(South Africa)
INTRODUCTION
Aqueous produced by the ciliary body flows out against a resistance in the anterior chamber. This resistance to outflow is responsible for an intraocular pressure (IOP) of approximately 15 mm Hg, which is necessary for the shape and optical properties of the globe. Several prospective, randomized clinical trials substantiated the benefit of lowering the intraocular pressure in the treatment of glaucoma.
Alterations in the IOP is mostly due to changes in the outflow resistance. Knowledge of the outflow routes and the factors that influence it is crucial in the understanding of glaucoma and its treatment. Two routes for aqueous humor outflow exist: the conventional (trabecular)and unconventional (uveoscleral and possibly uveovortex) routes.
In the conventional route aqueous humor flows through the trabecular meshwork into Schlemm's canal, and from there into the episcleral veins via collector channels. This outflow is pressure dependant (outflow is diminished by an increased IOP), and is increased with an increase in ciliary muscle tone. The major resistance is believed to reside in the cribiform portion of the meshwork and flow through this juxtacanalicular connective tissue into Schlemm's canal seems to be through both intracellular and intercellular transport mechanisms. This transport system appears to be primarily passive, but active transport may also contribute.
Unconventional outflow occurs through the root of the iris and interstitial spaces of the ciliary muscle reaching the suprachoroidal space. From there it reaches the episcleral tissue via scleral emissary canals that contain ciliary nerves or via perforating ciliary vessels, vessels of optic nerve membranes, or directly through the collagen substance of the sclera. Outflow appears to be pressure independent, and is decreased with increased ciliary muscle tone. The extracellular matrix appears to contribute to the outflow resistance of both the conventional and unconventional pathways.
EMBRYOLOGY
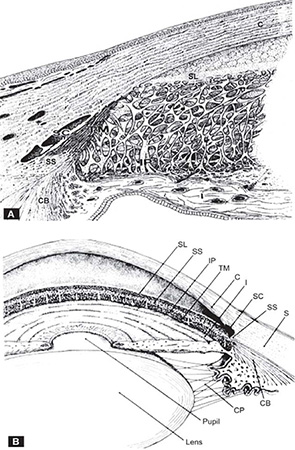
The study of childhood glaucoma necessitates an understanding of the development of the anterior chamber (Table 1.1). The lens vesicle develops as an invagination of the surface ectoderm, and separates from it by the fifth to sixth week of gestation.1 The mesenchyme situated on the anterior surface of the lens condenses to form the pupillary membrane. The two layers of neuroectoderm forming the edge of the optic cup extend onto the posterior surface of the pupillary membrane.2 Cells from this tissue move anteriorly in three successive waves between the sixth and eighth weeks, forming the corneal endothelium, iris stroma and the corneal stroma.3 The beginnings of the angle results from the ingrowth of the first wave of mesenchymal cells (the future corneal endothelium)and the posterior extension of the second wave of cells (the primary pupillary membrane) from the rim of the optic cup (Figures 1.1A and B). The sphincter and dilator muscles of the iris are derived from the pigment cells of the neuroectoderm. The mesenchyme forms the connective tissue and blood vessels of the iris.4
FIGURES 1.1A and B: Diagrams showing the development of the iris and ciliary body. The two layers of neuroectoderm forming the edge of the optic cup cover the ciliary muscle and then extend onto the posterior surface of the papillary membrane.From Snell RS, Lemp MA. Clinical Anatomy of the Eye, 2nd edition, Blackwell Science, 1998)
The anterior chamber arises as a slit in the mesenchyme between the surface ectoderm and the developing iris. The anterior chamber angle is formed by the third and fourth months of gestation as described above. By the fifth month the anterior chamber angle is rounded and lies at the level of Schlemm's canal. At birth the apex of the angle is at the level of the scleral spur. A progressive deepening and posterior movement of the angle recess continues for a considerable period after birth5. Gonioscopically, the neonatal angle appears to have a relatively anterior insertion of the iris and ciliary body.
The trabecular meshwork begins developing during the third to fourth months of gestation from undifferentiated mesenchymal cells of neural crest origin.6 Most of the anterior face is covered by corneal endothelium. No demarcation exists between the cells destined to become the meshwork and those that will differentiate into the ciliary muscle. With the development of the scleral spur between 22nd and 24th weeks of gestation the meshwork can be divided into the outer corneoscleral portion and the inner uveal meshwork. By the ninth month the uveal meshwork is well-formed with wide intercellular spaces. As the angle recess deepens and the trabecular beams differentiate, the open spaces of the meshwork come into direct communication with the anterior chamber.7
At the end of the third month mesodermal mesenchyme differentiates into a small plexus of venous canaliculi that will develop into Schlemm's canal. The vascular channels are formed at several points before anastomosing.8 The canal is surrounded by mesenchymal cells that will form the juxtacanalicular region. Transcellular channels that allow for the bulk flow of aqueous humor are formed at the beginning of the fifth month as vacuolar configurations in the endothelial cells lining Schlemm's canal.9 This corresponds to the time of differentiation of the ciliary processes and the onset of the circulation of aqueous humor.
ANATOMY
ANTERIOR CHAMBER
The anterior chamber is bound anteriorly by the inner surface of the cornea, and posteriorly by the lens centrally, anterior surface of the iris and anterior face of the ciliary muscle peripherally. It communicates with the extracellular spaces of the iris, ciliary body, trabecular meshwork, and with the posterior chamber of the eye through the pupillary aperture.10
The volume of the anterior chamber is approximately 220 μl, and the average depth is 3.15 mm.11 Chamber depth decreases by 0.01 mm per year of life and is shallower in the hypermetropic than myopic eye. The diameter of the anterior chamber varies between 11.3 and 12.4 mm.12
THE OUTFLOW APPARATUS
The bulk of the aqueous humor produced by the ciliary body is drained through the trabecular meshwork into the canal of Schlemm, and from there into the intra-and episcleral venous systems.
The Sulcus
The sulcus extends anteriorly from Schwalbe's ring, which is the termination of Descemet's membrane, to the scleral spur posteriorly (Figures 1.2A and B). It accommodates the canal of Schlemm externally, and the corneoscleral portion of the trabecular meshwork internally.
Schwalbe's Ring
This termination of Descemet's membrane also marks the transition between the corneal endothelium and the trabecular cells.5
FIGURES 1.2A and B: Semi-diagrammatic representation of the structures of the angle of the anterior chamber. (A) Enlarged view of the trabecular meshwork. Superimposed trabecular sheets with intra-and intertrabecular spaces through which aqueous humor flows to reach Schlemm's canal. (B) Composite gonioscopic and cross-sectional view of the anterior segment of the eye. SL = Schwalbe's line, SS = scleral spur, IP =iris process, TM = trabecular meshwork, C = cornea, I = iris, SC = Schlemm's canal, S = sclera, CB = ciliary body, Z = zonules.(From Tripathi RC and Tripathi BJ in Duane TA and Jaeger EW (eds) (1982) Biomedical Foundations of Ophthalmology, Vol. 1, published by Harper and Row)
Scleral Spur
Gonioscopically it is seen as a pale strip of scleral tissue located anterior to the ciliary band. It marks the posterior boundary of the corneoscleral meshwork and receives the insertion of the anterior tendons of the longitudinal ciliary muscle on its inner aspect. Contraction of the ciliary muscle pulls the spur posteriorly, opening up the trabecular spaces. Miotics probably reduce the outflow resistance in this way.13 The collagen and elastic tissue of the scleral spur blends with that of the trabecular beams of the corneoscleral and juxtacanalicular meshwork.
Scleral spur cells are myofibroblast-like cells orientated circumferentially within the scleral spur.14 These cells are innervated by unmyelinated axons derived from neurons that run forward in the supraciliary space. The complex innervation of the scleral spur cells may form a basis for modulating the resistance of the outflow pathway through the tendon-like contacts of these cells with the elastic fibers of the trabecular meshwork.
Trabecular Meshwork
The trabecular meshwork is a sieve-like structure of connective tissue beams arranged as superimposed perforated sheets. It bridges the scleral sulcus, extending from Schwalbe's ring anteriorly, to the scleral spur and junction of iris and ciliary body posteriorly. The meshwork is divided into three layers. The uveal meshwork is innermost; closer to Schlemm's canal, and connected to the spur is the corneoscleral meshwork. Closest to the endothelial lining of Schlemm's canal is the cell-rich zone of the juxtacanalicular or cribiform meshwork (Figure 1.3). The spaces in the meshwork sheets progressively decrease in size moving outward. Hydrophilic glycosaminoglycans and collagenous material in the extracellular spaces must influence the outflow resistance.
- Uveal meshwork: The cord-like trabeculae of the inner uveal meshwork are connected with the ciliary muscle fibers posteriorly. They taper anteriorly, joining the periphery of Descemet's membrane and the inner part of Schwalbe's ring.
- Corneoscleral meshwork: This consists of 8 to 15 layers of flat, perforated sheets, converging anteriorly to merge with the inner corneal lamellae. The intratrabecular spaces are smaller than those of the uveal meshwork, decreasing in size from within outwards.The trabecular structure is composed of an inner collagenous core, surrounded by a subcellular cortex and a covering of trabecular cells.FIGURE 1.3: Three layers of the trabecular meshwork in cutaway views.(From Allingham RR, Damji K, Freedman S et al. Shields' Textbook of Glaucoma, 5th edition, Lippincott, Philadelphia, 2005)These cells are phagocytic and are thought to perform synthetic activities (secretion of basal laminar material, collagen and glycosaminoglycans).15 These cells provide a lining for the inter-and intratrabecular spaces and they might be involved in a self-cleansing mechanism which keeps the trabecular meshwork clean.The collagen fibrils in the core of the trabecular sheets are orientated along its long axis, and this orientation is probably determined by the direction of pull exerted by the ciliary muscle on the uveal beams and through the scleral spur on the corneoscleral beams. The elastic tissue in the core imparts a recoil, so that meshwork spacing would be resumed after widening of the trabecular spaces.
- Juxtacanalicular connective tissue: This connective tissue lies between the endothelial lining of Schlemm's canal and the outer corneoscleral trabecular meshwork. Schlemm's canal is invested in this connective tissue zone of loosely arranged cells (2 to 5 layers) embedded in an extracellular matrix. Aqueous humor can move through spaces between the cells to reach the endothelial lining of Schlemm's canal.The current thinking is that this part of the drainage system makes a major contribution to the outflow resistance, due to the narrow and tortuous pathways as well as the presence of extracellular proteoglycans and glycoproteins. The open spaces in this region may also contain a gel-like substance which could contribute to the outflow resistance.16
Schlemm's Canal and Collector Channels
Schlemm's canal is a narrow circular tube, oval or triangular in cross-section, that lies in the outer portion of the internal scleral sulcus.17 It is some 36 mm long and lined by endothelium.
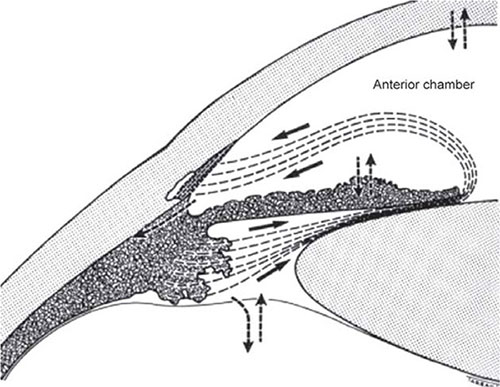
The vacuolar configuration of the endothelium provides a mechanism for direct communication between the extracellular spaces of the trabecular meshwork and the canal of Schlemm. This is probably the major route of aqueous flow into Schlemm's canal.18
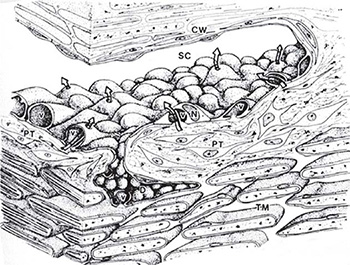
Giant vacuoles, probably formed by invagination of the basal plasmalemma, are a prominent feature of the inner wall of Schlemm's canal (Figure 1.4). They provide access for aqueous humor in the pericanalicular region,19 and some also communicate with Schlemm's canal through an apical opening, thus forming a transcellular channel.20 Red blood cells can pass through these channels due to their deformability, but macrophages, red cell ghosts and sickled red cells become trapped in the inner portion of the meshwork. Some vacuoles have openings on the inner and outer sides, so they can be considered transcellular microchannels. Some authors have assumed that these structures provide a valvular function, because the openings on the meshwork side are often larger than those on the luminal side and because their apical pores are usually not located directly opposite the basal openings (Figure 1.5).21
FIGURE 1.4: A composite three-dimensional schematic rendering of the walls of Schlemm's canal (SC) and the adjacent trabecular meshwork (TM). The spindle-shaped endothelial cells lining the trabecular wall of Schlemm's canal are characterized by luminal bulges corresponding to unique macrovacuolar configurations (v) and nuclei (N). The macrovacuolar configurations are formed by surface invaginations on the basal aspect of individual cells which gradually enlarge to open eventually on the apical aspect of the cell surface thus forming transcellular channels (arrows) for the bulk flow of aqueous humor down a pressure gradient. Pericanalicular tissue (PT). Corneoscleral wall (CW).(From Tripathi RC and Tripathi BJ in Duane TA and Jaeger EW (eds) (1982) Biomedical Foundations of Ophthalmology, Vol. 1, published by Harper and Row)
FIGURE 1.5: Concept of cyclical sequence of events in the formation of vacuolar transcellular channels and mechanism of bulk flow of aqueous humor across the endothelial barrier of Schlemm's canal. Initially, vacuoles are formed by a membranous depression or infolding of the basal aspect of the cell surface (stage 2). Progressive enlargement of this infolding leads to formation of a macrovacuolar structure (stages 3 and 4), which eventually opens on the luminal aspect of the cell surface (stage 5) thus forming a transient vacuolar transcellular channel (continuous arrow). After a time, the basal infolding is occluded and the cell returns to its non-vacuolated state (stage 1).(From Tripathi RC. Mechanism of the aqueous humor outflow across the trabecular wall of Schlemm's canal. Exp Eye Res 1971;11:116).
The formation of the vacuoles seems to be pressure dependant, the number and size being decreased at low pressures and reversed at high pressures.22 The transcellular route is responsible for most of the aqueous entering Schlemm's canal, and only about 1 percent of the inner wall conductance can be attributed to the non-vacuolar channels.23
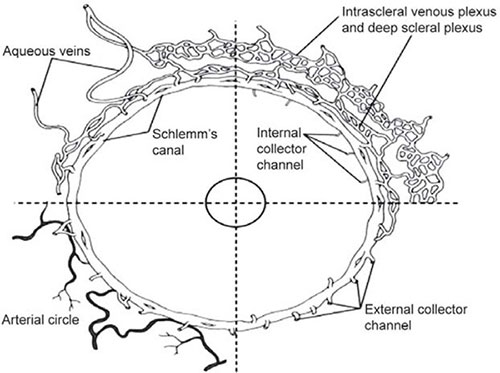
The 25 to 35 collector channels drain the canal of Schlemm at irregular intervals into three interconnecting venous plexuses: the deep, mid and episcleral venous plexuses (Figure 1.6). The aqueous veins drain directly onto the episcleral plexus.24 There are no valves in the system.
FIGURE 1.6: Schematic representation of aqueous humor outflow distal or beyond the conventional or trabecular pathway and into the canal of Schlemm. The internal collector channels of Sondermann are labeled in the upper right sector as they extend into the trabecular meshwork. The external collector channels are seen in the upper and lower right sectors, arising from the canal and uniting with the deep intrascleral plexus extending directly to the episcleral veins. The deep and intrascleral venous plexuses are external to the canal. In the upper left sector an aqueous vein arises from the deep scleral plexus and another arises from Schlemm's canal and runs directly to the episcleral venous plexus. External collector veins are seen to arise from the canal and join the deep scleral plexus. In the lower left sector, the arteries of the deep sclera are seen to be in close relation to the canal of Schlemm.(From Hogan MA, Alvarado J, Weddell J. Histology of the human eye. Philadelphia: WB Saunders, 1971)
INNERVATION OF THE OUTFLOW APPARATUS
The innervation of the outflow apparatus derives from the supraciliary nerve plexus and the ciliary plexus in the region of the scleral spur. Nerve endings are more plentiful in the region of the inner portion of the pericanalicular zone.
The inner layers of the eye are innervated by sensory nerves of trigeminal origin. Numerous axons run forwards parallel to the muscle bundles in the meridional part of the ciliary muscle, near its insertion into the scleral spur. Some of the transciliary myelinated fibres pass forwards beyond the anterior insertion of the ciliary muscle and give rise to a loose network of circumferentially oriented axons at the level of the scleral spur. In places these spur axons lose their myelin sheaths, branch profusely and terminate in club-shaped nerve endings which have the morphological features of mechanoreceptors. Three potential roles have been proposed for these presumed mechanoreceptors: (a) acting as propioceptive tendon organs for the ciliary muscle, (b) influence on the contraction of the myofibroblastic scleral spur cells or (c) performing a baroreceptor function in response to changes in intraocular pressure.25
AQUEOUS HUMOR
DRAINAGE
Conventional Pathway
As noted earlier, most of the aqueous humor leaves the eye at the anterior chamber angle through the system consisting of the trabecular meshwork, Schlemm's canal, intrascleral channels and episcleral and conjunctival veins (Figure 1.7). This pathway is referred to as the conventional or trabecular outflow pathway.
The trabecular meshwork accounts for approximately 70 percent, in younger eyes, to 95 percent of the aqueous humor egress in older eyes. Both total outflow facility and trabecular outflow facility decrease with age. The relative contributions of trabecular and uveoscleral outflow show an age-related shift, with a relative increase in the contribution of the trabecular outflow. Because uveoscleral outflow is relatively independent of IOP in the physiologic range, decreased uveoscleral outflow and increased trabecular outflow resistance with age mean that IOP must rise sufficiently to drive a higher proportion of the total flow (which remains rather constant with age) across the increased trabecular resistance.26
Uveoscleral Pathway
The anterior portion of the ciliary body extends into the anterior chamber angle and is inverted internally by the uveoscleral meshwork, behind the scleral spur. Since there is no continuous cellular layer on the anterior iris face aqueous humor has direct access from the anterior chamber into the ciliary body. From the ciliary body the aqueous drains into the supraciliary and suprachoroidal compartments, and from there either diffuses through the sclera, pass via scleral pores surrounding blood vessels and nerves, or gets absorbed into the uveal vascular system, including the vortex veins (the uveovortex outflow).27 This drainage pathway accounts for about 10 percent of the total bulk aqueous outflow in the human eye.
Drainage via the uveoscleral pathway is virtually independent of pressure at IOP levels greater than 7 to 10 mm Hg.28 The facility of uveoscleral outflow is approximately one twentieth the facility of trabecular outflow. The reason for the pressure independence of the uveoscleral pathway is not entirely clear, but might be the consequence of the complex nature of the pressure and resistance relationship between the various fluid compartments within the soft intraocular tissues along the route.29 8
FIGURE 1.7: Diagrammatic representation of the drainage pathways of the aqueous humor. The aqueous humor, formed in the posterior chamber (pc) by the ciliary processes, flows into the anterior chamber through the pupil. In normal eyes, the main drainage route for bulk outflow is the conventional drainage pathway constituted by the trabecular meshwork and Schlemm's canal (SC). Subsidiary drainage routes include (1) the uveoscleral and uveovortex, that is, through the anterior face of the ciliary body into the suprachoroidal space; (2) exchange across the anterior vitreous face; (3) exchange across the iris vessels; and (4) exchange across the corneal endothelium. The role of 2,3 and 4 in the net removal of aqueous humor appears to be insignificant because the net loss is equivalent to net gain through a two-way exchange.(From Tripathi RC and Tripathi BJ in Duane TA and Jaeger EW (eds)(1982) Biomedical Foundations of Ophthalmology, Vol. 1, published by Harper and Row)
Under normal circumstances there is always a positive pressure-gradient between the suprachoroidal and intraorbital spaces, with the result that fluid and solutes can easily exit the eye. At low IOP levels, the net pressure gradient across the uveoscleral pathways is apparently so low that uveoscleral drainage decreases.30 The absence of an outflow gradient from the suprachoroid may contribute to the development of choroidal detachments seen during the ocular hypotony that sometimes follow intraocular surgery.31
Uveovortex Pathway
Some investigators believe that some fluid may be drawn osmotically into the vortex veins by the high protein content in the blood of these vessels.32 The unidirectional flow of aqueous into the lumen of iris vessels by vesicular transport is not energy-dependent. The aqueous penetrates the vessels of the iris, ciliary muscle and anterior choroid to eventually reach the vortex veins. The role of net fluid movement of aqueous into the iris vasculature is probably not clinically significant.33
FUNCTION AND COMPOSITION
The circulating aqueous humor has the following functions: (a) its flow against a resistance generates an IOP of approximately 15 mm Hg, which is necessary for the proper shape and optical properties of the globe, (b) it nourishes the cornea, lens and trabecular meshwork, (c) it provides a transparent and colorless medium of refraction with refractive index of 1.33332,34 (d) it delivers high concentrations of ascorbate, and (e) participates in local paracrine signaling and immune responses.35
The composition of the aqueous humor depends not only on the nature of its production, but also on the metabolic interchanges that occur with various tissues, especially diffusional exchange across the iris. The two most striking characteristics of aqueous humor compared to plasma is a marked excess of ascorbate (15 times greater than that of arterial plasma), and a marked deficit of protein. The source of aqueous humor proteins seems to be the ciliary body, diffusing anteriorly through the iris, or secreted into the vitreous.36 Aqueous is slightly hypertonic compared with plasma, as well as acidic with a pH of 7.2.37 The concentration of most ions and non-electrolytes are very close to that of plasma. Molecules considered to be potential paracrine signaling molecules, being circulated and distributed to local tissues, have been identified in human aqueous humor. These include: norepinephrine, nitric oxide, coagulation and anticoagulation compounds and growth factors. Myocilin has been detected in normal aqueous humor, but is absent in the aqueous humor of patients with myocilin-associated glaucoma.38
REGULATION OF INTRAOCULAR PRESSURE
AQUEOUS HUMOR DYNAMICS
Aqueous humor is secreted into the posterior chamber by the ciliary epithelium lining the ciliary processes, from where it flows around the lens and through the pupil to enter the anterior chamber. In the anterior chamber convection flow of aqueous humor exists: downward close to the cornea, where the temperature is cooler, and upward near the lens, where the temperature is warmer.
The IOP builds up in response to the inflow of aqueous humor to the level that is sufficient to drive fluid across the resistance. The steady-state IOP occurs when the rate of aqueous production equals its outflow. Outflow resistance is usually determined by measuring its inverse, the facility of aqueous flow. Aqueous flow is the sum of the drainage through both the conventional (Ftrab) trabecular pathway and the uveoscleral (Fu) pathway, where F = flow in μl/min. Then:
Fout = Ftrab + Fu and
Ctot = Ctrab + Cu + Cps
where C = facility of conductance of flow (μl/min/mm Hg) = 1/R
R = resistance to flow (mm Hg × min/μl)
Ctot = total aqueous humor outflow
Ctrab = facility of outflow via trabecular pathway
Cu = facility of outflow via uveoscleral pathway
Cps = facility of inflow
The Goldman equation views aqueous flow as passive, non-energy-dependent bulk fluid movement down a pressure gradient, 9with aqueous leaving the eye only via the trabecular route, where ÄP = Pi – Pe, where P = pressure (mm Hg), Pi = IOP and Pe = episcleral venous pressure. This translates into the oversimplified relationship of F = Ctrab (Pi – Pe).
Even though aqueous flow through the trabecular meshwork can be measured experimentally, the uveoscleral outflow needs to be calculated. It represents the difference between aqueous flow and trabecular outflow. Flow through the trabecular meshwork is directly proportionate to the pressure drop across this outflow mechanism, or the IOP minus the episcleral venous pressure (Pe). It is also inversely related to the resistance to outflow (R).
Pi-Pe
F = R or F = Ctrab(Pi –Pe), by invoking a coefficient of outflow facility (Ctrab), where outflow facility and resistance are inversely related (Ctrab = 1/R). The main factors that determine IOP according to the Goldman equation is therefore:
Aqueous flow is the sum of drainage through both the uveoscleral pathway (Fu), and trabecular pathway (Ftrab). Hence:
Consequently aqueous flow through the anterior chamber is represented by the following equation:39
LOCATION OF RESISTANCE TO OUTFLOW
The pressure gradient from the anterior chamber (between 10 and 21 mm Hg IOP) to the episcleral veins (approximately 9 mm Hg) is explained by a resistance to aqueous humor flow residing somewhere in the conventional outflow pathway. The precise distribution of the resistance along the outflow pathway is still under debate, but it seems that the conventional outflow pathway accounts for approximately 90 percent of the pressure-dependent outflow, while the uveoscleral pathway accounts for the rest.40
Approximately 60 to 80 percent of the resistance to aqueous humor outflow resides in the tissues between the anterior chamber and the lumen of Schlemm's canal.41 One monkey study has suggested that 60 to 65 percent of outflow resistance is in the trabecular meshwork, 25 percent in the inner one-third to one-half of the sclera, and 15 percent in the outer one-half to one-third of the sclera.42 Most investigators believe that the major resistance resides in the cribiform portion of the meshwork, which is the outermost part of the mesh consisting of several layers of endothelial cells embedded in a ground substance consisting of a wide variety of macromolecules, including hyaluronic acid, other glycosaminoglycans, collagen, fibronectin and other glycoproteins.43 Aqueous can flow inbetween the cell-covered lamellae of the corneoscleral meshwork, whereas the aqueous directly penetrates the tissue layer of the cribiform layer. Some investigators however consider the main resistance to lie slightly proximal to the juxtacanalicular (cribiform portion) tissue.44
On the inner wall of Schlemm's canal, the basal lamina is deficient in places, which leaves the basal aspect of the cells bare.45 This interrupted nature suggests that normally it may not provide significant resistance to the flow of aqueous humor. Some investigators feel a small percentage of the resistance, perhaps 10 to 20 percent, resides in the inner wall of Schlemm's canal.46 Other factors that can contribute to resistance in the inner wall region is proteoglycans (as well as other substances that are not visualized with conventional methods of fixation and processing) in the gaps and optically empty spaces of the trabecular meshwork, the aqueous flow through non-gap regions of the basement membrane and the resistance offered by the inner wall endothelial cells.
In glaucomatous eyes there is an increase in extracellular matrix beneath the inner wall of Schlemm's canal and in the cribiform region of the trabecular meshwork. The trabecular lamellae are also thickened, compared with age-matched healthy controls,47 and in advanced cases of primary open-angle glaucoma there is additional loss of trabecular cells.48 The origin of the increased extracellular matrix is unknown, and it has to be noted that antiglaucoma drugs themselves can induce changes in the trabecular cells.49
REGULATION OF AQUEOUS HUMOR OUTFLOW
The manner in which the IOP is controlled is not fully understood. It is likely that both aqueous production and outflow resistance are regulated.
Myofibroblast-like scleral spur cells, in close association with varicose axons characteristic of mechanoreceptor nerve endings, suggest a mechanism for measuring stress or strain in the scleral spur, as might occur with ciliary muscle contraction or changes in IOP.50 The anterior tendons of the longitudinal ciliary muscle fibers insert on the scleral spur and posterior portion of the corneoscleral meshwork. This anatomic arrangement suggests an important mechanical role for the cholinergic innervation of ciliary muscle on trabecular meshwork function.51
The macromolecules in the cribiform meshwork are probably produced by the meshwork endothelial cells. Their synthesis and turnover might suggest one mechanism by which outflow resistance is regulated.52 It has been proposed that trabecular cells can modulate flow by changing the ionic microenvironment of the extracellular matrices near their surfaces within the flow channels.53 The control mechanism is unknown, but there is evidence suggesting that modulation of the trabecular meshwork-inducible glucocorticoid response (TIGR)/myocilin gene expression and its proteins may play an important role.54 The role of myocilin expression in the trabecular meshwork is not fully understood, but clinically important given its role in juvenile glaucoma.55
Glucocorticoids may influence the outflow facility by a direct effect on the extracellular matrix metabolism of trabecular cells, and may also alter the cytoskeletal organization of trabecular meshwork cells.10
FACTORS INFLUENCING AQUEOUS HUMOR OUTFLOW
An elevation of intraocular pressure is associated with increased resistance to aqueous humor outflow, which appears to be related to a collapse of Schlemm's canal.56 At normal pressures, outflow resistance depends on an inner wall of Schlemm's canal which is pressed against an unyielding outer wall, blocking the intrascleral outlet channels.57 An elevated IOP leads to a distention of the trabecular meshwork, increase in endothelial vacuoles, and a ballooning of the inner wall endothelial cells into the canal. Expanding Schlemm's canal decreases the resistance to outflow, which presents the proposed mechanism of action of the viscocanalostomy procedure.58
Fibrinolytic activity in the trabecular meshwork and endothelium of Schlemm's canal protects the system from occlusion by fibrin and platelets.59 Tissue plasminogen activator may influence resistance to aqueous humor outflow under normal circumstances by altering the glycoprotein content of the extracellular matrix.
There is an age-related reduction on the size of Schlemm's canal, leading to a decrease in the number of giant vacuoles and of the cell count in Schlemm's canal.60 The trabecular beams progressively thicken, the intertrabecular spaces narrow and there is an increase in extracellular material. All these structural changes lead to an increased resistance. Morphologically similar, but exaggerated changes are observed in primary open-angle glaucoma.61
An increase in episcleral venous pressure, leads to an equal or greater rise in intraocular pressure.62
In the primate eye, cholinergic agonists such as pilocarpine augment aqueous humor drainage via the trabecular route and diminish drainage via the uveoscleral route.
Epinephrine increases both trabecular and uveoscleral outflow. It appears that its effect is mediated by α2-adrenergic receptors on the trabecular endothelial cells and the subsequent G protein adenylate cyclase-cAMP cascade.63 The IOP-lowering action of epinephrine may be mediated, at least in part, by prostaglandins or other cyclo-oxygenase end products.64
Prostaglandin-induced relaxation of the ciliary muscle widens the intermuscular spaces, thereby redirecting the aqueous humor outflow from the trabecular to the uveoscleral pathway. This redirection would both rid the eye of excess protein and maintain physiologic IOP. This could also explain the low IOP that often accompanies uveitis.
Topical or systemic glucocorticoids may induce elevation of IOP in susceptible persons as a result of decreased outflow. Glucocorticoids may play a major role in the normal physiologic regulation of outflow and IOP.65 Patients with glaucoma have increased plasma levels of cortisol compared with healthy individuals.66 A stress and glucocorticoid-inducible response gene product (TIGR protein; myocilin), whose progressive induction over time matches the time course of clinical steroid effects on IOP and outflow, has been associated with outflow obstruction and glaucoma pathogenesis.67 Alteration in TIGR/myocilin expression affects trabecular meshwork cell adhesion, proliferation and phagocytosis. The TIGR gene has been directly linked to patients with primary open-angle glaucoma (POAG). Precisely how steroids affect aqueous humor outflow remains controversial, but it may be that intercellular junctions that are necessary for the development and maintenance of transendothelial flow resistance are involved in the increased resistance associated with glucocorticoid exposure.
Glycosaminoglycans (GAG) contribute to the filtration barrier of aqueous humor outflow. There seems to be a depletion of hyaluronic acid and an accumulation of chondroitin sulfates in the POAG trabecular meshwork.68 Research supports potential roles for hyaluronic acid in the regulation of the physiologic aqueous humor outflow resistance or in the maintenance of the outflow channels, or both.69
A thorough knowledge of the anatomy and pathophysiology of the anterior chamber, its angle and the circulating aqueous humor is clearly essential in the proper understanding of all types of glaucoma. This knowledge will always form the basis of any new treatment modality for this blinding disease.
REFERENCES
- Mann IC. The Development of the Human Eye (3rd ed). Grune Stratton; New York, NY: 1964.
- Beauchamp GR, Knepper PA. Role of the neural crest in anterior segment development and disease. J Ped Ophthal Strab 1984;21: 209.
- Wulle KG. Electron microscopy of the fetal development of the corneal endothelium and Descemet's membrane of the human eye. Invest Ophthalmol 1972;11: 397.
- Snell RS, Lemp MA. Clinical Anatomy of the Eye (2nd ed). Blackwell Science; 1998.
- Tripathi BJ, Tripathi RC, Wisdom J. Embryology of the anterior segment of the human eye, in The Glaucomas (2nd edition). Mosby, St Louis; 1995.
- McMenanin PG. A quantitative study of the prenatal development of the aqueous outflow system in the human eye. Exp Eye Res 1991;53: 507.
- Hansson HA, Jerndal T. Scanning electron microscopic studies on the development of the iridocorneal angle in human eyes. Invest Ophthalmol 1971;10: 252.
- Hamanaka T, Bill A, Ichinihasama R, et al. Aspects of the development of Schlemm's canal. Exp Eye Res 1992;55,479.
- Wulle KG. Electron microscopy of the fetal development of the corneal endothelium and Descemet's membrane of the human eye. Invest Ophthalmol 1972;11: 897.
- Bron AJ, Tripathi RC, Tripathi BJ. Wollf's anatomy of the eye and orbit. (8th edition). Chapman & Hall. 1997.
- Weekers R, Grieten J, Lavergne G. Study of the dimensions of the human anterior chamber. Ophthalmologica 1961;142: 650.
- Tripathi RC, Tripathi BJ. Functional anatomy of the anterior chamber angle. In Duane TD, Jaeger EA (Eds): Biomedical Foundations of Ophthalmology, Lippincott, Philadelphia, 1982;10:1.
- Grierson I, Lee WR, McMenamin PG. The morphological basis of drug action on the outflow system of the eye. Res Clin Forums 1981;3: 1.
- Tamm ER, Flügel C, Stefani FH, et al. Contractile cells in the human scleral spur. Exp Eye Res 1992;54: 531.
- Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microscopy Research and Technique 1996;33: 336.
- Ethier CR, Kamm RD, Palaszewski BA, et al. Calculation of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci 1986;27: 1741.
- Ashton N. Anatomical study of Schlemm's canal and aqueous veins by means of Neoprene casts: I. Aqueous veins. Br J Ophthalmol 1951;35: 291.
- Tripathi RC. The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res Suppl 1977a;25:65.
- Tripathi RC. Mechanism of the aqueous outflow across the trabecular wall of Schlemm's canal. Exp Eye Res 1971;11: 116.
- Fink AI, Gelix MD, Fletcher RC. Schlemm's canal and adjacent structures in glaucomatous patients. Am J Ophthalmol 1972;74: 893.
- Johnstone MA, Grant WM. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Ophthalmol 1973;75: 365.
- Bill A. The drainage of aqueous humor. Invest Ophthalmol 1975;14: 1.
- Ascher KW. The aqueous veins: I Physiologic importance of the visible elimination of intraocular fluid. Am J Ophthalmol 1942;25: 1174.
- Bron AJ, Tripathi RC, Tripathi BJ. Wolff's anatomy of the eye and orbit, (8th edition). Chapman & Hall, London, 1997.
- Allingham RR, Damji K, Freedman S, et al. Shields' Textbook of Glaucoma, (5th edition). Lippicott. Philadelphia, 2005.
- Tripathi RC, Cole DF. Uveoscleral drainage in the rabbit. AVRO Suppl: Invest Ophthalmol Vis Sci 1976;1: 1.
- Bill A. Conventional and uveoscleral drainage of aqueous humor in the cynomolgus monkey at normal and high intra-ocular pressures. Exp Eye Res 1966;5: 45.
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev 55:383, 1975.
- Bill A. Further studies on the influence of the intra-ocular pressure on aqueous humor dynamics in cynomolgus monkeys. Invest Ophthalmol 1967;6: 364.
- Kaufman PL, Alm A. Adler's Physiology of the Eye, (10th edition). Mosby, St. Louis, 2003.
- Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. In Albert DM, Jakobiec FA (Eds): Principals and Practice of Ophthalmology, WB Saunders Philadelphia, 2000.
- Bill A. Blood Circulation and Fluid Dynamics in the Eye. Physiol Rev 1975;55(3): 383.
- Millar C, Kaufman PL. Aqueous humor: secretion and dynamics. In Tasman W, Jaeger EA (Eds): Duane's Foundations of Clinical Ophthalmology, Lippincott-Raven Philadelphia, 1995.
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol 2002;21(2–3):123.
- Haddad A, Laicine M, de Almeida JC. Origin and renewal of the intrinsic glycoproteins of the aqueous humor. Graefes Arch Clin Exp Ophthalmol 1991;229(4): 371.
- Becker B. Chemical composition of human aqueous humor; effects of acetazolamide. AMA Arch Ophthalmol 1957;57(6): 793.
- Rao PV, Allingham RR, Epstein DL. TIGR/myocilin in human aqueous humor. Exp Eye Res 2000;71(6): 637.
- Morrison JC, Pollack IP. Glaucoma Science and Practice, Thieme, New York, 2003.
- Bron AJ, Tripathi RC, Tripathi BJ. Wolff's Anatomy of the Eye and Orbit (8th edition). Chapman & Hall. London, 1997.
- Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm: an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol 1972;50: 295.
- Peterson WS, Jocson VL, Sears ML. Resistance to aqueous outflow in the rhesus monkey eye. Am J Ophthalmol 1971;72(2): 445.
- Kaufman PL, Alm A. Adler's Physiology of the Eye (10th edition). Mosby 2003.
- Murphy CG, Johnson M, Alvarado JA. Juxtacanalicular tissue in pigmentary and primary open-angle glaucoma: the hydrodynamic role of pigment and other constituents. Arch Ophthalmol 1992;110: 1779.
- Tripathi RC. Comparative physiology and anatomy of the aqueous outflow pathway. In Davson H (Ed): The Eye. Academic Press, London, 1974; 163.
- Svedbergh B. Aspects of the aqueous humor drainage: functional ultrastructure of Schlemm's canal, the trabecular meshwork, and the corneal endothelium at different intraocular pressures. Acta Univ Uppsl 1976;256: 1.
- Lütjen-Drecoll E, Rohen JW. Morphology of aqueous outflow pathways in normal and glaucomatous eyes. In Ritch R, Shields MB, Krupin T (Eds): The Glaucomas, Mosby At Louis, 1989.
- Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology 1984;91: 564.
- Lütjen-Drecoll E, Kaufman PL. Morphological changes in primate aqueous humor formation and drainage tissues after long-term treatment with antiglaucomatous drugs. J Glaucoma 1993;2: 316.
- Tamm ER, Flugel C, stefani FH, et al. Nerve endings with structural characteristics of mechanoreceptors in the human scleral spur. Invest Ophthalmol Vis Sci 1994;35(3): 1157.
- Kater A, Shahsafaei A, Epstein DL. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest Ophthalmol Vis Sci 1992;33:424.
- Polansky JR, Wood IS, Alvarado JA. Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmology 1985;91: 580.
- Gard T, VanBuskirk EM, Acott TS. Ionic modulation of flow resistance in an immobilized proteoglycan model of the trabecular meshwork. J Glaucoma 1993;2: 183.
- Polansky JR, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 1997;211: 126.
- Allingham RR, Damji KF, Freedman S, et al. Shield's Textbook of Glaucoma (5th edition). Williams and Wilkins; Lippincott, 2005.
- Johnstone MA, Grant WG. Pressure-dependant changes in structure of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol 1973;75(3): 365.
- Moses RA. The conventional outflow resistance. Am J Ophthalmol 1981;92(6): 804.
- Smit BA, Johnstone MA. Effects of vicoelastic injection into Schlemm's canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology 2002;109(4): 786.
- Pandolfi M, Kwaan HC. Fibrinolysis in the anterior segment of the eye. Arch Ophthalmol 1967;77(1): 99.
- Ainsworth JR, Lee WR. Effects of age and rapid high-pressure fixation 1990;31(4):745.
- Brubaker RF. Determination of episcleral venous pressure in the eye: a comparison of three methods. Arch Ophthalmol 19 on the morphology of Schlemm's canal. Invest Ophthalmol Vis Sci 1990;31(4): 745.
- Brubaker RF. Determination of episcleral venous pressure in the eye: a comparison of three methods. Arch Ophthalmol 1967;77(1): 110.
- Nilsson SF, Bill A. Physiology and neurophysiology of aqueous humor inflow and outflow. In Kaufman PL, Mittag TW (Eds): Glaucoma, Mosby-Year Book Europe, Ltd. London, 1994.
- Anderson L, Wilson WS. Inhibition by indomethacin of the increased facility of outflow induced by adrenaline. Exp Eye Res 1990;50: 119.
- Schwartz B. The response of ocular pressure to corticosteroids. In Schwartz B (Ed): Corticosteroids and the eye, Little, Brown and Co Boston, 1966.
- Schwartz B, McCarty G, Rosner B. Increased plasma free cortisol in ocular hypertension and open-angle glaucoma. Arch Ophthalmol 1987;105: 1060.
- Stone EM, et al. Identification of a gene that causes primary open angle glaucoma. Science 1997;275: 668.
- Knepper PA, et al. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 1996;37: 1360.
- Lerner LE, et al. Hyaluronan in the human trabecular meshwork. Invest Ophthalmol Vis Sci 1997;38: 1222.