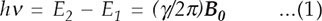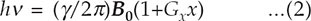INTRODUCTION
Nuclear magnetic resonance imaging (usually abbreviated MRI, or sometimes in clinical applications, just MR) has become a very widely used and extremely valuable medical diagnostic method and a valuable tool for a broad range of biomedical research studies.* As a medical technology, MRI is a multi-billion dollar industry, with thousands of clinical scanners available in every major hospital in the world.
For radiologists and other clinicians, MRI burst onto the scene in the early 1980s, when commercial clinical instruments became available. MRI appeared as a new method closely related superficially to X-ray computed tomography (CT), which had been developed a few years earlier as a diagnostic tool for examining internal human anatomy. In fact, early clinical MR images were evaluated as adjuncts to CT. For chemists, however, MRI was a brilliant, but logical, extension of the nuclear magnetic resonance (NMR) instruments and methods that had been unraveling molecular structures for 30 years. And for physicists, MRI was one more application of the phenomenon of NMR that was discovered in 1938.
In this article, I will try to describe the evolution of biomedical MRI in the context of the long-range, overall development of NMR itself. I will discuss a few important NMR concepts and methods and show how they eventually came to play key roles in today's MRI applications. I have drawn heavily on information contained in the 200 historical articles in the Encyclopedia of NMR.1
ORIGINS OF NMR
In the mid-1920s, the advent of quantum mechanics provided a coherent explanation of many chemical and spectroscopic observations in terms of quantized energy levels and the concept of a spin of the electron and of many atomic nuclei. In rough terms, spin can be pictured as a rotation of the electron or nucleus about an axis, much as the earth rotates about its polar axis. Because nuclei and electrons are charged particles, such a motion would be expected to generate a magnetic moment, and these particles could interact with an externally applied magnetic field. In 1933, Otto Stern (Nobel Prize, 1943) and his co-workers found that a beam of hydrogen molecules in a highly 2evacuated container could be deflected by an inhomogeneous magnetic field.2 The beam split into two components, depending on whether the nuclear magnetic moments of the hydrogen nuclei were oriented parallel or antiparallel to the magnetic field.
Stern's molecular beam technique was able to provide estimates of the magnitude of nuclear magnetic moments and spawned increasingly more elegant experiments, particularly in the laboratory of II Rabi at Columbia University. In seeking ways to improve the precision of the measurement, Rabi was led to the idea that in a magnetic field a nucleus with spin orientation parallel to the field would exist in a different energy level from one with antiparallel orientation, and that absorption of a quantum of energy at precisely the correct frequency could cause nuclei to “flip” from one orientation to the other. The energy v needed to satisfy the Bohr relation
turns out to be in the radiofrequency (RF) range, generally 1 to 900 megahertz (MHz), depending on the magnetic field strength. In equation 1, h is Planck's constant, Ei are the magnetic energy levels, γ is the nuclear magnetic moment divided by the nuclear spin quantum number, and B0 is the strength of the applied magnetic field. In December 1937, Rabi and co-workers subjected a beam of LiCl molecules to an RF of 3.518 MHz as the molecules passed through a static magnetic field. They varied the strength of the magnetic field and found that at one particular value the beam was deflected and its intensity on the detector abruptly dropped.3 This was the first observation of nuclear magnetic resonance (NMR), the term “resonance” describing the very precise relation in equation 1 that had to be fulfilled among the magnetic field, the magnetic moment of the lithium nucleus, and the RF. Rabi received the Nobel Prize in 1944 for the discovery of NMR.
The NMR in molecular beams provided physicists with an elegant method to measure nuclear magnetic moments with much higher precision than could be obtained previously. However, the practical use of NMR really stems from its first observation in bulk materials—solids, liquids and even gases at normal pressures. The physicist CJ Gorter had made two unsuccessful attempts to observe NMR in bulk materials (1937 and 1943),4 and it was not clear that the weak NMR signals could be detected. The signal from a molecular beam arises from all nuclei in the beam; but in bulk materials, the theory of absorption and induced emission makes it clear that the signal would come from only the difference in the populations in the two energy levels—about 1 in 100,000. However, just after the World War II two groups succeeded in detecting NMR. On December 15, 1945, Purcell, Torrey and Pound (MIT/Harvard) found the resonance of hydrogen nuclei in a large sample of paraffin wax in a waveguide cavity.5 About a month later, Bloch, Hansen and Packard (Stanford) used a different experimental approach to find the resonance in a small sample of liquid water.6 These experiments, carried out independently and concurrently, were published in January 1946 and earned Bloch and Purcell the Nobel Prize in 1952. Purcell's address on this occasion presaged the myriad applications that would be found for NMR:7
“I remember in the winter of our first experiments, just seven years ago, looking on snow with new eyes. There the snow lay around my doorstep—great heaps of protons quietly precessing in the earth's magnetic field. To see the world for a moment as something rich and strange is 3the private reward of many a discovery. But I'm afraid it has little bearing on the sober question we, as physicists, must ask ourselves: What can we learn from all this about the structure of matter?”
APPLICATIONS IN PHYSICS AND CHEMISTRY
Once NMR could be studied in ordinary substances, myriad applications soon emerged. As the details of NMR were elucidated through theoretical and experimental work in the late 1940s and early 1950s, it became apparent that the method could be used for much more than measuring nuclear magnetic moments. Shortly after the initial publication, Bloch showed that relaxation of the nuclear spins occurs after a burst of RF power (an RF pulse). Relaxation processes are characterized by a time T1, measuring the transfer of energy from the spins to their surroundings (the lattice), and a time T2, measuring the interchange of energy among the spins themselves. Soon thereafter, Bloembergen, Purcell and Pound showed that these relaxation processes are intimately related to random molecular motion (Brownian motion), a result that would ultimately be a very important factor in the feasibility of biomedical MRI, as we see later.
About 1950, several investigations made it clear that the nuclear resonance frequency depends on magnetic shielding from the electrons around the nucleus, hence on the specific chemical environment. This chemical shift is the cornerstone of the use of NMR to elucidate molecular structure. Other phenomena, including spin-spin coupling and the nuclear Overhauser effect, were soon discovered that further enhanced the use of NMR in chemistry. Soon there were hundreds of NMR spectrometers dispersed in almost every chemistry laboratory throughout the world, and an industry developed to make dramatic improvements in technology, particularly higher and more homogeneous magnetic fields and vastly improved sensitivity. This piece of infrastructure would later play a key role in the rapid commercialization of MRI.
Methods for obtaining NMR spectra were vastly improved with the advent of pulse Fourier transform (FT) techniques that were realized just as the computer revolution permitted their incorporation into NMR instruments. The pulse FT approach resulted in a dramatic gain in effective sensitivity. Later, the development of two-dimensional FT methods provided a conceptually new way of correlating information for chemical applications and also played a key role in the realization of MRI. Richard Ernst received the Nobel Prize in 1991 for his development of FT and 2D FT methods.
BIOLOGICAL APPLICATIONS OF NMR
Since water constitutes a large percentage of all biological tissues, animals and humans have been a natural target for NMR studies. Bloch, probably, carried out the first biological NMR experiment when he put his finger into an NMR probe and found, not surprisingly, a strong signal from the water protons. However, the first real NMR investigations of biological samples, begun in 1950, aimed at studying the water content and the relaxation properties of water in bits of tissue excised from plants, animals and humans. Over the next 20 years, scores of studies of 1H, 2H, 17O and 23Na NMR in cells and tissues agreed on one common and striking feature—in nearly all cases studied, the relaxation times of water nuclei and the diffusion constants of water 4molecules are much lower than the values observed for free water, probably, the result of restricted and anisotropic motion of water molecules in the vicinity of biological macromolecules.
In 1971, Raymond Damadian made an important discovery that would have long-term consequences.8 With the background of observations of cellular water, Damadian anticipated possible differences in NMR relaxation times for water in normal cells and cancer cells. He measured T1 and T2 in pieces of tissue excised from normal rats and from fast-growing tumors that had been implanted into other rats, and found that each of the eight tumor samples had longer relaxation times than any of the normal samples. However, a number of further studies in several laboratories showed that with slow-growing mouse tumors and with excised human tissues the distinction between normal and malignant tissue was not so clear-cut. Although tumors tended to have longer relaxation times (partly, at least, because of higher water content), there was such a wide variability that relaxation measurements could not be used as a definitive method to distinguish among tissues that are normal, malignant, or pathological but not malignant. Nevertheless, the attention of a number of NMR investigators had been drawn to the possibility of using this method in medical diagnosis.
Meanwhile, in a number of laboratories, attention was being given to NMR studies within a living animal—initially worms and a rat tail, which fit into standard NMR tubes, but later to small animals that could be accommodated in a large bore magnet. Little useful information could be obtained from a spectrum of the entire animal, but localization by means of surface coils permitted spectra to acquired, particularly with 31P NMR, that gave insights into physiological function. Surface coils, however, were limited to studies of tissue near the surface, not deep within the body. The need for better localization methods for NMR research and the potential for using NMR information obtained in the living human body for medical diagnosis provided the impetus for development of new techniques.
INVENTION OF NMR IMAGING
The breakthrough came in 1971, with Paul Lauterbur's conception of the use of linear magnetic field gradients to localize NMR signal information along one dimension and to construct a two- or three-dimensional image from a collection of such one-dimensional data sets.9 Lauterbur recognized that the basic resonance equation (1) would be modified when a magnetic field gradient Gx is applied along direction x:
The resonance frequency thus provides a measure of the location of the signal along the x direction for a non-uniform macroscopic object such as an animal or a human. Lauterbur first demonstrated the application of this idea a year later, using a commercial NMR spectrometer (Varian Model A-60) and a sample consisting of two capillaries filled with water inside a 5 mm diameter sample tube filled with D2O. He used combinations of the normal x and y shim coils to generate magnetic field gradients in various directions in the xy plane, then applied a “back projection” method to generate a 2D image from these several data sets. These results were published in a short paper in Nature early in 1973.10 His paper emphasized the basic physics and the key role of the interaction of the static and RF fields. To reflect the importance of these two factors, he coined the name zeugmatography from the 5Greek word zeugma, “that which is used for joining.” He showed that the experimental image could be made dependent on relaxation times, and he correctly forecast the future wide applicability of the technique:
“The variations in water contents and proton relaxation times among biological tissues should permit the generation, with field gradients, large compared to internal magnetic inhomogeneities, of useful zeugmatographic images from the rather sharp water resonances of organisms, selectively picturing the various soft structures and tissues. A possible application of considerable interest at this time would be to the in vivo study of malignant tumours, which have been shown to give proton nuclear magnetic resonance signals with much longer water spin-lattice relaxation times than those in corresponding normal tissues.”
“The basic zeugmatographic principle may be employed in many different ways, using a scanning technique, as described above, or transient methods. Variations on the experiment, to be described later, permit the generation of two- or three-dimensional images displaying chemical compositions, diffusion coefficients and other properties of objects measurable by spectroscopic techniques. Although applications employing nuclear magnetic resonance in liquid or liquid-like systems are simple and attractive because of the ease with which field gradients large enough to shift narrow resonances by many line widths may be generated, NMR zeugmatography of solids, electron spin resonance zeugmatography, and analogous experiments in other regions of the spectrum should also be possible. Zeugmatographic techniques should find many useful applications in studies of the internal structures, states, and compositions of microscopic objects.”
A very different approach to MRI was initiated concurrently and independently by Peter Mansfield.11 Mansfield and his co-workers were exploring high resolution NMR in solids. In 1972, Mansfield devised a method using pulsed magnetic field gradients to create a diffraction pattern that could provide information on atomic separations in crystals. The first demonstrations of the feasibility of the concept were given in a paper in the Journal of Physics in 197312 and at a Colloque AMPERE in Krakow, Poland in September 1973. There Mansfield learned of Lauterbur's initial imaging paper and recognized that his method could also be used for imaging of general macroscopic objects, not just crystalline solids. Mans-field's recollection of his initial reaction to Lauterbur's paper is interesting:11
“I was struck by the stark contrast between our two approaches to imaging. In my pulsed approach, I had formally proposed the optical analogy of plane-wave scattering through a mathematical framework where reciprocal lattice space or k-space was introduced and in which the k vector was proportional to the product of time and gradient vector. The concept of k-space in MRI, of course, plays an important role these days in categorizing different imaging sequences in terms of the k-space trajectories.”
Mansfield's laboratory soon showed how a magnetic field gradient and frequency-selective pulses could be used to select specific slices in a three-dimensional object to permit 2D imaging of each slice; and later, he developed the technique of echo-planar imaging, which provides images much more rapidly than the conventional methods. Lauterbur and Mansfield shared the Nobel Prize in 2003 for their independent development of MRI.
OTHER EARLY APPROACHES TO NMR IMAGING
Lauterbur's initial paper did not attract wide 6attention in the NMR community, but his presentations at two NMR conferences had dramatic effects.
2D FT Method
At the Experimental NMR Conference (ENC) in Raleigh, North Carolina, in April, 1974, Richard Ernst heard Lauterbur's talk on zeugmatographic imaging and immediately recognized that the two-dimensional NMR method he was beginning to develop could be used with switched magnetic field gradients to obtain images. In Mansfield's terminology, Ernst's method scanned k-space in a different and more efficient manner than Lauterbur's initial back projection method. Shortly thereafter, Kumar, Welti and Ernst13 obtained an image, again of two tubes of water, with repetitive experiments using an x gradient that is stepped through successive time periods after the exciting RF pulse is applied (so-called phase encoding), while the y gradient is applied during data acquisition (frequency encoding). As this 2D imaging method began to be used in more sophisticated practical applications, it became clear that distortions could be introduced with the variable time periods for the phase encoding step. This artifact was overcome by Edelstein, Hutchison, Johnson and Red path14 by using a constant time but variable strength field gradient for phase encoding. With this so-called spin warp refinement, the basic Lauterbur concept, implemented in Ernst's 2D manner, has become the standard method for MRI diagnostic imaging.
Sensitive Point Method
Other approaches to NMR imaging, developed in the mid-1970s, were initially useful; but ultimately, proved to be inefficient for most practical imaging applications. One arose from a talk by Lauterbur in January 1974, at the meeting of the International Society of Magnetic Resonance (ISMAR) in Bombay (now Mumbai). Two members of the audience from the University of Nottingham—Waldo Hinshaw and William Moore—were inspired to begin a new approach as soon as they returned home. They developed a simple method that was easy to implement with equipment then available, the sensitive point method. They applied oscillating magnetic field gradients in the x, y and z directions concurrently across the sample, so that only the sample in a very small volume remains in resonance, hence contributes to the imaging signal. By altering the electrical parameters of the coils used to create the gradients, the sensitive point could be moved through the sample and eventually produce a 3D image.15 Within a few months, this simple method had been developed to produce images of small objects such as fruits and vegetables. The problem is that a signal is generated from only one point at a time, a very inefficient procedure that requires a lengthy period for data acquisition for a large sample. Improvements were made to generate a sensitive line, rather than a point, and this method was used in early human imaging, as described later, but this technique was never developed extensively in commercial application.
FONAR
A somewhat similar method that relied on obtaining signal from only one small portion of sample at a time was developed by Raymond Damadian. Following his observation that relaxation times in excised tissue from some tumors were longer than those in normal tissue, Damadian began to think about obtaining NMR information from 7within a human body. In a patent application in 1972 that covered his work on excised tissue, he also speculated on a large in vivo NMR system in which the RF could some-how be focused into a narrow beam:16
“In Figure 2 (not shown here) an electromagnet shown in cross-section is designed to have sufficiently large dimensions to hold a mammal or human being to be examined. A transmitter probe is provided with a beam focusing mechanism for focusing the radiated magnetic energy from the radiofrequency generator into a beam having a narrow cross-section. This probe is slidably mounted on a helical track and positioned so that the radiated beam is orthogonal to the direction of the field provided by the electromagnet. The transmitter probe is moved on the track by means not shown so that the probe may scan the entire body to be examined. Also mounted on the track is a receiver probe of the same design as the transmitter probe which detects a beam having the same cross-sectional width as the beam radiated by the probe…”
Focusing RF of several meter wavelength into a narrow beam flies in the face of well- known optical principles, Damadian turned his attention to the design and construction of a large, low field magnet where the static field B0 could be shaped with suitable shim coils to obtain a small homogeneous volume, with the homogeneity rapidly falling off outside this volume. Damadian called the method “field focusing NMR” and coined an acronym FONAR. FONAR is similar to the sensitive point method in obtaining data inefficiently, from only one small volume at a time. Moreover, FONAR has the additional disadvantage that the location of the homogeneous volume is fixed by the design of the magnet, so the subject must be physically moved in three orthogonal directions to scan a macroscopic volume, and the magnet must be large enough to accommodate this movement.
In spite of these disadvantages, Damadian and co-workers were able to construct a magnet for animal studies and obtain an image of the thoracic cavity of a mouse in 1976, with an estimated resolution of 3 mm.17 They also showed the image of a mouse in which a tumor had been implanted in the upper thorax. By 1977, they had constructed a 0.05 T magnet of 53 inch diameter, with an NMR frequency of 2 MHz, and were able to demonstrate an image of a human chest.18 With the very low field and point-by-point method, the signal/noise was poor and it took several hours to complete a scan. The resolution was poor; but major features, such as the heart and lungs, could be identified. Damadian pursued this method with the establishment of the FONAR Corporation, which produced a few instruments based on the FONAR method. However, by 1981, the FONAR Corporation joined other companies in adopting the Lauterbur field gradient principle as embodied in the Ernst 2D method with spin warp modification.
DEVELOPMENT OF HUMAN MRI
The first live human NMR image was published by Mansfield and Maudsley in 1977—a line scan of the internal anatomy of a finger, an appendage small enough to be inserted into a standard NMR magnet.19 Contrast between bone, bone marrow, nerve, artery and other tissues was demonstrated. Also, in 1977, Andrew et al20 used the multiple sensitive point method and a larger gap electromagnet to image a hand, while Hinshaw, Bottomley and Holland examined Bottomley's wrist and discussed potential medical uses.21
Serious application to most anatomical studies required a magnet capable of holding 8a human being. The necessary scale-up from traditional NMR sample tube sizes of 5 to 20 mm diameter to nearly a one meter bore for a whole human body was a daunting task, which could be accomplished only with a corresponding reduction in the magnitude of the magnetic field. Most early efforts were directed toward electromagnets with an air core, designed for a field of about 0.1 T (4 MHz for 1H NMR), except for Damadian's 0.05 T superconducting magnet. In 1978, Mansfield et al observed the first image of the human abdomen,22 and Clow and Young reported the first NMR image of a section through a human head.23 Features such as the eyeballs and ventricles in the brain were clearly visible. By 1980, Moore and his colleagues at Nottingham were able to demonstrate one advantage of NMR imaging over X-ray computed tomography (CT) in showing the first sagittal and coronal sections of a human head.24 In another paper, the same year they showed that NMR images could reveal a wide range of pathology in the head and commented on the “dramatic … new advance in neuroradiological capability with its potential impact on clinical management.”25 Their clinical results provided examples that included tumors, aneurysms, circulatory malformations and chronic sinus infection.
As these successful applications became more widely known, interest accelerated among a number of companies in developing MRI instruments commercially for a potentially very large and lucrative market in medical diagnostic imaging. There is a fascinating story—too lengthy to be presented here—of the concerted efforts in several companies in the USA and Europe to rapidly convert laboratory prototypes to clinically useful instruments. Superconducting magnets with a 1 meter bore were developed initially at 0.5 T; but by the early 1980s, a field of 1.5 T (about 60 MHz for proton NMR) became the industry standard. By October, 1987, sufficient clinical data had been obtained for the National Institutes of Health in the United States to convene a Consensus Conference, in which a “jury” of radiologists and other clinical experts could assess the state of the art and make recommendations on optimum uses of MRI. The following excerpts from the Conference Statement highlight the prevailing views:26
“Even in the short period of its use, it has proved to be unusually rewarding in the detection, localization, and assessment of extent and character of disease in the central nervous, musculoskeletal, and cardiovascular systems. In the brain, for example, it has a proven capacity to define some tumors and the plaques of multiple sclerosis provided by no other technique. It is a competing imaging method in the evaluation of many other organs. Additional prospective studies comparing MRI with other diagnostic methods are essential in those areas where the method has shown promise but where its precise role has not yet been defined. This consensus development conference does not purport to include all of the applications of MRI to the pediatric patient, a subject that will require separate consideration… The full potential of MRI has not been reached, and continuing refinement of equipment, contrast agents, and software may be anticipated…”
The final sentence quoted was prophetic. MRI has become widely available and is regarded as an indispensable diagnostic modality. New generations of magnets have been developed at both higher and lower magnetic fields for specific purposes. Faster scanning methods have become available, and the quality of images has steadily 9improved. Moreover, imaging methods have been used to study function, as well as anatomy, and imaging principles have been used for localized in vivo NMR spectroscopy, as described in a number of other articles in this book.
SOME ANTECEDENTS OF MRI METHODS
I would like to close this account by recalling some of the early methods and technological developments in NMR which occurred long before the invention of NMR imaging in the early 1970s but which were crucial to the development of MRI as we now know it.
Chemical Applications
One important aspect that is sometimes forgotten is the long history of chemical applications of NMR. Once the chemical shift and spin coupling had been discovered about 1950, chemical applications dominated commercial development of NMR, just as imaging would do three decades later. During this 30-year period, NMR became an invaluable tool for chemical research only because of:
- improvements in the homogeneity of B0
- introduction of a field/frequency lock to stabilize B0
- continued increase in the magnitude of B0 in order to accentuate chemical shift differences and to improve sensitivity
- improvements in probe design and electronic circuitry that increased sensitivity by orders of magnitude
- introduction of pulse Fourier transform methods to speed up data collection and further enhance sensitivity
- introduction of multiple pulse experiments, including two-dimensional NMR, that greatly expanded the scope of NMR
Lauterbur's initial demonstration of imaging was carried out on a Varian A-60 spectrometer that was widely used for chemical analysis. Indeed, his experiment was simple to implement: He had only to twist the x or y electric shim control from its optimal position for maximum field homogeneity to an extreme position where it introduced the usually unwanted magnetic field gradient that is at the heart of imaging. Almost all early imaging experiments in academic labs used magnets developed commercially primarily for chemical applications. The rapid scale-up of magnets to human size and the development of software designed for MRI were remarkable achievements, but they were possible only because of the advanced technology already available for chemical NMR.
The First NMR “Image”
There is no question that Lauterbur first conceived the idea of employing field gradients in separate directions and using the resulting NMR data to construct two- or three-dimensional images of macroscopic objects. However, he was not the first to apply a magnetic field gradient to discriminate objects along a single axis. In fact, what is probably the first one-dimensional NMR image was obtained by Herman Carr in Purcell's lab at Harvard and reported in Carr's PhD thesis in 1952.27 There is an interesting story behind this experiment: Long before Fourier transform NMR methods had revolutionized the field, physicists were aware of the Fourier transform relation between a slow passage continuous wave (cw) NMR spectrum and the free induction decay (FID) following a 90° RF pulse. Carr and others made “mental” Fourier transforms of FIDs. For two or three 10lines of equal intensity, it was not too difficult to make the mental transform and picture the cw spectrum. However, for systems with unequal line separations and unequal intensities, the process was more difficult. A “hot” topic at the time was the recently discovered 1H chemical shift in ethanol (CH3CH2OH), which displayed a readily interpretable cw spectrum—three lines of intensities 3:2:1. The corresponding FID, however, was a complicated beat pattern. To convince visitors of the equivalence of the two responses, Carr made what in current NMR imaging would be called a “phantom”, three pieces of rubber of volumes 3:2:1, which were placed in a magnetic field gradient that simulated the chemical shifts in ethanol. The FID obtained from this phantom agreed with that from liquid ethanol itself, thus verifying Carr's interpretation.
Echoes
Whatever pulse sequence is used to obtain an NMR image, a spin-echo is invariably involved in some way. The discovery of the spin echo by Erwin Hahn in 1949 ranks as a milestone in NMR,28 since it introduced a principle of reversibility that might at first appear to be physically impossible. The spin-echo has proved to be widely applicable in all sorts of NMR experiments. The physics of the spin-echo will be covered elsewhere; here I comment only on its accidental discovery. Hahn was investigating the use of short RF pulses when he observed what he calls “a weird signal” that appeared when no pulse was being applied.29 He initially considered it an artifact but persisted in exploring the phenomenon and soon recognized that he had observed a refocusing of magnetization that had apparently decayed to zero. Carr and Purcell investigated the spin echo in detail and developed a more effective pulse sequence,30 later improved by Meiboom and Gill (the CPMG sequence),31 that formed the basis for making reliable measurements of T2. Carr and Purcell also introduced the 180°-τ-90° pulse sequence to measure T1. These sequences have had enormous impact in MRI, as T1- and T2-weighted images play a critical role in diagnosis.
The pulse sequences for diffusion-weighted images can also be traced back to the early 1950s when the CPMG sequence was used to measure diffusion in liquids under the influence of a steady magnetic field gradient. A much more effective method was introduced in 1963 when Stejskal and Tanner32 showed that a pulsed field gradient offered independent control of the magnitude and length of the gradient without interfering with the measurement of the spin echo. In fact, the pulsed field gradient had been introduced originally by Hahn and co-workers33 in 1955 to avoid spurious echoes, an application that survives in imaging methods as crushers and spoiler gradients.
Other applications of echoes abound in imaging. The stimulated echo from a 90°-τ-90°-τ-90° pulse sequence was also discovered by Hahn and now appears in imaging methods. The gradient-recalled echo (GRE) was first reported by Herman Carr in his PhD thesis in 1952.27 Rather than reversing the direction of the gradient to create the echo, he rotated the sample in the gradient (the first example of sample spinning). However, there was little application of the GRE until it was introduced in the form of a pulsed gradient as a key element of imaging methods.
CONCLUSION
This short chronology of some early developments in NMR only scratches the surface 11in describing the conceptual advances, the innovative techniques that have been introduced, and the extremely wide range of applications for NMR that have come about in the 60 years since the discovery of NMR in bulk materials. Modern MRI and its close relative, localized NMR spectroscopy, are feasible only because this whole array of technology has now come together to create the powerful methods we now employ. There is no sign of any abatement in further rapid advances in this field.
REFERENCES
- Becker ED (Ed) Historical Perspectives, Vol. 1 of Grant DM, Harris RK (Eds) Encyclopedia of Nuclear Magnetic Resonance Chichester, John Wiley and Sons, England: 1996.
- Estermann I, Stern O. Z Phys 1933; 85:17;
- Frisch R, Stern O Z Phys 1933; 85:4.
- Rabi II, Zacharias JR, Millman S, Kusch P. Phys Rev 1938; 53:318.
- Gorter CJ. Physica (The Hague) 1936; 3: 995;
- Gorter CJ, Broer LJF. Physica (The Hague) 1942; 9: 591.
- Purcell EM, Torrey HC, Pound RV Phys Rev 1946; 69: 37.
- Bloch F, Hansen WW, Packard M Phys Rev 1946; 70: 474.
- Purcell EM, Les Prix Nobel 1952.
- Damadian R. Science 1971; 171: 1151.
- Lauterbur PC. “One path out of many—how MRI actually began”. Ref. 1, 445–49.
- Lauterbur PC. Nature (London) 1973; 242: 190.
- Mansfield P. “A personal view of my involvement in the development of NMR and the conception and development of MRI”. Ref. 1, 478–81.
- Mansfield P, Grannell PK. J Phys C 1973; 6: L422.
- Kumar A, Welti D, Ernst RR. J Magn Reson 1975; 18: 69.
- Edelstein WA, Hutchison JMS, Johnson G, Redpath T. Phys Med Biol 1980; 25: 751.
- Hinshaw WS. Phys Lett 1974; 48A: 87.
- Damadian RV. US Patent 3 789 832 (Filed March 17, 1972, Issued February 5, 1974).
- Damadian R, Minkoff L, Goldsmith M, Stanford M, Koutcher J. Science 1976; 194: 1430.
- Damadian R, Goldsmith M, Minkoff L. Physiol Chem Phys 1977; 9: 97.
- Mansfield P, Maudsley AA. Br J Radiol 1977; 50: 188.
- Andrew ER, Bottomley PA, Hinshaw WS, Holland GN, Moore WS, Simaroj C. Phys Med Biol 1977; 22: 971.
- Hinshaw WS, Bottomley PA, Holland GN. Nature (London) 1977; 270: 722.
- Mansfield P, Pykett IL, Morris PG, Coupland RE Br J Radiol 1978; 51: 921.
- Clow H, Young IR. New Scientist 1978; 588.
- Holland GN, Hawkes RC, Moore WS J Comput Assist Tomogr 1980; 4: 429.
- Hawkes RC, Holland GN, Moore WS, Worthington BS. J Comput Assist Tomogr 1980; 4: 577.
- Magnetic Resonance Imaging: National Institutes of Health Consensus Development Statement, Office of Medical Applications of Research, NIH, Bethesda, MD, 1987.
- Carr HY PhD Thesis, Harvard University, Cambridge, MA, 1952. See also “Early Years of Free Precession Revisited,” Reference 1, 253–60.
- Hahn EL. Phys Rev 1950; 77: 297.
- Hahn EL. “Pulsed NMR—A personal history,” Reference 1, 373–78.
- Carr HY, Purcell EM. Phys Rev 1954; 94: 630.
- Meiboom S, Gill D. Rev Sci Instrum 1958; 29: 688.
- Stejskal EO, Tanner JE. J Chem Phys 1965; 42: 288.
- Anderson AG, Garwin RL, Hahn EL, Horton JW, Tucker GL, Walker RM. J Appl Phys 1955; 26: 1324.