INTRODUCTION
The mystery of development is the question of how the fertilized egg under right conditions gives rise to a complex multicellular organism consisting of differentiated cells arranged in a precise pattern. The first month of the human embryo development is characterized by two main morphogenetic processes: gastrulation and neurulation. During this period the embryo is in the intimate contact with the endometrial wall and therefore hidden from the view of ultrasound.
In the early embryonic development period, dating of pregnancy in humans is very imprecise. From this reason Carnegie staging system was established. It uses precise criteria based on groups of morphological characteristics classified into 23 stages determined on specimens from Carnegie collection of human embryos. The Carnegie staging system is also very commonly used in comparative and experimental investigations of early mammalian development.
EARLY EMBRYONIC DEVELOPMENT
First Week of Development: Pre-embryonic Period
Fertilization
Human development as a repeating process from generation to generation starts by fertilization, which usually takes place in the ampulla of the uterine tube. Fertilization is a complex process (not a moment) that involves some preparations as well as set of interactions between sperm and egg.
The ejaculated sperm first must undergo a process of maturation, known as capacitation, which is the alteration of the glycoprotein surface of sperm under the influence of secretion products of the femal reproductive tract.
The ovulated oocyte is a large cell approximatelly 80 µm in diameter, surrounded by folicular granulosa cells called corona radiata, and by a 13 µm thick, transparent acellular glycoprotein envelope—zona pellucida. The zona is composed of three glycoproteins: ZP1, ZP2 and ZP3. ZP3 functions as a species-specific receptor for complementary molecules on the head of the sperm and it influences sperm binding to the zona pellucida. After process of binding, ZP3 is also responsible to induce the acrosomal reaction of the sperm.1, 2 The acrosome membrane fuses with the outer sperm membrane and releases enzymes, which start to digest the zona. In this way sperm reaches the oocyte membrane, binds and fuses with it. After initial fusion of sperm's and egg's plasma membranes, the head and tail of the sperm sink into the egg. Once the sperm has fused with the egg, the polyspermy is prevented by the cortical reaction of the egg. The sperm-egg syngamy propagates a number of sequential events, i.e.:
- Metabolic activation of the egg
- Decondensation of the sperm nucleus
- Completion of second meiotic division of the egg
- Development and fusion of male and female pronuclei
- Restoration of the normal diploid number of chromosomes
- Formation of a zygote
- Determination of the sex of the future embryo.
Cleavage
The process of fertilization results in the zygote formation. Zygote divides after 24 to 36 hours into two cells and begins the process of cleavage. During cleavage, through subsequent divisions, the daughter cells called blastomeres are gradually smaller in diameter, and they form the morula. The size of the morula is constant because it is still enclosed within zona pellucida.4
In the two- and four-cell stage the blastomeres are identical in their capabilities expressing totipotency (Fig. 1.1). That indicates one of possibilities of twin formation. At the eight-cell stage the first communications between cells appear through formation of the intercellular contacts. This process is called compaction.3,4 The densely packed cells form a continuous epithelial layer, thus defining inside and outside of the embryo. When the morula reaches 32- to 58-cell number it is referred as free (early) blastocyst. Inside it contains a cavity with secreted fluid known as blastocele. The cells segregate into an internally situated inner cell mass or embryoblast and surrounding, outer trophoblast cells (see Fig. 1.1)
The events described above belong to the first week (preimplantation) period during which the transportation of zygote, morula and early blastocyst occurs from ampullar tube region to the uterus.
Second Week of Development: Implantation and Differentation of Bilaminar Embryonic (Germ) Disk
Implantation
Human implantation is difficult to identify in the uterus. The evidence is based on morphological studies of human and animal embryos, and on experimental data mostly in the mouse.
Successful implantation depends on coordination of both the blastocyst and the endometrium. The complex preparation for the implantation (dialog between mother and embryo) starts during the preimplantation period, soon after fertilization. It includes secretion of hormones, numerous signal molecules, factors and receptors from both blastocyst and endometrium. By that time the endometrium undergoes hormonaly induced preparation—decidualization. This process is characterized by series of changes in glandular and surface epithelium, stromal cells, stromal vessels and extracellular matrix components. The 5changes are known as “the window of implantation,” which corresponds to a range of morphological and biochemical modifications of the endometrium during its maximal receptivity of blastocyst, which are favorable to the further development of the embryo and enable immunological tolerance.5–7
After the blastocyst reaches the uterine cavity the first step in preparing implantation is “hatching” from the zona pellucida by enzymes secreted by trophoblastic cells.
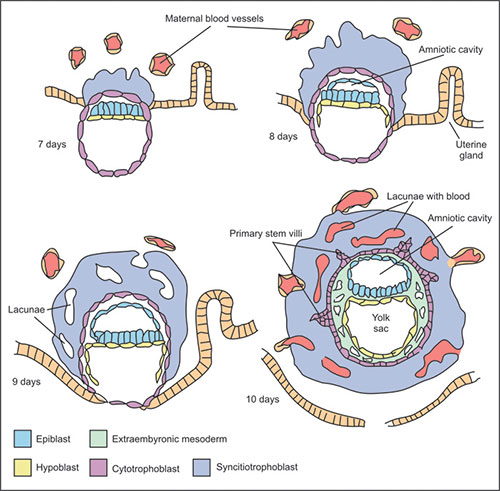
Approximately 5 to 6 days after fertilization, the blastocyst begins the “attachment” to the surface epithelia of the endometrium (Fig. 1.2). The apical surface of the endometrial cells expresses a variety of adhesion molecules called integrins. Both in vivo and in vitro studies have shown that attachment of the blastocyst occurs at the area above the embryoblast (inner cell mass) referred as embryonic pole. In this area the trophoblast proliferates and differentiates into inner cellular cytotrophoblast and outer multinucleated syncytiotrophoblast, where cells loose border membranes, and coalesce to form syncytium.
The next step of implantation is penetration (invasion) of the uterine epithelium by highly invasive syncytiotrophoblastic projections. By days 9 after fertilization, they expand and erode quickly into the endometrial stroma. Within the syncytiotrophoblast lacunae appear. On day 10, the embryo is embedded in the endometrium, and syncytiotrophoblast erodes endometrial blood vessels walls.
Fig. 1.2: Implantation period and bilaminar embryonic disk with the amniotic cavity and yolk sac formation
The maternal blood begins to fill the lacunae that have been forming in the trophoblast (Fig. 1.2). Maternal capillaries near the syncytiotrophoblast expand to form maternal sinusoids that rapidly anastomose with the trophoblastic lacunae, and the primitive uteroplacental circulation is established.
Bilaminar Embryonic (Germ) Disk
During the implantation period and differentiation of the trophoblast, the cells of the embryoblast (inner cell mass) split into two layers, i.e. the upper epiblast or primary ectoderm, and the lower hypoblast, primary or primitive endoderm. These two layers form the bilaminar germ disk, which develops into the embryo proper. A cavity called amniotic cavity or amnion develops within the epiblast. The cells that form the amniotic membrane are amnioblasts. The amniotic cavity is very small, but by the time it expands steadily (by the 8th week it encloses the whole embryo). The hypoblast gives rise to the endodermal lining of the primary yolk sac. On day 8, cells at the periphery of the hypoblast migrate out over the inner surface of the cytotrophoblast. On day 10, the flattened hypoblastic cells form exocelomic (Heuser's) membrane, lining the cavity called primary yolk sac or exocelomic cavity. At that stage the bilaminar germ disk is located between the primary yolk sac on ventral side and the amniotic cavity on its dorsal surface (see Fig. 1.2).
By day 12 the cells of hypoblast proliferate and migrate again forming a secondary or definitive yolk sac, while the primary yolk sac becomes constricted and pushed in front of it. The secondary yolk sac remains as an important structure through the fourth week giving rise to endothelial and hematopoietic stem cells (blood islands), as well to the primordial germ cells that migrate to the gonads.
Starting at about days 12 some cells of the hypoblast give rise to another extraembryonic tissue, the extraembryonic mesoderm. This mesoderm becomes the first connective tissue that supports the epithelium of amnion and yolk sac as well as the chorion (chorionic villi), which arises from the trophoblast.
Third Week of Development: Gastrulation
Formation of the Trilaminar Embryonic (Germ) Disk
At the end of the second week the primitive streak appears as a thickening on the dorsal surface of the epiblast near the caudal end of the bilaminar germ disk. The superior end of the primitive streak contains small elevation called primitive or Hensen's node.
On day 16, the cells of epiblast proliferate, migrate and form a groove along the midline of the region of the primitive streak. As the cells of epiblast reach the primitive streak, they change the shape and migrate down between the epiblast and hypoblast. Some of them displace the hypoblast cells replacing them with a layer of definitive or secondary endoderm. Others migrate laterally and cranially between epiblast and hypoblast and form a new germ layer, the intraembryonic or secondary mesoderm. The bilaminar embryonic disk is converted into trilaminar embryonic disk consisting of three definitive germ layers, i.e. ectoderm, mesoderm and endoderm. This process called gastrulation is the most important event in the early development establishing the main body axis and the source of embryo tissues and organs.
The cells passing through the primitive streak change their structure and organization. While the epiblast cells have typical epithelial structure and properties, upon invagination cells elongate, loose basal lamina and take a characteristic bottle shaped morphology. Reaching the space between epiblast and hypoblast, the bottle cells assume the structure of mesenchymal cells (Fig. 1.3).
Regression of the Primitive Streak
As mentioned before the primitive streak appears on day 16 and expands cranially until day 18. After that time it regresses caudally and soon disappears. The remains of primitive streak cells can produce tumors in sacrococcygeal region, referred as teratomas. Teratomas consist of many different tissues (cartilage, muscle, hair, fat glands epithelial tissue) as they contain cells, which can give rise to derivatives of all three germ layers.
Notochord
The epiblast cells in the area of the primitive node invaginate and migrate in midline and cranial direction (day 16 to 17) and give rise to the notochordal plate and notochordal process, which differentiate in the definitive notochord completed at day 31. The notochordal cells produce extracellular matrix molecules including collagen and form the primitive axial skeleton of the embryo (see Fig. 1.3). The notochord plays an important role in the induction of the overlying ectoderm in early development of the nervous tissue, a process called neurulation. The notochord also induces the vertebral body formation. The failure of these inductive interactions results in various vertebral column and spinal cord abnormalities, including spina bifida.
Abnormal Gastrulation
The period between 3 and 8 weeks of development is the critical period for abnormal development of the embryo, as this is the time when organs and systems are being established. The defects in gastrulation can have wide consequences and could influence a body plan. For example, abnormal mesoderm formation in the third week may cause several syndromes affecting caudal part of the body referred to as caudal agenesis, sacral agenesis and caudal dysplasia. Syrenomelia is severe reduction of the caudal structures, which results in fusion of the lower extremity limb buds. Milder anomalies of this type exhibit varying degrees of neurologic deficit particularly in the caudal regions. Some caudal malformations are associated with cranial abnormalities called VATER, including some or all of these: vertebral defects, anal atresia, tracheal-esophageal fistula and renal defects.
Differentiation of Germ Layers
Differentiation of Ectoderm: Neurulation
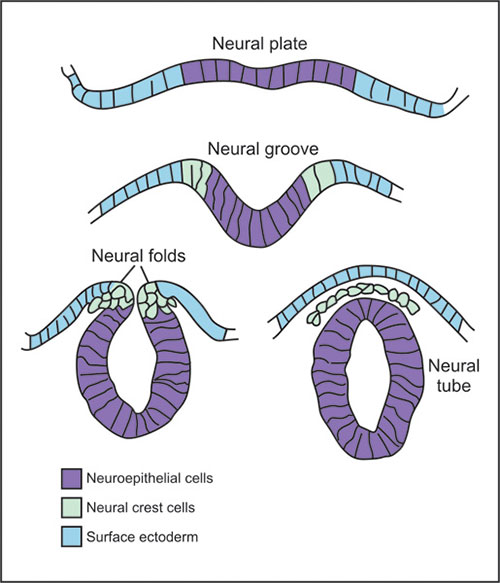
Upon influence of the notochord the above ectoderm starts the process of neurulation, i.e. formation of the neural tube (Fig. 1.4). This process consists of series of steps. First the ectoderm positioned in the midline, which corresponds to the region above the underlying notochord, thickens and forms the neural plate. The formation of neural plate is a sign for division of ectoderm in two separate regions, i.e. neuroectoderm in the midline which will give rise to the nervous system, and surface ectoderm laterally which will give rise of the epidermis of the skin. Lateral parts of the neural plate start to bend and form neural folds surrounding the neural groove in the middle. The neural folds approach dorsally in the midline and close the structure in the neural tube. The closure of the neural tube starts at the hindbrain/spinal cord boundary and then proceeds in anterior and posterior directions. Therefore until neurulation is completed two openings are present at the both sides of the closing neural tube: anterior neuropore and posterior neuropore. During the process of the neural tube closure cells located at the border between neuroectoderm and surface ectoderm migrate out and form the neural crest. The neural crest cells migrate to variety of destination throughout the body and give rise to the range of derivatives including those belonging to the nervous system like spinal and autonomous ganglia, but as well those not related to the nervous system like mesenchymal cells of head and neck, or melanocytes of the skin.
The process of neurulation is rather complex and it involves a cascade of events generated both inside the neuroectoderm, as well as in the surrounding tissues. One of the forces that influence change of shape of neuroectodermal cells is a constriction of apical part of the cells mediated by microfilament bundles. In addition one medial and two lateral hinge points within the neuroectoderm aid the elevation of neural folds. The bending of neural plate is as well supported by expansion of the neighboring mesodermal cells. Cell-cell affinities and cell-cell communications are important for the final fusion of the neural folds when the neural folds have approached each other.7,8
The failure of neural tube to close or its reopening leads to neural tube defects (NTDs), which are among the most common congenital defects in human. The NTDs are manifested as anencephaly in the cranial region or spina bifida in the lumbosacral region.
Differentiation of Mesoderm
During the third week the mesoderm cells which migrate through the primitive streak and laterally from it begin to condense into sheet-like structure. After notochord formation the mesoderm forms paired cylindrical condensations called the paraxial mesoderm. Paraxial mesoderm condenses in groups of cells positioned along the notochord and the neural tube and forms somites. Continuous with the paraxial mesoderm is a lateral region of the intermediate mesoderm which laterally splits into lateral plate mesoderm forming two layers: the parietal (somatic), and visceral (splanchnic) sheath (Fig. 1.5).8
Between them the intraembyonic celom develops.
The paraxial mesoderm differentiates into the axial skeletal structures, voluntary musculature, and connective tissue (dermis) of the skin. The intermediate mesoderm produces urinary system and part of the genital system. The lateral mesoderm (somatic and splanchnic) forms the bulk of the body wall, the wall of the digestive tube and limbs (Fig. 1.6).
Differentiation of Endoderm
Endoderm gives rise to the epithelial lining of the gastrointestinal and respiratory tracts and some glands (Fig. 1.6).
Development of the intraembryonic endoderm germ layer is connected with the folding of the embryo in the craniocaudal direction forming a C-shaped structure. Early in the third week the roof of the yolk sac constitutes future intraembryonic part. By the folding of embryo this flat part transforms into a tubular primitive gut delineated from the yolk sac. The primitive gut differentiates into the foregut, midgut and hindgut. The midgut remains open to the yolk sac ventrally. The anterior end of the embryo foregut is temporarily connected to the surface ectoderm forming a bilayer called oropharyngeal membrane, which separates the future mouth - stomodeum. The caudal part of hindgut — cloaca forms another endodermal-ectodermal attachment referred as the cloacal membrane.
At the end of the fourth week the region of the yolk sac is reduced to form the yolk stalk (omphalomesenteric or vitelline duct).
EARLY CIRCULATION DEVELOPMENT
Starting on day 17, first vessels primordia begin to develop in the extraembryonic mesoderm of yolk sac wall forming aggregations of cells called blood islands. This process occurs under inductive interaction of endoderm of yolk sac. On the day 18 vasculogenesis (blood vessels formation) begins. The blood island cells are pluripotent stem cells—hemangioblasts, peripherally differentiating into the endothelial vascular wall and centrally into primitive hematopoietic cells.9
Fig. 1.5: Transverse 1 µm thick section stained by toluidine blue of the human embryo (2 mm crown-rump length, 21 to 22 days)
The vessels fuse to form networks of endothelial channels. The primitive blood vessels by the same time extend into the mesenhyme of the connecting stalk (allantoic) and chorionic villi, and by the end of the third week the vascular extraembryonic network is established.
On days 18, vasculogenesis in the embryo begins in the splanchnic mesoderm and it does not involve any more formation of blood cells. The mesenchymal cells differentiate in the angioblasts, which develop into the flattened endothelial cells forming angiocysts. Later angioblastic cords develop by the fusion of the angiocysts forming a network of angioblastic plexuses that establish the initial circulatory system of the embryo. The yolk sac is the first supplier of blood cells to the embryonic circulation. Later in the week 5, the blood cell production (hematopoiesis) starts in embryonic organs, including liver, spleen, thymus and bone marrow. The source of these cells is unclear.
The primitive heart forms in a similar manner from mesenchymal cells in the cardiogenic area. The cardiac primordium is located in the front of the head fold. Paired endocardial heart tubes develop before the end of the third week. Soon after these tubes approach each other and fuse into a primitive heart tube.10
The heart elongates and develops dilatations and constrictions: the truncus aretriosus, bulbus cordis, primitive ventricle, primitive atrium and sinus venosus.
By the end of the third week the heart tubes are linked up with blood vessels in the embryo, connecting stalk, chorion, and yolk sac to form a primitive cardiovascular system. The circulation of blood starts by the end of the third week when contractions of the primitive heart begins (day 22). The cardiovascular system is the first organ system to reach a functional state.
The blood from the primitive heart is distributed by aortic arches to the tissue of branchial arches (future head and neck region), and by the aortae and their branches to the caudal part of the embryo body.
REFERENCES
- Cohen N, Wasserman PM. Association of egg zona pellucida glycoprotein mZP3 with sperm protein sp56 during fertilization in mice. Int J Dev Biol 2001; 45:569–76
- Esterhuizen AD, Franken DR, Luorens JGH, van Royen LH. Clinical importance of zona pellucida-induced acrosome reaction and its predictive value for IVF. Hum Reprod 2001; 16:138–44
- Carlson BC. Human Embryology & Developmental Biology, Mosby 1999.
- Tao J, Tamis R, Fink K, Williams B, Nelson-White T, Graig R. The neglected morula/compact stage embryo transfer. Hum Reprod 2002; 17:1513–8.
- Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA. 2003; 100:2963–8.
- Lukassen HG, Joosten I, van Cranenbroek B, van Lierop MJ, Bulten J, Braat DD, van Der Meer A. Hormonal stimulation for IVF treatment positively affects the CD56bright/CD56dim NK cell ratio of the endometrium during the window of implantation. Mol Hum Reprod 2004; 10:513–20.
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn 2001;221:117–45.
- Melton KR, Iulianella A, Trainor PA. Gene expression and regulation of hindbrain and spinal cord development. Front Biosci 2004;9:117–38.