7 The ovary develops receptor for gonadotropin in second half of pregnancy. But development of primordial follicles and the process of meiosis are not gonadotropin dependant.8
After birth the ovary contains only 1–2 million follicles. Most of these follicles are in the stage of primordial follicles. A few are in the antral stage.
Neonatal and childhood period:
In the neonatal and childhood period, the gonadotropin level is low, hypothalamic activity is suppressed and there is absence of pituitary response to GnRH. The ovarian follicles, however, are not quiescent. Though majority are in the primordial follicular stage, a few antral follicles, respond to gonadotropins by forming follicular cysts. However, they are not pathological; they form and regress. The follicles begin to grow and because of absence of gonadotropin support cannot reach the stage of full maturity and ultimately become atretic. The continued follicular growth followed by atresia leads to increase of the stromal compartment of the ovary. This leads to prepubertal enlargement of ovary both in size as well as in volume.
At puberty and during reproductive years:
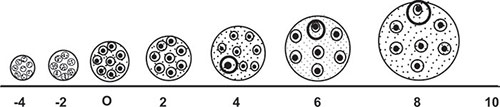
With the advent of puberty because of continued atresia the germ cell population has been reduced to a total of 3,00,000 to 5,00,000 units. Out of these only 400 to 500 will be selected for ovulation. For every follicle that ovulates, approximately 1,000 will grow for variable length of time to achieve incomplete grades of maturity and will eventually become atretic.
It is now apparent that the time required for growth of primary follicle to an ovulatory follicle is approximately 85 days (Figure 1.2).9
Major part of the growth occurs in environment deficient of gonadotropin. The number of follicles that will mature will depend on amount of gonadotropin available to the gonad and the sensitivity of the follicles to the gonadotropins. During reproductive years, the recycling process of follicle recruitment and growth, ovulation and corpus luteum formation are dependant on the complex but well-defined regulatory sequence of hypothalamic pituitary, gonadal interactions.5
Recycling of Menstruation and Ovulation
From menarche to menopause recycling of menstruation and ovulation will depend on delicately balanced interactions of 5 endocrine glands (unless interrupted by pregnancy and lactation). Of these three are directly and two indirectly involved. The directly involved endocrine glands are CNS-hypothalamus, pituitary and ovary. The supporting endocrine glands, thyroid and adrenal cortex indirectly support events, in menstruation and ovulation. It is essential that these glands should remain intact meaning thereby that they should not be damaged or their function should not be retarded by tumor, disease, chromosomal, genetic, autoimmune or receptor dysfunction. Even if they remain intact, the biological expression of their function will depend on regulatory ‘feedback’ mechanism of their products, namely endocrine, autocrine and paracrines.
Endocrine Control of Menstruation and Ovulation
Elaborate description of endocrine control of ovulation is beyond the scope of this chapter. The important events leading to ovulation is summarized below:
Hypothalamus releases gonadotropin releasing hormone (GnRH) under the influence of neurotransmitter, norepinephrine. GnRH stimulates the pituitary through receptors to synthesize and release two gonadotropins, namely follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH in different periods of menstrual cycle stimulate two cells in the follicle, granulosa and theca to produce estrogen and progesterone respectively. The mechanism of follicular maturation and ovulation is primarily controlled by this “two cell-two gonadotropin system”, working through feedback mechanism.
Feedback Mechanism in Different Phases of Ovulatory Menstrual Cycle
From ovulation point of view, menstrual cycle is divided into four distinct phases: (a) late luteal phase of previous menstrual cycle,(b) menstrual and early follicular phase, (c) mid-follicular phase, (d) late follicular phase and periovulatory events.
The events which occur in these different phases of ovulatory menstrual cycle are as follows:
- Late luteal phase of previous menstrual cycle: Estrogen and progesterone start declining. Anterior pituitary is released from negative feedback effect of estrogen and progesterone. FSH primarily and LH to some extent start rising. Under the influence of FSH, fresh batch of follicles are recruited which will compete for maturity in the next menstrual cycle.
- Menstrual and early follicular phase: Rise of FSH continues. LH rise is relatively slower than FSH. There are four functions of FSH: (i) multiplication of granulosa cells—thereby increasing the size of the follicles; (ii) production of enzyme aromatase which will convert androgen to estrogen; (iii) increasing FSH and LH receptors in the follicles (more receptors for both FSH and LH in follicle destined to become dominant compared to those in the remaining follicles); (iv) production of inhibin-B.Therefore, rise of FSH in early follicular phase will lead to increase in size of follicles and increased concentration of estrogen in the intrafollicular environment. Small amount of LH in early follicular phase is responsible for production of androgen primarily from the theca cells, which will be converted to estrogen by the enzyme aromatase. Excess androgenization instead of adequate estrogen intrafollicular environment will lead to arrested follicular growth and improper oocyte maturation.
- Mid-follicular phase: During the process of follicular growth, one of the follicles gets maximum FSH exposure. As a consequence, this follicle grows at a much faster rate than its fellow follicles. This follicle is known as dominant follicle (DF). The criteria for selection of this dominant follicle are not very clear. The dominant follicle is destined to rupture and ovulate. Selection of dominant follicle is completed by d5 or d6. As the levels of follicular estrogen and inhibin-B increase, there is negative feedback effect on the pituitary. This results in decline of FSH and gradual rise of LH. The growth of dominant follicle continues in spite of declining FSH. Obviously the control of development of dominant follicle is taken over by rising level of LH.6
- Late follicular phase and periovulatory events: FSH in the early and mid-follicular phase has already produced receptors for LH in the dominant follicle. Under the influence of rising LH, the dominant follicle continues to grow. The remaining follicles become atretic. There is luteinization of granulosa cells with release of small amount of progesterone (0.8 to 1.8 ng/ml). This will lead to oocyte maturation by completing the first meiotic division. Before the FSH starts declining in the later part of mid-follicular phase, the estradiol has reached the peak (around 100 pg/ml). This peak level is maintained for about 48 hours (plateau). Estradiol at this peak and pleateu level exerts negative ‘feedback’ effect on the pituitary for which FSH declines and LH starts rising. At the same time, the rise of estradiol finally exerts a positive feedback effect on hypothalamus for which a bolus of GnRH is released. Pulsatile rise of GnRH stimulates the pituitary to release a bolus amount of LH (about 75 to 90 mIU/ml) which is known as ovulatory “LH surge”. The follicle ruptures with release of mature oocyte. This is known as ovulation. Besides the dynamic event of ovulatory LH surge, intrafollicular prostaglandin and an enzyme collagenase, generated by plasminogen activator, plasmin are also involved in the mechanism of follicular rupture. The liberated mature oocyte is picked up by fimbria of the fallopian tube and proceeds toward ampulla, which is now ready to be fertilized if a mature sperm is available.
The entire mechanism of ovulatory menstrual cycle may be schematically represented in Table 1.1.
Endocrine events and folliculogenesis in ovulatory menstrual cycle have been diagrammatically represented in Figures 1.3 to 1.5.
Spermatogenesis
In the testes two types of spermatogonial cells (germ cells) have been recognized: Type-A and Type-B cells. All Type-A spermatogonia have been considered to play a stem cell role. Some of the A-type spermatogonia act, as reserve stem cells while others are active stem cells. From these active stem cells Type -B Spermatogonia are produced which ultimately lead to the development of spermatocytes.10 The reserve Type-A stem cells lie dormant and become active on demand.
These germ cells are scattered between radially directed supporting cells called Sertoli cells. The Sertoli cells lie within the seminiferous tubules. By forming tight junctions with each other, the Sertoli cells divide seminiferous tubules into two compartments called the basal and the adluminal compartment. Sertoli cells produce inhibin and a protein known as androgen binding globulin (ABG). Androgen binding globulin transports the androgens produced by Leydig cells (which lie outside the seminiferous tubules) into the basal compartment for completing the maturational changes of the spermatozoa. Maturational changes of spermatozoa occur in four stages (Figure 1.6).
Type-B spermatogonia differentiate into primary spermatocyte.
By first meiotic division one primary spermatocyte divides into two secondary spermatocytes.
By second meiotic division two secondary spermatocytes will further divide into four spermatids. Maturational changes upto this stage occurs within the seminiferous tubules. Finally, adult spermatozoa are formed which by this time has reached the caudal epididymis. The sperms reach the caudal epididymis approximately 72 days after the initiation of spermatogenesis. The sperms are stored in the caudal epididymis for final ejaculation. During the maturation of spermatids to spermatozoa, several events occur, including formation of acrosome, changes in nuclear morphology and formation of the flagellum.
The sperm functions are preserved during this period of storage by adequate amount of circulating plasma testosterone.
Anatomy of Human Spermatozoa
A normal spermatozoon is 60 µm in length. The different parts of spermatozoa are: (a) head, (b) neck (also known as connecting piece) and (c) tail. The tail is subdivided into three segments viz. midpiece, principal piece and end piece. The sperm head is the part-containing nucleus, covered in its anterior aspect by the acrosomal cap. The acrosomal cap has two layers—outer acrosomal and inner acrosomal layer. The acrosomal cap contains various types of enzymes of which two are significant, namely acrosin and hyaluronidase. Covering the outer acrosomal layer is the plasma membrane, which is the outermost layer of the sperm head.8
The neck contains proximal centriole and the remnants of the distal centriole. Sperm centriole has an important function during fertilization. Following entry of sperm head into the oocyte, sperm centriole triggers up formation of female pronucleus inside the ooplasm. This area has been termed as the ‘black box’ (preserving all the informations for fertilization) of the spermatozoa (Figure 1.7).
Figure 1.7: “Biochemical anatomy of sperm head and neck”
PM | — | Plasma membrane |
OAM | — | Outer acrosomal membrane |
IAM | — | Inner acrosomal membrane |
AS | — | Acrosomal sac containing enzymes; acrosin and hyaluronidase |
C | — | Centriole |
Tail has the important function as it provides energy for the sperms to move. Presence of dyenin bands (a type of protein) in the tail is the source of energy and motility of sperm. Absence of dyenin or deformity of tail is the factors for sperm immotility.
Endocrine Regulation of Spermatogenesis
Like ovulatory control, endocrine regulation of spermatogenesis depends on ‘two gonadotropin-two cell’ system. There is a regulatory ‘feedback’ mechanism in the hypothalamic-pituitary-testicular axis. Spermatogenesis is a continuous process. Hence, unlike ovulatory cycles, ‘feedback’ mechanism in the hypothalamic-pituitary-testicular axis in spermatogenesis has no specific cyclicity.
The endocrine regulation of spermatogenesis has been diagrammatically represented in Figure 1.8.
Under the influence of GnRH stimulation released by hypothalamus, pituitary produces two gonadotropins, viz. follicle stimulating hormone (FSH) and luteinizing hormone LH. LH stimulates the Leydig cells of the testes to generate adequate amount of testosterone. Leydig cells also produce some amount of estradiol. Leydig cells lie outside the lumen of the seminiferous tubules. Hence, testosterone production is extraluminal.
FSH secreted by anterior pituitary will stimulate the Sertoli cells to produce inhibin and another peptide known as androgen binding globulin (ABG). Androgen binding globulin produced by Sertoli cells has the capability of carrying extraluminally produced androgen inside the lumen of the seminiferous tubules. Androgens now within the lumen of the seminiferous tubules will help in the maturational changes of the spermatozoa.
EXISTENCE OF ANATOMICALLY AND PHYSIOLOGICALLY NORMAL MALE AND FEMALE REPRODUCTIVE ORGANS
Embryogenesis
The wolffian (mesonephric) and mullerian (paramesonephric) ducts develop during the ambisexual period of embryonic development (upto 8 weeks). Thereafter one duct will persist and give rise to sex-specific internal and external genital organs and the other will disappear by the third fetal month except for rudimentary vestiges.
The crucial factor which will determine as which duct will persist or regress is the presence or absence of antimullerian hormone (AMH) secreted by the testes. If antimullerian hormone is present, paramesonephric duct will disappear and wolffian duct will start developing. On the other hand, in the absence of antimullerian hormone, the paramesonephric ducts will develop and wolffian duct will disappear.
Internal genital organs have the intrinsic tendency to feminize. In the absence of a Y-chromosome, a functional testes and antimullerian hormone, the mullerian system will develop into fallopian tubes, uterus and upper vagina. Therefore, development of female reproductive organ is passive and does not require an active stimulus.
On the other hand, differentiation of wolffian system requires active stimulation through testosterone production by testes.
Developmental Anatomy of Female Reproductive Organs
In the absence of antimullerian hormone, the two paramesonephric ducts come into contact in the midline to form a Y-shaped structure, which will form the uterus, tubes and upper part vagina.11 The fallopian tubes, uterus and the upper portion of the vagina are created by fusion of the mullerian ducts by 10th week of gestation. By 22nd week of gestation, canalization of the uterine cavity, cervix and vagina is completed. Smooth muscle cells and uterine stroma will originate from the mesenchymal tissue underneath the epithelium. By 20th week, uterine mucosa has differentiated into the endometrium. Endometrium, one of the most complex tissues of the human body is essential for reproduction. Endometrium is cyclically changing in response to estrogen and progesterone of the ovulatory menstrual cycle and to a complex interaction among its own autocrine and paracrine factors.
Functional Anatomy of Male Reproductive Organs
Male reproductive organs consist of: (a) testes, (b) epididymis, (c) vas deferens, (d) ampulla of vas, (e) seminal vesicles, (f) prostate, (g) Cowper's and urethral glands, (h) penis (Figure 1.9).
Testes
Human testes is ovoid in shape and is located within the scrotal sac. The length and weight are approximately 4.5 cm and 34 to 45 gm respectively. The reproductive components within the testes are: (a) Leydig cells, (b) seminiferous tubules, (c) Sertoli cells.
Leydig cells are found in clusters and form about 5 to 15 percent of the total volume of testes. They lie outside the lumen of seminiferous tubules and are the source of androgen production. The number of Leydig cells in both testes in a 20-year-old male is 700 million and diminishes by one-half by the age of 60.
Seminiferous Tubules
Seminiferous tubules are the sites of spermatogenesis. They are long, loop-like convoluted ducts with both ends terminating in the rete testes. The number of seminiferous tubules is 600 to 1200 with an estimated total length of 250 m.12 Spermatozoa and fluid originating in the tubules are transported to the rete testes and then to the epididymis. Rete testes acts as a valve that controls the flow.13
Sertoli Cells
Sertoli cells line the inner aspect of the basement membrane of seminiferous tubules. The germinal cells are arranged and scattered inbetween the Sertoli cells. The undifferentiated spermatogonia are located near the basement membrane, and the more advanced forms are arranged successively at higher levels of Sertoli cells near the tubular lumen.10
Sertoli cells are more than nursing cells to the adjacent germinal cell. The adjacent Sertoli cells are joined to one another by inter-Sertoli ‘tight’ junctions. Sertoli-cell ‘tight’ junctions subdivide the seminiferous tubules longitudinally into basal and adluminal compartments. These tight junctions of the adjacent Sertoli cells form an impermeable “blood testes” barrier. Germ cells develop upto the stage of primary spermatocytes within the basal compartment which has free access to the extratubular environment. Secondary spermatocytes continue their development in the adluminal compartment. Because of the blood-testes barrier, any factor influencing the latter stages of spermatogenesis must be mediated through the Sertoli cells.
Epididymis
Epididymis is an elongated structure which extends from cranial to caudal pole of testes. It begins from efferent ducts and continues upto vas deferens. The epididymis consists of three parts: caput, corpus and cauda. Coiled efferent ducts emerge from the rete testes and constitute most of the caput epididymis. The length of epdidymis has been measured to be 5–6 m. Epididymis synthesizes certain compounds that are secreted into the lumen of the canal. These include protein, carnitine, lipids, glycerophosphoryl choline (GPC), carbohydrates, steroids and other small molecules. Carnitine is an epididymal marker and helps in preserving sperm viability and stimulating motility after ejaculation.
Vas deferens
Vas deferens is a tube, 35 to 45 cm long with a diameter of 0.85 ± 0.7 mm. It extends from the tail of the epididymis, runs along the medial side of the spermatic cord, through the inguinal canal and ends in a glandular enlargement on the medial side of the seminal vesicle. This terminal glandular enlarged portion is known as ampulla of vas. Ampulla of the vas finally fuses with the neck of the seminal vesicle to form the ejaculatory duct. Ejaculatory duct passes through the prostate and opens into the floor of prostatic urethra at the level of verumontanum. Ampulla of vas helps in continuous maturation process of spermatozoa which has started in the epididymis.
Prostate
Prostate is the largest accessory male sex gland. In young and middle aged adults, the gland is 3 to 4 cm in 11diameter and approximately 20 gm in weight. The two ejaculatory ducts pierce the prostate obliquely and pass into the interior of the gland. Within the prostate, they converge, decrease in diameter and terminate in the floor of the prostatic urethra, in the region known as verumontanum.
Prostate fluid accounts for 15–30 percent of the total ejaculate volume. Prostatic fluid contains a number of constituents of which acid phosphatase, plasminogen activator, seminin, zinc, magnesium and calcium are significant. Plasminogen activator and seminin cause lysis of seminal clot. Acid phosphatase can be used as prostatic marker.
Seminal Vesicles
Seminal vesicles are paired, highly convoluted pyriform glands. Each vesicle is 5–6 cm long and 1–2 cm wide. They lie lateral to ampulla of vas deferens, posterior to urinary bladder and superior to prostate. About 70 percent ejaculate originate in seminal vesicles.
Of the various secretion of seminal vesicles, fructose and prostaglandins are important. Though constituents of seminal plasma are not absolutely essential for fertilization, the secretion may optimize conditions for sperm motility, survival and transport in both the seminal pathway and the female reproductive tract.
Cowper's (Bulbourethral) and Urethral Glands
Cowper's glands are paired bodies 3–5 mm in diameter which are homologous to Bartholin's glands in the female. They secrete droplets of mucin which help in urethral lubrication. Scattered accessory glands can be found throughout the male reproductive tract.
Penis
Penis is composed of three cylindrical masses of erectile cavernous tissue, blood vessels, lymph and nerves. The erectile tissue within the penis is a labyrinth of irregular blood sinus and spaces. Male sexual function consists of: (a) erection: this is both reflexogenic and psychogenic, (b) accessory glandular secretion and seminal emission and (c) ejaculation.
EVENTS LEADING TO RELEASE OF GAMETES, THEIR TRANSPORT, FUSION AND FERTILIZATION, CLEAVAGE AND IMPLANTATION
Egg Release and Transport
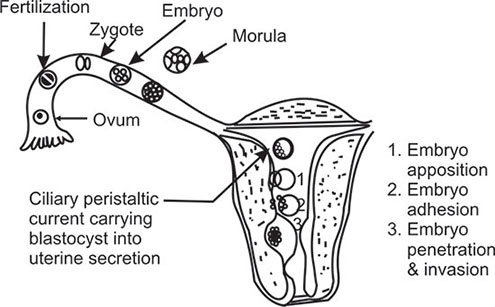
Prior to ovulation, the oocyte completes its first meiotic division under the influence of midcycle ‘surge’ of LH. Thereafter, it enters into second meiotic division and is arrested at second metaphase. The dominant follicle gradually moves up to the surface of the ovary. After follicular rupture, the ovum is picked up by fimbriated end of the fallopian tube by sweeping movement. Entry into the tube is facilitated by muscular movements that bring the fimbriae into contact with the surface of the ovary. Variations in the method of ovum pick up surely exist, because women may achieve pregnancy even with one ovary and a single tube located on contralateral side. Furthermore, pregnancies have been recorded following direct intraperitoneal insemination (DIPI).1412
Egg and subsequently, zygote and embryo transport involves the time that elapses from ovulation up to the time of entry of compacted morula into the uterus. The egg is fertilized in the ampulla of the fallopian tube.
The epithelium of fallopian tube consists of two types of cells—ciliated and non-ciliated. They undergo cyclic changes of the menstrual cycle.15 The non-ciliated cells secrete cytoplasmic components during passage of the egg or embryo providing important metabolic factors for transport and implantation. Ciliary movement and tubal muscular contractions are both involved for transport of egg from ampulla toward the uterus.
Following ovulation, the egg is inside the ampulla within 2–3 minutes. The transport time from ampulla to uterus for the fertilized oocyte is approximately 3 days. In human, 80 percent of this time, period are spent in the ampulla (Figure 1.10).
In most species, the fallopian tube appears to be essential for full development of embryos, because uterine fluid during the first 48 hours following ovulation remains toxic to the egg. In humans also, if the endometrium is in the reduced or advanced stage of development compared with the developmental stage of the fertilized egg, implantation may fail. This may not be always true. Because, pregnancies have been recorded following Estes operation where the eggs are ovulated directly inside the uterine cavity. Moreover, when fertilized donor eggs are transferred to women, who are receiving hormone supplementation, a larger implantation window is created in the endometrium when the blastocyst will implant. Hence, a perfect synchrony between the incoming embryo and developing endometrium is not absolutely essential.
Sperm Release and Transport
The sperms reach caudal epididymis approximately 72 days after initiation of spermatogenesis. The caudal epididymis is the storehouse of the sperms, which should be available for ejaculation. Semen coagulates immediately following ejaculation. But this is liquefied in 20 to 30 minutes following ejaculation by an enzyme derived from prostate gland. Most of the sperms become immotile in the acid pH of vaginal secretion. The alkaline pH of semen offers some transient protection for the spermatozoa to survive but majority of sperms are immobilized within 2 hours. The more active sperms by their own motility enter into the cervical canal and then into the uterine cavity. It is generally believed that cervical mucus has a filtering action. Sperm antibodies on the sperm head interacts with cervical mucus and inhibits sperm motility and entry into uterine cavity. Similarly, less active sperms are unable to swim up into the uterine cavity. The number of active sperms remaining within the cervical mucus remains constant for 24 hours and after 48 hours only few sperms are left behind in the cervical canal.16 In many animals isthmus of the fallopian tube is believed to be the storehouse of sperms but in humans, cervical mucus rather than fallopian tube seems to be the reservoir of sperms.17 Approximately 80 to 100 million sperms are deposited in the vagina and out of these, only a few are able to achieve proximity of the egg in the ampulla.17 Majority of sperms are lost in the vagina either by enzymatic digestion or by phagocytosis.
In the uterus and in the fallopian tube, the sperms acquire two very important functions, viz. capacitation and hyperactivated motility.
Capacitation and Hyperactivation
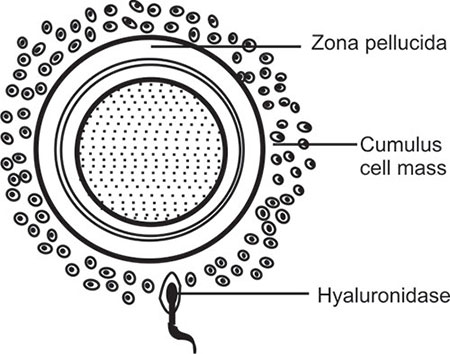
While in the female genital tract (cervix, uterus or in the fallopian tube) the sperms undergo some physiobiochemical modifications which is called “Capacitation”.18 Capacitation involves removal of seminal plasma factors coating the surface of the sperm, and modification of some biochemical properties of the sperm head 13membrane. Capacitation will help in acrosome reaction and acquisition of hypermotility; further requirements for sperm penetration through cumulus cells and zona pellucida; the outer vestments surrounding the oocyte.
Preparatory Changes in Gametes before Fertilization
Further Sperm Maturation (Acrosome Reaction)
The fusion of plasma membrane with outer acrosomal layer followed by breakdown of the membranes to allow escape of acrosome cap contents is known as ‘acrosome reaction’.19 Sperm capacitation is a prerequisite change for acrosome reaction. While passing through cumulus the sperms do not release acrosin.20 The acrosome cap contains enzymes—the important ones are hyaluronidase and acrosin. Hyaluronidase digests the cumulus cells (Figure 1.11) and acrosin helps penetration of zona pellucida. Sperm hypermotility induced by capacitation is also an essential step for rapid sperm entry through zona pellucida.
Further Oocyte Maturation
Apart from meiotic divisions of the oocytes, there is an influx of extracellular calcium in response to estradiol which improves the chances of fertilization. This is followed by secondary rise in calcium ions from intracellular stores. This is characterized by wavelike oscillations within the ooplasm.21 This transient increase in intracellular calcium, which is estrogen dependant, improves quality of oocyte and increases the chances of fertilization. These events are not related to oocyte meiosis. However, improved fertilization following estradiol induced calcium increases indicates the important role of intrafollicular estradiol for overall oocyte maturation.
Fertilization
Fertilizable life span of oocyte ranges between 12 and 24 hours. Similarly, fertilizable life period of the spermatozoa ranges between 48 and 72 hours. Majority of pregnancies occur when coitus takes place within 3 days prior to ovulation.22 The process of fertilization consists of series of events occurring in both sperms and eggs. Contact of a single sperm with egg is due to chemotactic activity exerted by the egg on the sperm (Figure 1.12).
Events in Sperm-egg Interaction
There are three types of glycoproteins in zona pellucida. These are known as ZP1, ZP2 and ZP3 of which ZP3 is most abundant.23 ZP3 is the primary ligand for the sperm and ZP2 is responsible for zona reaction following sperm penetration to prevent polyspermy.
Penetration through the zona is rapid and mediated by acrosin, a trypsin like proteinase.24
Spermatozoa enters perivitelline space at an angle. Then there is binding between inner acrosomal membrane of the sperm head and oelema (the outer membrane of ooplasm). This induces cortical and zona reactions which prevent entry of further spermatozoa into the oocyte, thereby blocking polyspermy.
Pronucleus Formation-syngamy-embryonic Cleavage
Approximately, 3 hours after entry of sperm head in the oocyte, the second meiotic division is completed and the second polar body is released with a haploid complement of chromosomes. The remaining haploid number of chromosomes in the oocyte will form the female pronucleus. The nucleus of the sperm head undergoes decondensation and the male pronucleus is formed. The male and female pronuclei migrate toward each other. When they come in close proximity, the limiting membranes break down. There is exchange of chromosome material between the male and female pronucleus. The process is known as syngamy. A spindle is formed on which chromosomes become arranged. The stage for first mitotic cell division has now been organized and with first cell division a zygote is formed. Embryonic-genomic activity starts between 4 to 8 cell stages of cleavage, 2–3 days after fertilization.25 Normal embryonic-genomic activity will now control further cell division into morula and blastocyst.
Preimplantation Preparatory Changes
Prior to implantation both incoming embryo and developing endometrium and incoming embryo undergo some preparatory changes.
Endometrial Preparation for Implantation
Endometrial preparation for implantation involves: (a) histologic changes, (b) synthesis of PGE2 and (c) production of many cytokines, peptides, and lipids.
Preparatory histologic changes in the endometrium: After ovulation, the endometrial growth is under the influence of estrogen and progesterone. The striking feature is that height of endometrial growth is fixed initially at its preovulatory extent inspite of continued availability of estrogen. Epithelial proliferation is inhibited and this is brought about by progesterone. Though epithelial proliferation is restricted, the growth of individual components of the endometrium continued by edema and swelling under the influence of progesterone and prostaglandins. But confinement in a fixed structure leads to progressive tortuosity of glands and coiling of spiral vessels. Subnuclear vacuoles appear in the cells lining the glands. They contain glycogen and mucin. The cells being distended finally rupture pouring their contents into the gland lumen. Stroma becomes increasingly edematous and spiral vessels are prominent and densely coiled. By day 3 post-ovulation, the endometrium by these secretory changes is differentiated into three distinct zones viz., the innermost zone known as stratum basalis (less than twenty-five percent thickness; not affected by hormones); the stratum spongiosum, the middle zone of the endometrium (comprising at least 50 % of thickness) and stratum compactum, the outer layer (about 25% of the thickness). At the time of implantation the main endometrial change is stromal edema with increased vascularity induced by estrogen-progesterone and increase in prostaglandin production (mainly PGE2) by the endometrium.
The ‘window’ of endometrial implantation is restricted to days 16–22 of 28 day normal cycle and day 16–19 of cycles stimulated with exogenous gonadotropins.26, 27
During the peak secretory phase of endometrium ‘pinopodes’ form on the surface epithelium. Pinpodes form as a result of cystic change in the surface epithelial microvilli. Pinpodes serve to absorb uterine fluid from the uterine cavity forcing the blastocyst to be in contact with the endometrial epithelium.
Origin and role of prostaglandins in the endometrial preparatory process:
Apart from estrogen-progesterone stimulation, prostaglandin synthesis at the implantation site increases in response to blastocyst factors like platelet activating factor (PAF). The increase in prostaglandin concentration 15involves prostaglandin E2 component and not PGF2-∝. While PGE2 is essential at the implantation site, decidual synthesis of prostaglandin in general is reduced, apparently a direct effect of progesterone and perhaps a requirement in order to maintain pregnancy.28
Role of endometrial cytokines and growth factors in the process of implantation:
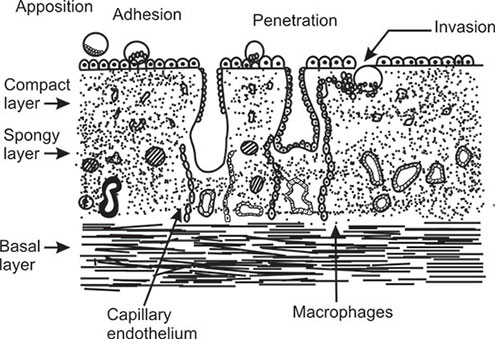
Numerous members in the cytokine family and various growth factors have been identified in all tissues associated with implantation. They are believed to be the biochemical vehicles through which the physical process of embryonic apposition, adhesion, penetration and trophoblastic invasion are completed. Of various types of molecules (proteins) identified, some will help in adhesion (adhesive molecules), others will prevent adhesion (e.g. some members in the family of cytokines, e.g. human IL-1 receptor antagonists). Antiadhesive molecules are commonly found on tubal epithelium. If they are deficient in tubal epithelium, ectopic pregnancy might result.
The important adhesion molecules include integrin, immunoglobulin superfamily, selection and caderhins—which are expressed on epithelial cell surface and help in attachment and adhesion of the embryo. Basement membrane and matrix substrates include collagen, fibronectin, laminin, enactin and tenascin which help to guide the trophoblast through basement membrane and stromal matrix for anchorage on maternal decidua.
Embryonic Preparation for Implantation and Preimplantation Signals
Signals: Embryo while still in the fallopian tube signals to the mother as it prepares for implantation. In response to this signal, mother produces early pregnancy factor (EPF).29 EPF has immunosuppressive property and is associated with cell proliferation and growth.
After reaching the uterine cavity in the compacted morula or blastocyst stage, the embryo produces βhCG which is essential for embryo hatching and embryonic implantation. hCG liberated by the embryo (even before implantation) will signal the corpus luteum to secrete higher level of estradiol and progesterone which can be detected in the maternal serum.30 Function of corpus luteum is essential for endometrial bed preparation, implantation and maintenance of pregnancy daring the first 9 to 10 weeks of gestation.
Embryo Hatching
A prerequisite change for the embryo is embryo hatching. After its entry into the uterine cavity in the morula (16 to 64 cells) or blastocyst stage (30 to 200 cells), it remains in the uterine cavity for 2–4 days still encapsulated by zona pellucida. Zona must be lysed before the embryo can attach to the maternal decidua. Lysis of zona and escape of embryo is known as zona hatching. Zona hatching is accomplished by components of uterine fluid as well as by blastocyst movement. By this time, blastocyst has differentiated into inner cell mass (embryo) at one pole and trophectoderm (placenta) at the other pole with a cystic cavity (blastocele) inbetween. Zona pellucida becomes thin and ultimately disrupts through which the inner cell mass wriggles out to differentiate into three primitive layers of future fetus, namely ectoderm, mesoderm and endoderm. Initially, primitive embryonic plate is bilaminar consisting of ectoderm and endoderm. After certain period of growth, a third layer, mesoderm originating from ectoderm insinuates between ectoderm and endoderm so that embryonic plate now becomes trilaminar. All tissues and organs of the growing fetus will develop from these three layers. Trophoectoderm and inner cell mass are essential for implantation. Even if the endometrial preparation is adequate for implantation, this may not occur if the embryo is not at the proper stage of development. This disparity is often observed in in vitro fertilization procedure when there is a risk of failure of zona hatching after the embryo has been transferred into the uterine cavity. In in vitro procedure the zona may become hard because of its exposure to culture medium. However, under favourable circumstances, the process of implantation starts with embryo-endometrial contact.
Implantation
Embryo-Endometrial Contact
Apposition and adhesion: As the blastocyst comes into close contact with the endometrium, the microvilli on the surface of the trophectoderm will interdigitate with those on the luminal surface of the decidual epithelial cells. At this stage, the cell membranes are in close contact and junctional complexes have been formed. Thereafter the early embryo cannot be easily dislodged.
Penetration and invasion (anchorage and placentation):
Trophoblast is invasive in nature. The early embryo secretes a variety of enzymes (e.g. collagenase and plasminogen activators) and these are important for digesting the intracellular matrix that holds the decidual cells together. This highly proliferative phase of trophoblast in early embryogenesis is regulated by many growth factors and cytokines produced in both fetal and maternal tissues. This is essential for effective anchorage and at the same time limiting the extent of trophoblastic invasion of maternal decidual tissue. Invasion requires the expression of integrins stimulated by insulin like growth factor II and inhibited by transforming growth factor-2.31
The trophoblast differentiates into two layers— cytotrophoblast (the cellular layer) and the syncytiotrophoblast (the acellular layer). Cytotrophoblast invades the uterine spiral anterioles and allows the maternal blood to enter into the decidual lacunae created by trophoblastic invasion. These lacunae are built up by cytotrophoblastic cells which remain in contact and constantly bathed by maternal blood for fetal nutrition and exchange of gaseous materials.
Penetration of the maternal decidua will depend on factors which are capable of suppressing the maternal immune response to paternal antigens. The endometrial tissue is responsible for immune suppression by synthesizing proteins in response to the blastocyst even before implantation.32 Usually the genetically abnormal embryos are rejected by the decidua. It may be possible that the abnormal embryo cannot produce a signal in early pregnancy that can be recognized by the mother.
CONCLUSION
The fundamentals of normal reproduction involves the fulfilment of three basic requirements. These are: availability of male and female gametes, anatomically 17and functionally competent reproductive organs of both the partners and the ability of the gametes to achieve biological competence for fertilization and implantation in the female genital tract. The gametes, originating as germ cells in the hind gut and yolk sac, migrate to the genital ridge (future gonad) to become either oocyte or spermatocyte. Genital ridge differentiates into ovary or testes in response to chromosomal complement of the embryo. For spermatocyte, the maturational changes are nearly completed within the testes as the diploid spermatogenia are reduced to haploid before leaving the testes. In case of oocytes the first meiotic division which starts in the intrauterine life remains arrested at prophase and is completed before ovulation. The maturational changes of gametes and their periodic release from testes and ovary are influenced by endocrine, autocrine and paracrine factors. DNA in the nucleus of the sperm head, centriole in the neck and release of mature haploid oocyte from the dominant follicle are the vital segments of gametes which are actively involved for successful fertilization.
Anatomically normal and physiologically active male and female reproductive organs are essential for further maturation and for accelerating fertilizing potential of the gametes. For this, sperm requires additional energy because it has to travel a long distance to penetrate the outer vestments of the oocyte, namely the cumulus matrix and zona pellucida. The additional energy is acquired by the sperm in different phases. As it travels through the seminal pathway in the male genital tract, sperm acquires motility and other biochemical back up from secretion of epididymis, vas deferens, seminal vesicles and prostate. In the female genital tract climax fertilizing potential of the sperm is completed by two very significant changes, namely capacitation and hyperactivation. Oocyte after ovulation, travels relatively a shorter distance before it reaches the sperm for sperm-ovum interaction. During this short journey, there is intracellular influx of calcium which accelerates its fertilizing potential.
The biological competence of gametes induces sperm-ovum interaction, fertilization, formation of pronucleus and cleavage into a two-celled zygote. Zygote sends signals to the maternal tissues which now prepare to receive the incoming embryo for implan-tation.
Implantation occurs in four stages, namely attachment, adhesion, penetration and anchorage. Besides endometrial bed preparation under the influence of steroid hormones and prostaglandins (PGE2) the process of implantation is additionally controlled by various members of the endometrial cytokine family and growth factors. These cytokines and growth factors are the biochemical vehicles through which the various phases of embryonic adhesion, trophoblastic invasion and placentation are completed.
REFERENCES
- Yoshinaga K, Hess DL, Hebdrucjt AG, Zamboni L: The development of the sexually indifferent gonad in the prosimian, Galago crassicaudatus. Am J Anat 181: 89, 1998.
- Baker TG: A quantitative and cytological study of germ cells in human ovaries. Prac Roy Sec land 158: 417, 1963.
- Motla PN, Makabe S, Nottola SA: The Ultrastructure of human reproduction, I. The natural history of the female germ cell: Origin, migration and differentiation inside the developing ovary. Hum Reprod Update 3: 281, 1997.
- Jost A, Vigier B, Prepia J, Perchellet JP: Studies on sex differentiation in mammals. Recent Prac. Hormone Res 29: 1, 1973.
- Gondos B, Bhiraleus P, Hobee C: Ultrastructural observation on germ cells in human fetal ovaries. Am J Obstet Gynecol 110: 644, 1971.
- Gondos B, Westergard L, Byskov A. Initiation of oogenesis in the human fetal ovary: Ultrastructural and squash preparation study. Am J Obstet Gynecol 155: 189, 1986.
- Thomas GB, Meweilly AS, Gibson F, Brooks AN: Effects of pituitary-gonadal suppression with a gonadotrophin- releasing hormone agonist on fetal gonadotrophin secretion, fetal gonadal development and maternal sternid secretion in the sheep. J Endocrinal 141: 317, 1997.
- Robinovici J, Jaffe RB: Development and Regulation of growth and differentiated function of human and subhuman primate fetal gonads. Endocr Rev 11: 532, 1990.
- Gougeon A: Dynamics of follicular growth in the human: A model from preliminary results. Hum Reprod 1: 81, 1986.
- Clermont Y, Antar M: Duration of the cycle of the seminiferous epithelium and the spermatogonial renewal in the monkey Macca Auctoides. Am J Anat 136: 153, 1973.
- Acien P: Embryological observations on the female genital tract. Hum Reprod 7: 437, 1992.
- Lennox B, Ahmad KN: The total length of tubules in the human testes. J Anat 107: 191, 1970.
- Sharma V, Mason B, Riddle A, Campbell S: Peritoneal oocyte and sperm transfer. Filth World Congress on in vitro Fertilisation and Embryo Transfer, Norfolk, Virginia, 1987.
- Crow J. Amso NN, Lewin J, Shaw RW: Morphology and Ultrastructure of fallopian tube epithelium at different stages of the menstrual cycle and menopause. Hum Reprod 9: 2224, 1994.
- Perloff WH, Steinberger E: In vivo survival of spermatozoa in cervical mucus. Am J Obstet Gynecol 88: 439, 1964.
- Williams M, Hill CJ, Seudamore I, Denphy B, Cooke ID, Barratt CLR: Sperm numbers and distribution within the human fallopian tube around ovulation. Human Reprod 8: 2019, 1993.
- Chang MC: Fertilising capacity of spermatozoa deposited into the fallopian tubes. Nature 168: 696, 1951.
- Yanagimachi R: Capacitation and the acrosome reaction. In Asch R Balmaceda JP, Johnston I (Eds): Gamete Physiology, Seronosymposia, Massachusets, Norewell, 31, 1990.
- Talbot P: Sperm penetration through oocytes investments in mammals. Am J Anat 174: 331, 1985.
- Tasarik J, Mendoza C: Nongenomic effects of 17-β-estradiol on maturing human oocytes: Relationship to oocytes developmental potential. J Clin Endocrinol Metab 1438: 80, 1995.
- Wilcox AJ, Weinberg CR, Baird DD: Timing of sexual intercourse in relation to ovulation in the probability of conception, survival of the pregnancy and sex of the baby. New Engl J Med 833: 1517, 1995.
- Shabanowitz RB, Orand MG: Characterization of the human zona pellucida from fertilized and unfertilized eggs. J Reprod Fertil 82: 151, 1988.
- Zaneveld LJD, Polakoski KL, Williams WL: Properties of a proteolytic enzyme from rabbit sperm acrosome. Biol Reprod 6: 30, 1972.
- Brande P, Bolaton V, Moore S: Human gene expression first occurs between the four and eight cell stages of preimplantation development. Nature 332: 459, 1988.
- Rosenwaks Z: Donor eggs: their application in modern reproductive technologies. Fertil Steril 47: 895, 1987.
- Psychoyos A: Uterine receptivity for nidation. Ann NY Acad Sci 476: 36m, 1986.
- Vander Weiden RMF, Helmerhorst FM, Keirs: MJNC, Influence of prostaglandins and platelet activating factor on implantation. Human Repord 6: 436, 1991.
- Morton H, Raefe BE, Cavanagh AC: Early pregnancy factor, seminars. Reprod Endocrinol 10: 72, 1992.
- Stewart DR, Overstreet JW, Nakajima ST, Lasley BL: Enhanced ovarian sternit secretion before implantation in early human pregnancy. J Clin Endocrinol Metab 76: 1470, 1993.
- Irving JA, Tala PV: Functional role of cell surface integrins on human trophoblast cell migration: Regulation by TGF-β, IGF-II, and IGFBP-1. Exp Cell Res 217: 419, 1995.
- Clark DA, Slapsys RM, Croy BA, Kreck J, Rossant J: Local active suppression by suppressor cells in the deciduas: A review. Am J Reprod Immunol 6: 78, 1984.