Fundamental to the understanding of orbital pathology and its surgical management is a sound working knowledge of the anatomy of the normal orbit in three dimensions. The goal of this chapter is to review the location of critical ocular adnexal, orbital and related craniofacial structures and the anatomic relationships between them.
Overview
The orbit is defined as the bony cavity containing the globe, extraocular muscles, fat, nerves and blood vessels. Although the orbit is often described as pyramidal in shape, the space is actually pear-shaped, with its largest horizontal and vertical diameters lying 1 cm past the orbital rim and adjacent to the equator of the globe. Average orbital volume is approximately 25-30 cc, of which the globe occupies approximately 7 cc of space. The lateral walls are oriented about 90° to one another and run 40 to 45 mm in length to the apex. The medial walls of each orbit run parallel to each other and measure 45 to 50 mm in length. The optical axes themselves are also parallel to the course of the medial walls, rather than the diverging central axes of the orbits. Therefore, each globe is tonically held in adduction by the extraocular muscles to maintain ocular alignment. These relationships and other important dimensions of the orbit are illustrated in Figure 1.1.
Orbital Osteology
The orbital rim is roughly in the shape of a spiral, with its starting and end points at the anterior and posterior lacrimal crests.1 There are seven bones which make up the four orbital walls: the frontal, sphenoid (greater and lesser wings), ethmoid, lacrimal, maxillary, palatine, and zygomatic bones (Figure 1.2).
The roof of the orbit is comprised of the frontal bone anteriorly, and the lesser wing of the sphenoid bone posteriorly (Figure 1.3). The overall thickness of the roof is significantly greater than that of either the medial wall or orbital floor and is therefore, relatively resistant to fracture. Within the lesser wing lies the optic foramen, through which the optic nerve exits the orbit via the optic canal. In about 30% of individuals, just above the frontosphenoid suture, lies the meningeal foramen through which the recurrent meningeal artery (a branch of the external carotid system) passes to anastomose with the lacrimal artery (a branch of the internal carotid system). This communication provides an important potential source of collateral blood flow to the orbit should its primary supply via the internal carotid system become disrupted.
Figure 1.1: An axial view of the orbits demonstrating the dimensions and relationships between associated structures
When the meningeal foramen is absent, the middle meningeal artery courses directly via the superior orbital fissure.2 Other important bony landmarks include the lacrimal gland fossa in the temporal roof and the trochlear fossa anteromedially. Just superolateral to the trochlear fossa and at the medial one-third junction of the superior rim, lies the supraorbital notch which gives passage to supraorbital artery, vein, and nerve. In some individuals, this point of egress is completely enclosed and appears as the supraorbital foramen.3
The medial wall includes the ethmoid, maxillary, and lacrimal bones, as well as the lesser wing of the sphenoid (Figure 1.4). Within the bony suture line separating the frontal from the ethmoid bone, there are two important apertures, the anterior and posterior ethmoidal foramina. These foramina are the exit points for the anterior and posterior ethmoidal arteries and nerves, respectively. The anterior ethmoidal foramen is typically located approximately 24 mm posterior to the orbital rim and the posterior ethmoidal foramen lies approximately 36 mm posterior to the rim. The optic foramen, in turn, is located approximately 6 mm posterior to the posterior ethmoidal foramen. These foramina help the surgeon delineate the frontoethmoidal suture which is an important surgical landmark for the roof of the ethmoid sinus, or fovea ethmoidalis. The orbital roof slopes downward as it travels medially. Medial to the orbital space, just beyond the frontoethmoidal suture line, the fovea ethmoidalis continues in a downward plane and ends sagittally just above the nasal cavity and below the anterior cranial fossa at the cribriform plate. Bony dissection of the medial wall above the suture line exposes the dura of the frontal lobe.
The ethmoid portion of the medial wall, the lamina papyracea, is extremely thin and is thus prone to fracture with trauma and to easily transmit infection from the ethmoid air cells into the orbit as subperiosteal abscesses. The medial wall thickens again in the area of the inferior suture between the ethmoid and maxillary bones. This maxilloethmoid strut4 provides support to the inferomedial orbital wall and often survives trauma which fractures the more superior aspects of the wall. At the anterior aspect of the medial wall is the lacrimal sac fossa, bounded by the anterior and posterior lacrimal crests. The anterior and posterior limbs of the medial canthal tendon insert on the anterior and posterior lacrimal crests, respectively.
The floor of the orbit consists of the maxillary, zygomatic and palatine bones. The maxillary bone forms the bulk of the floor while the zygomatic bone contributes anterolaterally and the palatine bone contributes to the posterior floor. A major landmark in this area is the infraorbital groove, which originates approximately 25-30 mm posterior to the orbital rim. The groove deepens and becomes an enclosed canal as it travels anteriorly within the floor to open again on the face of the maxillary bone at the infraorbital foramen on the maxillary face, 4-6 mm from the rim in adults.5 This pathway contains the infraorbital neurovascular bundle which is easily injured by floor fractures or inadvertent surgical dissection. Just medial to the infraorbital groove is the thinnest portion of the maxillary bone. Not only does this render the posteromedial part of the floor particularly susceptible to blowout fractures, but it also provides an area where bone can be removed with relative ease for inferior orbital decompression. The thicker, maxilloethmoid strut lies in the medial floor and provides support for the orbital soft tissues and the globe.4, 6 In the anteromedial floor, the bony nasolacrimal duct travels from the base of the lacrimal fossa in an inferior and usually slightly posterolateral direction through the maxillary bone in the lateral nasal wall to empty into the inferior meatus of the nose. The vector of the nasolacrimal duct shows considerable variability.6
The lateral wall contains the zygomatic bone and the greater wing of the sphenoid which separates the posterolateral orbit from the middle cranial fossa. The posterior borders of the lateral wall are defined by the superior and inferior orbital fissures. The boundary between the lateral wall and roof is formed by the frontosphenoid suture, which transmits the recurrent meningeal artery. The anterior part of the lateral wall is comprised of the zygoma. Important landmarks in this region include the lateral orbital tubercle, or Whitnall's tubercle, which is the insertion point of the posterior head of the lateral canthal tendon, the lateral horn of the levator aponeurosis, the check ligament of the lateral rectus muscle, and Lockwood's ligament. The tubercle can be found just inside the orbital rim and approximately 11 mm below the frontozygomatic suture.7 The supero-anterior zygoma also contains the zygomaticotemporal and zygomaticofacial canals through which branches of the lacrimal artery and the lacrimal and zygomatic nerves pass (Figure 1.5).
The Periorbita
The periorbita refers to the tough, fibromembranous lining of the bony orbit which acts as a physical barrier to infection and provides a scaffold to which other intraorbital connective tissues can attach. The regions of greatest adherence between this sheath and bone are at the orbital rim, suture lines, bony fissures, trochlear fossa and the lacrimal crests. At the orbital margins, at the arcus marginalis, the periorbita thickens and gives rise to the orbital septum. Deep in the orbit, the periorbita continue through the superior orbital fissure and optic canal to become continuous with the dura. The potential space outside the periorbita is an important surgical plane. Access to the orbital walls in decompression surgery, for example, entails dissection between the bone and this overlying periosteal sheet.
The Orbital Apex
The orbital apex merits special attention, as the region in which many critical orbital structures converge and communicate with other important, periorbital spaces (Figure 1.6). Cranial nerves II through VI, major orbital vessels, and all of the extraocular muscles excluding the inferior oblique sit in tight proximity within the apex, and pathology in this region can produce profound deficits in vision and ocular motility.8, 9
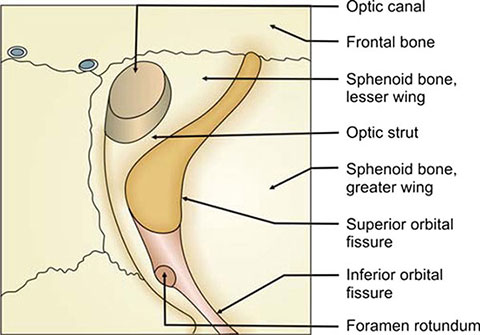
The apex is defined by only three walls; the floor is absent in the far posterior orbit.1 Major bony landmarks include the superior orbital fissure, inferior orbital fissure, and the optic canal. The superior orbital fissure divides the sphenoid into the greater (lateral) and lesser (medial) wings and lies inferiorly and laterally to the optic foramen. This fissure measures approximately 20-22 mm in overall length and is separated into superolateral and inferomedial sections by the tendon of the lateral rectus muscle. The superotemporal part of the fissure lies above the annulus of Zinn, the fibrous ring formed by the common origin of the rectus muscles. The lacrimal, frontal and trochlear nerves, and the superior ophthalmic vein pass through this region as they traverse the apex.
Figure 1.7: The superior and inferior orbital fissures and associated apex structures of the right eye
The inferomedial segment of the superior orbital fissure, also called the oculomotor foramen, is located inside the annulus and transmits the superior and inferior divisions of the oculomotor nerve, the abducens nerve, sympathetic fibers, and the nasociliary nerve, a terminal sensory branch of the ophthalmic division of the trigeminal nerve10–12 (Figure 1.7).
The inferior orbital fissure bounds the greater wing of the sphenoid, separating it from the maxillary bone inferomedially. This fissure communicates primarily with the pterygopalatine fossa (Figure 1.8). The maxillary branch of the trigeminal nerve passes through the pterygopalatine fossa and subdivides into the infraorbital nerve which, in turn, travels anteriorly into the orbit via the infraorbital groove. The zygomatic nerve, another branch of the maxillary branch of cranial nerve V, enters the orbit through the inferior orbital fissure to provide sensory innervation to lateral orbit and cheek after passing through the zygomaticofacial foramen. Also within the pterygopalatine fossa is located the maxillary artery which gives rise to the infraorbital artery, part of the neurovascular bundle traveling through the infraorbital groove. Parasympathetic fibers originating from the pterygopalatine ganglion and terminating in the lacrimal gland are transmitted by the inferior orbital fissure as well.2 The inferior ophthalmic veins also pass from the orbit into the pterygoid plexus via the inferior orbital fissure.
The optic canal penetrates the superomedial orbital apex through the lesser wing of the sphenoid bone as the optic foramen. The canal is approximately 6 mm in diameter and 8-10 mm in length and houses the optic nerve and the ophthalmic artery. The canal runs along the upper, lateral wall of the anterior sphenoid sinus and in the floor of the anterior cranial fossa.
The Cavernous Sinus
The cavernous sinus is a large venous sinus posterior to the orbital apex contained within a dural cleft which is situated lateral to the sphenoid sinus (Figure 1.9). Its tributaries consist of the ophthalmic, cerebral middle meningeal, and pterygoid veins. The left and right cavernous sinuses communicate with one another via small channels which run superior to the roof of the sphenoid sinus. Several critical structures pass through the cavernous sinus as they travel into the orbit. The carotid siphon and the sympathetics which ride along on its sheath traverse centrally. In the lateral wall of the cavernous sinus area embedded the oculomotor nerve, trochlear nerve, the ophthalmic and maxillary divisions of the trigeminal nerve, and the abducens nerve. The optic nerves course superomedially to the cavernous sinus. The optic chiasm is formed just above the anterior aspect of the cavernous sinus.
As in the orbital apex, pathological processes involving the cavernous sinus such as the formation of carotid-cavernous fistulas, inflammation or infection typically cause multiple cranial neuropathies affecting the eye.13 Further, because the right and left cavernous sinuses are interconnected, disease processes extending posteriorly from the orbit on one side can spread to the other via these spaces.
The Globe
The average-size globe measures approximately 23.5 mm in the horizontal meridian and 23 mm vertically, with an anterior-posterior dimension of about 24 mm. Its overall volume is about 7cc. The globe is surrounded by a loose fascial sheath, or Tenon's capsule, which is interconnected to the sclera by fine fibrous bands. The potential space between these layers is the episcleral space, and the areas of greatest adherence between them are approximately 1.5 mm from the limbus anteriorly, and posteriorly, at the optic nerve sheath. Tenon's capsule is suspended inside the orbit by interconnections with fine connective tissue septae within the surrounding orbital fat. This sheath must be traversed by the nerves and blood vessels which supply the globe. Likewise, the extraocular muscles must penetrate Tenon's layer to attach to the globe. As the muscles pass from outside to inside the episcleral space to fuse with the sclera, Tenon's capsule makes attachments with the intermuscular septum, a fibrous network which encases and interconnects the extraocular muscles (Figure 1.10).14–18 Thus, following enucleation, orbital implants that are placed within Tenon's capsule may demonstrate a fair amount of motility even if they are not sutured to the extraocular muscles themselves.18 The intermuscular septum and rectus muscles delineate the intraconal versus extraconal space.
Interconnections between the extraocular muscle sheaths and the periorbita comprise the check ligaments. Superiorly, the check ligaments consist of the fascial complex surrounding the upper lid retractors, the superior rectus and levator palpebrae muscles.19 Similarly, the inferior check ligament consists of the muscle sheaths surrounding the lower lid retractors, the inferior rectus and inferior oblique.
Medially, the muscle sheath of the medial rectus inserts just inside the posterior lacrimal crest, to the medial orbital septum and caruncle to collectively form the medial check ligament. The analogous check ligament of the lateral rectus inserts onto the lateral orbital tubercle of Whitnall.7
The Extraocular Muscles
The four rectus muscles originate at the annulus of Zinn, within the orbital apex. Specifically, the superior and medial recti originate adjacent to the lesser wing of the sphenoid, next to the optic canal. The inferior rectus originates from a portion of the annulus which extends from the body of the sphenoid bone to its great wing. The lateral rectus has a bifid origin from a tendinous segment of the annulus which extends across the superior orbital fissure from the greater to the lesser sphenoid wing and a more inferior portion which extends directly from the greater wing itself.
The superior rectus lies just underneath the levator palpebrae. Immediately beneath and medial to the superior rectus run the nasociliary nerve and ophthalmic artery. The superior edge of the medial rectus travels just under these structures. From its origin at the annulus, the inferior rectus closely follows the floor of the orbit until reaching the anterior orbit, where it becomes separated from the floor by the inferior oblique muscle as the latter crosses from the medial to the lateral wall, and by fat. Medial and superior to the lateral rectus, is found the ciliary ganglion which is usually adherent to the intraorbital segment of the optic nerve.
The superior oblique muscle also begins along the annulus of Zinn, superomedially and extends forward superiorly and along the junction between the orbital roof and the medial orbital wall. As it courses toward the anterior orbit, the muscle belly transitions to a tendinous segment as it reaches the trochlea, a pulley-like cartilaginous structure which lies approximately 6-10 mm posterior to the superomedial orbital rim.20, 21 From this point, the tendon passes posterolaterally, making a 54° angle, to attach to the globe. It is this course which gives rise to the main actions of the superior oblique, namely intortion and depression of the globe with contraction. During orbital surgery, caution to avoid injuring the trochlea must be taken to avoid subsequent scarring and restriction of superior oblique muscle action, or Brown's syndrome.22
The inferior oblique, unlike the other extraocular muscles, does not originate at the annulus, but from the periosteum of the anterior, inferomedial orbit on the maxillary bone. It courses posterolaterally, just beneath the inferior rectus to insert on the inferolateral globe, which gives rise to its main actions: extortion and elevation of the globe. The capsulopalpebral fascia and Lockwood's ligament interdigitate with the muscular fascia of the inferior oblique.
The extraocular muscles approximate a spiral in the distance between their individual insertions and the corneal limbus, the so-called spiral of Tillaux (Figure 1.11). Beginning with the medial rectus, each successive muscle inserts farther from the limbus. Although there is individual variation, the average distances are: medial rectus, 5.5 mm; inferior rectus, 6.5 mm, lateral rectus, 6.9 mm, and superior rectus, 7.7 mm.23
Innervation to the extraocular muscles is largely carried by the oculomotor nerve (third cranial nerve), which supplies the medial, inferior, and superior rectus muscles, the inferior oblique, as well as the levator palpebrae superioris. The superior oblique and lateral rectus receive innervation from the trochlear nerve (fourth cranial nerve) and the abducens nerve (sixth cranial nerve), respectively.
The blood supply to the muscles is carried by muscular branches of the ophthalmic artery.
Lids
Understanding the general anatomy and dimensions of the major eyelid landmarks is important in the evaluation of structural disease of the lids and in planning surgical repair. The normal interpalpebral fissure height is 10-12 mm and the average length is 28-30 mm. The distance between the upper lid crease and lid margin measures approximately 8-11 mm at the pupillary axis. The highest point of the upper lid contour rests just nasal to the center of the pupil and the upper lid margin is typically located 1-2 mm below the superior limbus in adults. The lateral canthal angle sits approximately 2 mm higher than the medial canthus.
Both the upper and lower eyelids can be divided into the skin, orbicularis, orbital septum, orbital fat, lid retractors, tarsus, and conjunctiva. For descriptive purposes, the layers can be grouped into the anterior and posterior lamellae. The anterior lamella contains lid structures which lie outside the orbit per se, and is comprised of the skin and the orbicularis oculi (Figure 1.12).
The skin of the lids is the thinnest of the body and unlike skin elsewhere, has no subcutaneous fat layer. The portion which lies anterior to tarsus is relatively firmly attached to deeper structures while the preseptal portions are loosely connected. Anterior to the margin, the skin contains the lash follicles with their associated sebaceous glands of Zeis and apocrine glands of Moll, as well diffusely distributed eccrine sweat glands.
The orbicularis muscle is a C-shaped complex of muscle fibers which functions to close the lids (Figure 1.13). It is divided into pretarsal, preseptal and orbital sections, all of which receive innervation from the facial nerve (seventh cranial nerve). The pretarsal and preseptal parts of orbicularis are primarily involved in involuntary closure of the lids, as elicited by the blink reflex. These fibers insert at the medial canthal tendon as deep and superficial heads. A subset of pretarsal orbicularis fibers, also called Horner's muscle, inserts deep at the posterior lacrimal crest as the deep limb of the medial canthal tendon, along the posterior aspect of the lacrimal sac and surround the canaliculi.
These fibers are thought to provide a pumping action as they contract which facilitates tear drainage.24, 25 Horner's muscle is also critical in maintaining close contact between the posterior aspect of the lid and the globe. The remaining pretarsal orbicularis inserts superficially within the anterior limb of the medial canthal tendon. Laterally, slips from the upper and lower lid pretarsal orbicularis insert onto the lateral canthal tendon which in turn, inserts on the lateral orbital tubercle. Medially, the deep head of the preseptal orbicularis inserts into the fascia surrounding the lacrimal sac while the superficial head inserts onto the anterior limb of the medial canthal tendon.
The orbital orbicularis is chiefly responsible for forced lid closure, such as winking or in blepharospasm. Medially, its insertions lie along the orbital rim and anterior medial canthal tendon. As they course laterally, these fibers overlie the zygoma and the elevators of the lateral mouth, the zygomaticus major and minor.
Underlying the pretarsal orbicularis and resting anterior to the tarsus is the muscle of Riolan which consists of small, horizontally oriented slips of muscle. These fibers appear grossly as the “gray line” of the lid margin which is posterior to the lash line and function to turn the lashes toward the eye during blinking. The “gray line” is a useful landmark in aligning the wound edges in marginal lid laceration repair.
The septum, orbital fat and posterior lamella, which consists of the retractors, tarsus and conjunctiva, are considered to be intraorbital structures. The septum is comprised of tough fibrous connective tissue arranged in sheets which originate from the periosteum of the orbital rims at the arcus marginalis. This structure acts as an relative barrier between the orbit and lid in limiting the deep spread of superficial hemorrhage and infection. In the upper lid, the septum fuses with the aponeurosis of the levator muscle, the primary upper lid retractor muscle, approximately 2-5 mm above the superior tarsal edge.26 In the lower lid, the septum condenses with the capsulopalpebral fascia as the two layers converge toward the inferior edge of the lower tarsal plate.27
The orbital septum lies anterior to the preaponeurotic orbital fat pads, which prolapse forward with any violation of the septum. As a natural consequence of aging, as the septum thins and stretches, these fat pads tend to gradually herniate anteriorly. In the upper lid, there are two distinct fat pads, the medial and central fat pads. The medial pad can be distinguished by its relatively white color. The medial palpebral artery typically runs within this pocket and care should be taken to avoid inadvertent laceration or cauterization during surgery. Laterally, there is usually little fat in the upper lid. Instead, this space is usually filled by orbital lobe of the lacrimal gland.
The lower lids contain medial, central and lateral fat pads. The medial pad lies just medial to the inferior oblique and as in the upper lid, has a characteristically white color and contains the lower palpebral artery. The central fat pad lies between the inferior oblique muscle and a fascial band that separates it from the lateral fat pad. The latter extends to the inferior edge of the lacrimal gland.28
The upper lid retractors consist of the levator muscle which is innervated by cranial nerve III, and the sympathetically innervated Müller's muscle. The levator, which is the primary retractor, originates from a point just above the annulus of Zinn, from the lesser wing of the sphenoid bone. The levator complex includes a muscular component which is approximately 40 mm long extending from its origin on the lesser sphenoid wing just outside the annulus of Zinn, and the fibrous levator aponeurosis which is 14-20 mm in length. As the muscular portion courses forward in the orbit, it rides just above the superior rectus muscle and the two are inter-connected by interdigitated fibrous bands. As they reach the equator of the globe, the levator broadens and transitions into its aponeurotic component. Medially and centrally, the aponeurosis inserts onto the anterior tarsal surface and passes through the orbicularis to insert onto pretarsal skin. These insertions create the upper lid crease. Medially, the aponeurosis separates into a single medial horn which inserts into the posterior lacrimal crest and becomes continuous with the medial canthal tendon complex. Similarly, the aponeurosis courses into a lateral horn which inserts into the lateral orbital tubercle, also 12called Whitnall's tubercle, and becomes continuous with the lateral canthal tendon complex (Figure 1.14).
The normal magnitude of upper lid elevation is approximately 14-16 mm. Elevation of less than 10-12 mm is usually abnormal. Elevation of less than 5 mm is considered severe dysfunction and has important implications in ptosis surgery. Because of the close apposition and fibrous interconnections between the levator and the superior rectus muscle, when the globe is elevated, the upper lid follows. This relationship is not passive, and the levator and superior rectus actually co-contract. Likewise, when the globe is depressed, both muscles relax together and the upper lid moves downward.29
At the transitional zone between the anterior muscular component of the levator and its aponeurosis is a fascial sleeve called Whitnall's ligament, or the superior transverse ligament. This band runs both over and beneath the levator at this point and behaves as a fulcrum point for the levator where contraction of the muscular portion in the horizontal plane becomes directed in the vertical direction.30–32 However, Whitnall's ligament is not a stationary fulcrum,29 rather, it acts more as a swinging suspender of the levator.31 Whitnall's ligament also provides mechanical support for the superior orbital soft tissues. Medially, this structure inserts within the fascial tissue surrounding the superior oblique tendon and the trochlea. Laterally, the ligament inserts within the inner surface of the lateral wall into the periorbita of the lacrimal gland fossa, approximately 10 mm above the lateral orbital tubercle, or Whitnall's tubercle. (Note that despite the shared eponym, Whitnall's ligament does not directly insert into Whitnall's tubercle). Prior to its lateral insertion, the ligament courses across and divides the lacrimal gland into a superior orbital lobe and an inferior palpebral lobe.30 (Figure 1.15).
Müller's muscle is a secondary upper lid retractor, providing approximately 1-2 mm of elevation. It originates just deep to the levator aponeurosis at the level of Whitnall's ligament and is about 12-14 mm in length. Muller's muscle inserts at the superior edge of the tarsal plate.
An important landmark for this structure is the peripheral vascular arcade which lies between the levator aponeurosis and Müller's muscle just above the tarsus. Injury to Müller's or loss of sympathetic innervation, as occurs in Horner's syndrome, causes a characteristic mild (1-2 mm) ptosis.33
In the lower lid, the retractor complex is called the capsulopalpebral fascia. This structure is a condensation of fibrous attachments to terminal muscle slips from the inferior rectus which course anteriorly to surround the inferior oblique muscle and fuse with its sheath. From this point, an important component of this fascial complex forms Lockwood's ligament, which extends across the width of the inferior orbit somewhat like a hammock, inserting laterally at the lateral orbital tubercle and medially into the medial canthal tendon and providing some suspensory support to the orbital soft tissues.34 Anterior to Lockwood's ligament, the capsulopalpebral fascia send fibers into the inferior conjunctival fornix (thus forming the suspensory ligament of the inferior fornix), while additional fibers continue on to fuse with the septum and to finally insert into the inferior border of the tarsal plate. As in the upper lid, the lower lid retractors work in tandem with the inferior rectus to lower the lid with downgaze.
The analogous lower lid structure to Müller's muscle in the upper lid is the inferior tarsal muscle. Loss of sympathetic innervation may cause a small amount of “reverse ptosis” of the lower lid, elevating the inferior lid margin by approximately 1 mm above its usual resting position.33
The tarsal plates are comprised of dense connective tissue that act at the structural skeleton of the lids. In both lids, the tarsi are 1 mm in thickness. In the upper lid, the tarsus is approximately 10-12 mm in height at the pupillary axis, while the vertical extent of the lower tarsus is 4 mm. The tarsi contain the oil-producing meibomian glands which open on the margin, just posterior to the lash line. In the upper lid, approximately 2-3 mm from the tarsal margin, lies the marginal arterial arcade. In the lower lid this arcade typically lies within 1 mm of the lashes. Distichiasis is the abnormal growth of lashes from the meibomian gland orifices and may occur as a congenital anomaly or as an acquired state. In the latter case, distichiasis is often a result of severe chronic inflammation of the lids35.
At their medial and lateral borders, the tarsi taper. The upper and lower tarsi come together at the canthus to form the deep lateral canthal tendon, which inserts just anterior to the lateral orbital tubercle. Recall that the more superficial components of the lateral canthal tendon extend from the lateral pretarsal and preseptal orbicularis oculi muscles. Similarly, the medial aspects of the upper and lower tarsi contribute to the medial canthal tendon, with larger, more superficial components which arise from the orbicularis oculi.
The conjunctiva comprises the most posterior layer of the lids. Basal tear flow is provided by the accessory lacrimal glands of Krause in the upper conjunctival fornix, and the glands of Wolfring in the lower fornix. Additional mucin-producing glands are distributed within both the orbital and palpebral conjunctivae.
The Lacrimal System
The main lacrimal gland lies in the anterolateral orbital roof, within the lacrimal gland fossa of the frontal bone, and measures roughly 20 × 12 × 5 mm. The gland is separated into a palpebral and an orbital lobe by the lateral levator aponeurosis. The primary suspensory support for the main lacrimal gland comes from the Whitnall's ligament.1 Damage to the ligament leads to forward and downward prolapse of the gland in the orbit.36 Ducts from both lobes pass through the palpebral lobe to empty into the superolateral fornix. Therefore, ideally, lacrimal gland biopsies should not be performed on the palpebral lobe, since injury here may affect drainage from both lobes37 (Figure 1.15).
Innervation and blood supply are provided by the lacrimal nerve and lacrimal artery, which enter the gland posteriorly. Venous drainage occurs via the lacrimal vein, which empties into the superior ophthalmic vein. Parasympathetic inputs originate from the lacrimal nucleus of the pons. These preganglionic fibers pass through the geniculate ganglion and then travel with the greater petrosal nerve to synapse eventually within the pterygo-palatine ganglion. These fibers then directly synapse in the lacrimal gland.38, 39 Additional postganglionic 14fibers traveling along branches of the maxillary division of the trigeminal nerve that converge with the lacrimal nerve to enter the orbit also innervate the lacrimal gland.40
Tears drain medially via the upper and lower lid puncta, into the canaliculi, and into the lacrimal sac (Figure 1.16). The puncta are approximately 0.3 mm in diameter. The initial segment of each canaliculus extends 2 mm perpendicular to the lid margin then turns roughly 90° medially toward the canthus. These horizontal canalicular segments are approximately 8 mm in length. The lower canaliculus is typically slightly longer than its upper lid counterpart. In 90% of individuals, the upper and lower canaliculi then fuse to form a 2 mm long common canaliculus which lies between the anterior and posterior limbs of the medial canthal tendon and enters the lacrimal sac.41 The valve of Rosenmüller is located at this junction and prevents the reflux of tears from the sac retrograde into the canaliculi. The lengths of each component of the lacrimal drainage system become important when performing probing and irrigation to evaluate the patency of the outflow system.
The lacrimal sac sits within the lacrimal sac fossa. It is 12 mm long and its fundus lies 3-4 mm superior to the valve of Rosenmüller.42 The sac lies just anterior to the middle turbinate of the nose. The inferior sac is contiguous with the nasolacrimal duct which courses in the wall of the lateral nose and empties via the valve of Hasner just below the inferior turbinate. The valve of Hasner may be imperforate in young infants, and is the most common site of nasolacrimal duct obstruction in this age group.
The Nerves of the Orbit
The optic nerve: The optic nerve (the second cranial nerve) is actually part of the central nervous system, extending directly from the brain into the orbit. Like the rest of the central nervous system, the optic nerve is invested within a dural sheath and leptomeninges, surrounded by cerebrospinal fluid, and in part, is covered with myelin. The fact that cerebrospinal fluid surrounding the optic nerve communicates with the fluid surrounding the cerebrum and brainstem is the basis for the seizures and life-threatening cardiopulmonary depression which can occur with inadvertent perforation of the optic nerve sheath during retrobulbar anesthesia.43
There are four major segments to the optic nerve, including the intracranial, intracanalicular, intraorbital and the intraocular segments. The intracanalicular segment of the optic nerve is tightly surrounded by its dural sheath and tethered within the bone. Because of this, the intracanalicular segment of the optic nerve is particularly susceptible to blunt trauma.44, 45 Once it passes through the optic foramen, the length of the intraorbital portion of the nerve is roughly 24-30 mm as it traverses the 20 mm or so distance to the globe. Thus, the nerve has a slightly serpentine course inside the orbit that allows for movement of the globe and some degree of proptosis. However, severe proptosis puts the nerve on stretch, described radiographically as “globe tenting”.46 The intraocular length of the nerve is approximately 1 mm.
Sensory innervation of the orbit: Sensory innervation of the periorbital region is carried by the ophthalmic and maxillary divisions of the trigeminal nerve (fifth cranial nerve). Both branch from the trigeminal ganglion which is located within the lateral wall of the cavernous sinus.
The ophthalmic branch further subdivides into three segments: the frontal, lacrimal and nasociliary nerves.
The frontal and lacrimal branches enter the orbit in the superolateral part of the superior orbital fissure, outside the annulus of Zinn. The frontal nerve courses through the extraconal fat and separates in the anterior orbit into several smaller branches including the supraorbital branch which supplies the scalp, forehead, upper lid, and conjunctiva. The supraorbital nerve exits via the supraorbital notch or foramen and should be carefully avoided during dissection of the superior orbital rim. Injury to the deep, lateral branches of the supraorbital nerve which run beneath the frontalis muscle, as can occur during forehead lift surgery leads to scalp numbness to the vertex.47 The other major division of the frontal nerve, the supratrochlear nerve, exits just above the trochlea to innervate parts of the lower forehead and medial canthal region. The lacrimal nerve travels with the lacrimal artery superolaterally in the extraconal space, along the superior border of the lateral rectus (Figure 1.17). As it travels forward, it is joined by parasympathetic motor fibers within the orbit which began within the nervus intermedius and which supply the lacrimal gland, superolateral lid and conjunctiva.37
The nasociliary nerve enters the orbit via the superior orbital fissure within the annulus of Zinn, traversing just under the superior rectus muscle and over the optic nerve medially as it courses forward in the orbit in association with the ophthalmic artery. In the posterior orbit, it subdivides into long posterior ciliary nerves which run medially and laterally toward the globe, giving off sensory fibers which travel through the ciliary ganglion without synapsing. The long ciliary nerves enter the sclera and continue forward, innervating the iris, cornea and ciliary muscles. Additional fibers from the nasociliary nerve travel superomedially and are responsible for sensation from the nasal mucosa and the skin on the medial tip of the nose via the anterior ethmoidal nerve. It is this branch which is responsible for Hutchinson's sign in cases of herpes zoster ophthalmicus.
The final anterior branch of the nasociliary nerve is the infratrochlear nerve, which traverses the orbital septum inferior to the trochlea to supply the medial eyelid skin, lacrimal sac and the caruncle.
The maxillary division of the trigeminal nerve exits the middle cranial fossa via the foramen rotundum to enter the pterygopalatine fossa. From here, the zygomatic branch enters the inferior orbit via the inferior orbital fissure. It further subdivides into the infraorbital, zygomaticotemporal, and zygomaticofacial nerves. The infraorbital nerve exits the orbit via the infraorbital notch or groove to supply the skin of the lower lid, cheek and medial upper lip (Figure 1.2). Injury to this nerve by fractures involving the orbital floor result in hypesthesia over these areas. The zygomaticotemporal and zygomaticofacial nerves provide sensory innervation to the lateral brow and lateral cheek, respectively.
Motor innervation of the orbit: Motor innervation to the orbit involves the oculomotor, trochlear and abducens nerves, or the third, fourth and sixth cranial nerves, respectively. The oculomotor nerve exits the brainstem medially, leaving its dural sheath to enter the superolateral aspect of the cavernous sinus. Here, it divides into superior and inferior divisions which both pass into the orbit through the superior orbital fissure, within the annulus of Zinn. The superior division sends branches to the levator muscle and superior rectus while the inferior division branches into three parts to supply the medial rectus, inferior rectus and inferior oblique. The branch which innervates the inferior oblique also carries parasympathetic fibers which synapse in the ciliary ganglion. Thus, injury due to surgery or trauma to these inferior orbital structures can lead to an efferent pupillary defect and dilation.48
The trochlear nerve, the smallest and longest of the cranial nerves, arises from the dorsal midbrain, crosses the midline to emerge adjacent to the superior cerebellar peduncle. It enters the cavernous sinus along its lateral wall, reaching the orbit via the superior orbital fissure, above the annulus (along with the frontal and lacrimal nerves). It travels anteromedially above the levator just inferior to the periorbita, and enters the superior oblique at the muscle belly's posterior third. The trochlear nerve is unique among the cranial nerves. It is the only cranial nerve innervating an extraocular muscle which does not penetrate the intraconal surface of the muscle it serves. It is also the smallest cranial nerve, has the longest intracranial component, and is the only cranial nerve to exit dorsally from the brainstem. For these reasons, it is also the most prone to injury with closed head trauma.49
The abducens nerve originates from the pons and enters the cavernous sinus, initially following a course within the sinus near the internal carotid artery before coursing laterally along the wall. It passes into the orbit via the intra-annular portion of the superior orbital fissure, running along the inner surface of the lateral rectus and piercing the muscle belly at its posterior one-third. The intracranial course of the abducens nerve turns sharply as it crosses the petrosphenoidal ligament, making it particularly prone to injury50,51 with acute increases50 or decreases52 in intracranial pressure.
Sympathetic innervation of the orbit: Sympathetics to the orbit which supply the iris dilator, eyelid muscle, eccrine sweat glands, and blood vessels originate from the superior cervical ganglion. These fibers travel along the internal carotid artery, through the cavernous sinus and into the orbit along the ophthalmic artery, via the superior orbital fissure. The sympathetics pass through the ciliary ganglion (located lateral to the optic nerve at the apex) without synapsing.12
Parasympathetic innervation of the orbit: Parasympathetics innervate the iris sphincter muscle, ciliary muscle, lacrimal gland and orbital blood vessels to produce miosis, lacrimation and relaxation of vascular tone. These inputs originate in the Edinger-Westphal nucleus (third cranial nerve), the salivatory nucleus via the nervus intermedius10 (the parasympathetic nerve fibers originating from the facial nerve), and the parasympathetic ganglia supporting the orbit. Preganglionic parasympathetics course with the oculomotor nerve, along its inferior division, and enter the orbit via the inferior orbital fissure. These fibers run superficially in the oculomotor nerve as it exits the brainstem adjacent to the posterior communicating artery. Thus, aneurysms of the posterior communicating artery may produce a third nerve palsy with an associated dilated pupil. These nerves synapse in the ciliary ganglion and enter the globe as the short posterior ciliary nerves.17
The ciliary ganglion lies adjacent to the lateral aspect optic nerve at the orbital apex. The ganglion also contains sympathetics that travel into the orbit via the ophthalmic artery to reach the iris dilator and ocular blood vessels, as well as sensory fibers from the nasociliary nerve which supply intraocular structures. Neither the sympathetics nor the sensory fibers synapse within the ganglion.
Preganglionic fibers from the facial nerve nucleus pass through the geniculate ganglion and then travel with the greater petrosal nerve to eventually synapse within the pterygopalatine ganglion. These fibers course via the infraorbital fissure to the orbit and directly to the lacrimal gland.37, 38 Additional postganglionic fibers travel along branches of the maxillary division of the trigeminal nerve that converge with the lacrimal nerve to enter the orbit.
Vascular Anatomy of the Orbit: Arterial Supply
The ophthalmic artery, the first intracranial branch from the internal carotid artery, provides most of the blood supply to the orbit and globe. The ophthalmic artery arises just as its parent vessel exits the cavernous sinus, just inferior to the optic nerve and posterior to the anterior clinoid process. It immediately joins the optic nerve along its inferolateral surface, traveling within a common dural sheath, and entering the apex via the optic foramen.53, 54 Once inside the orbit, the artery crosses medially and gives off its major apical branches (Figure 1.18).
The major intraconal vessels include the central retinal artery, branches to the extraocular muscles, and the long and short posterior ciliary arteries. The first branch of the ophthalmic artery is the central retinal artery.
This vessel typically pierces the nerve inferomedially, at a point approximately 10 mm from the globe, and as it reaches the globe, gives off the end arteries to the retina. Branches to the extraocular muscles show greater individual variation in distribution. Generally, these vessels run within the muscle belly or along their medial surfaces. As they continue to travel anteriorly with the muscles, the terminal branches of these arteries enter the globe at the tendinous muscle insertions, becoming the anterior ciliary arteries. These branches provide anastomoses with the long posterior ciliary arteries to supply the iris, ciliary muscle and other anterior intraocular structures. Because of this contribution of the muscular arteries to the anterior segment, disinserting more than two extraocular muscles from the globe during surgery at one time is generally avoided.
Typically, there are two or three posterior ciliary arteries which branch from the ophthalmic artery near the apex and run medially and laterally within the orbit. Some of these vessels divide into 15-20 short posterior ciliary arteries which enter the posterior aspect of the sclera to supply the choroid and the optic nerve head. Others, the two long posterior ciliary arteries, continue to travel anteriorly within the sclera, entering the globe medially and laterally to supply the anterior segment and anastomosing with terminal branches of the muscular arteries.
The major extraconal, apical branches of the ophthalmic artery include the lacrimal and posterior ethmoidal arteries. The lacrimal artery, along with the lacrimal nerve, runs above the superior border of the lateral rectus to reach the lacrimal gland and lateral upper lid. It anastomoses with the middle meningeal artery via the recurrent meningeal artery, and the temporal arteries. The posterior ethmoidal courses medially, along the frontoethmoidal suture to exit via the posterior ethmoid foramen where it gives off branches supplying the sinus and nasal mucosa and the frontal dura.54, 55
As the ophthalmic artery continues forward in the orbit, it then gives rise to the anterior ethmoidal artery and finally, its terminal branches. The anterior ethmoidal vessel exits via the anterior ethmoidal foramen to supply frontal dura and ethmoid and frontal sinus mucosa. Anastomoses from this circulation and branches of the external carotid provide blood flow to the nose and septum. The frontoethmoidal suture, along which the anterior and posterior ethmoidal arteries and associated branches of the nasociliary nerve run, is an important landmark for the roof of the ethmoid, or fovea ethmoidalis which lies just beyond this line. Penetration of the medial wall above this suture would allow communication between the anterior cranial fossa and the orbit. Additionally, the posterior ethmoidal foramen characteristically lies 6 mm anterior to the optic canal and 12 mm posterior to the anterior ethmoidal foramen.2
The terminal branches of the ophthalmic artery are the supraorbital, supratrochlear, dorsal nasal and the medial palpebral arteries. The supraorbital and supratrochlear arteries provide the blood supply to the forehead and medial lids, while the dorsal nasal and medial palpebral arteries supply the medial lids and nose. The supraorbital artery travels above the levator via the supraorbital notch or foramen and should be carefully avoided during surgical dissection of the orbital roof. All of these vessels anastomose extensively with external carotid branches to the face.
It is clear that there is great degree of collateral flow to the orbit and lids between the internal and external carotid circulation. Therefore, a review of relevant branches of the external carotid artery, namely branches of the maxillary artery, is also important. Superiorly, the superficial temporal artery provides blood supply to the forehead, anastomosing with the circulation of the supraorbital and suprotrochlear arteries. The angular artery provides anastomoses with the dorsal nasal and palpebral arteries medially. Within the orbit itself, the sphenopalatine artery, like the ethmoidal circulation, supplies the nasosinus mucosa and nasal septum. The superficial branches of the infraorbital artery anastomose with the inferomedial palpebral arteries, while the deeper branches anastomose with the muscular arteries. The anastomosis between the lacrimal artery and the middle meningeal artery has already been discussed. This occurs via the recurrent meningeal artery, which enters the orbit through the sphenoid, through a foramen superolateral to the superior orbital fissure or directly via the fissure itself.219
Vascular Anatomy of the Orbit: Venous Outflow
The venous drainage pathways of the orbit run independently of the arteries and are a completely valveless system. There are three major outflow systems, involving the cavernous sinus, pterygoid plexus, and an anterior venous system which drains via the facial vein (Figure 1.19). The superior ophthalmic vein provides outflow from the superifical, superior periorbital and orbital regions, via the supraorbital, nasofrontal and angular veins. It can be divided into three segments as it runs anterior-posteriorly. The first segment courses adjacent to the trochlea and along the medial edge of the superior rectus. The second passes inferior to the muscle and into the cone. This segment receives the ciliary and superior vortex veins from the globe. The third portion of the superior orbital vein travels along the lateral edge of the superior rectus and exits the orbit via the extra-annular superior orbital fissure to drain into the cavernous sinus.
The inferior ophthalmic vein drains the inferior orbit, including tributaries from the inferior rectus and oblique muscles and from the inferior vortex veins. The inferior ophthalmic vein anastomoses with a branch of the superior ophthalmic vein. A portion of the outflow is directed into the pterygoid plexus and the rest directly into the cavernous sinus. Anteromedially, venous drainage occurs mainly via the angular and facial veins. Because of the high degree of anastamoses and absence of valves, some degree of venous obstruction can be redirected within the system. However, acute thromboses, particularly of the cavernous sinus, cause marked orbital congestion and subsequently, exophthalmos.
Paranasal Sinuses
There are four pairs of paranasal sinuses: the frontal, ethmoid, sphenoid and maxillary sinuses which directly neighbor the orbital roof, medial wall, and floor. Knowledge of their anatomy is useful since these spaces can share disease processes with the orbit such as infection and tumors, and can also provide surgical access to the orbit and lacrimal system.
The frontal sinus overlies the anterior portion of the orbital roof and drains into the frontonasal duct which travels though the anterior portion of the ethmoid sinus (ethmoid infundibulum) to empty into the middle meatus within the nose. This sinus develops in childhood and is usually difficult to appreciate radiographically until age 7 or so. Pneumatization is typically completed by early adulthood.56 The frontal sinus is a common site for mucocele formation.57
The ethmoid sinuses lie between the medial orbital walls and immediately posterior to the nose. The lateral wall of this sinus is comprised of the very thin lamina papyracea, which is easily fractured in surgery or trauma, or compromised by local infection. A frequent source of orbital cellulitis is ethmoid sinusitis which spreads secondarily. The ethmoid roof, or fovea ethmoidalis, is located just beneath the anterior cranial fossa and just medial to the frontoethmoidal suture line within the orbit. The most medial portion of the ethmoid roof is the cribriform plate, which overlies the nasal cavity (Figure 1.20). The sinuses themselves are comprised of many individual, thin-walled air cells and can be divided into three groups. The anterior and middle air cells drain into the middle meatus while the posterior air cells empty into the superior meatus of the nose. In performing dacryocystorhinostomy to create a passage between the lacrimal sac and nose, the ethmoid air cells are frequently encountered extending anterior to the posterior lacrimal crest.58,59
Figure 1.20: Computed axial tomography illustrating the relationship between the anterior cranial fossa, orbit and ethmoid sinus
The sphenoid sinuses are located midsagittally and posterior to the ethmoid air cells. Like the frontal sinuses, they pneumatize relatively late in life and do not reach full size until adolescence. Drainage occurs via the sphenoethmoid recess located in the anterior sinus wall. Because the contents of the orbital apex and nearby cavernous sinus exit the orbit through the sphenoid bone, the walls of the sphenoid lie in close proximity to a number of critical structures. Anteriorly and superolaterally, the optic nerve and intracavernous portion of the internal carotid artery run along the lateral sinus walls. Severe sphenoid sinusitis can therefore cause optic nerve injury.60 Likewise, congenital dysplasia of the sphenoid, as can occur in neurofibromatosis type 1, can produce pulsatile proptosis.61, 62 The sphenoid sinus also provides a useful surgical approach to the pituitary fossa which is located posteriorly to the sinus.63
The maxillary sinus underlies the orbital floor and is the largest of the paranasal sinuses. Drainage from this sinus occurs via the maxillary ostium into the middle meatus. The ostium is located high within the medial sinus wall, close to the level of the orbital floor. Thus, trauma to the orbital floor (i.e. orbital fracture or inferomedial decompression) can obstruct drainage from the sinuses. Inside the medial walls of the maxillary sinus, lie the bony nasolacrimal canals. The posterior most aspect of the sinus extends from the area of the infraorbital fissure, and the infraorbital nerve and artery run along the maxillary roof within the infraorbital canal. Behind the maxillary sinus is located the pterygopalatine fossa and the maxillary artery runs in its posterior wall.
Conclusion
The orbit and its surrounds represent a complex anatomical space, incorporating critical ocular, neural, and vascular structures. The purpose of this chapter has been to provide an overview of orbital anatomy, as well as basic anatomy of the eyelids, lacrimal 21system, and paranasal sinuses. A detailed understanding of this anatomy is fundamental to oculoplastic surgery and the management of orbital disease.
REFERENCES
- Whitnall SE. The Anatomy of the Human Orbit and Accessory Organs of Vision, 2nd ed. Oxford University Press; NewYork: 1932.
- Rootman J, Stewart B, Goldberg RA. Orbital Surgery: A Conceptual Approach Lippincott-Raven, New York: 1995.
- Webster RC. Supraorbital and supratrochlear notches and foramina: Anatomical variations and surgical relevance. Laryngoscope 1986;96:311.
- Kim JW, Goldberg RA, Shorr N. The inferomedial orbital strut: An anatomic and radiographic study. Ophthal Plast Reconstr Surg 2002;18:355–64.
- Rose JG, Lucarelli MJ, Lemke BN. Lacrimal, orbital and sinus anatomy. In: Woog JJ, (Ed): Manual of Endoscopic Lacrimal and Orbital Surgery. Elsevier, London: 2003.
- Goldberg RA, Schorr N, Cohen M. The medial orbital strut in the prevention of post-decompression dystopia in dysthyroid ophthalmopathy. Ophthalmic Plast Reconstr Surg 1992;8:32–34.
- Whitnall S. On a tubercle on the malar bone, and on the lateral attachments of the tarsal plates. J Anat Physiol 1911;45:426–32.
- Yeh S, Foroozan R. Orbital apex syndrome. Curr Opin Ophthalmol 2004;15:490–98.
- Lenzi GL, Fieschi C. Superior orbital fissure syndrome: a review of 130 cases. Eur Neurol 1977;16:23–30.
- Morard M, Tcherekayev V, deTribolet N. The superior orbital fissure: a microanatomical study. Neurosurgery 1994;35:1087–93.
- Jordan DR. The nervus intermedius. Arch Ophthalmol 1993;111:1691.
- Lyon D, Lemke BN, Wallow I, Dortzbach RK. Sympathetic nerve anatomy in the cavernous sinus and retrobulbar orbit of the cynomolgous monkey. Ophthalmic Plast Reconstr Surg 1992;8:1–12.
- Keane JR. Cavernous sinus syndrome. Analysis of 151 cases. Arch Neurol 1996;53:967–71.
- Koornneef L. New insights in the human periorbital connective tissue: results of a new anatomic approach. Arch Ophthalmol 1977;95:1269–73.
- Koornneef L. Orbital septa: anatomy and function. Ophthalmology 1979;86:876–85.
- Sutton J. The fascia of the human orbit. Anat Reconstr 1920;18:141.
- Dutton J. Atlas of Clinical and Surgical Orbital Anatomy. WB Saunders Co; Philadelphia: 1994.
- Jones LT. A new concept of the orbital fascia and rectus muscle sheaths and its surgical implications. Trans Am Acad Ophthalmol Otolaryngol. 1968;72:755–64.
- Fink W. An anatomical study of the check ligaments of the vertical muscles of the eyes. Am J Ophthalmol 1957;44:800–09.
- Helveston E, Merriam W, Ellis F, Schelhamer R, Gosling G. The trochlea: a study of the anatomy and physiology. Ophthalmology 1982;89:124–33.
- Sacks J. The shape of the trochlea. Arch Ophthalmol 1984;102:932.
- Neely K, Ernest J, Mottier M. Combined superior oblique palsy and Brownʼns syndrome after blepharoplasty. Am J Ophthalmol 1990;109:347.
- Apt L. An anatomic reevaluation of rectus muscle insertions. Trans Am Ophthalmol Soc 1988;78:365.
- Ahl NC, Hill JC. Hornerʼns muscle and the lacrimal system. Arch Ophthalmol. 1982;100:488–93.
- Shinohara H, Kominami R, Yasutaka S, Taniguchi Y. The anatomy of the lacrimal portion of the orbicularis oculi muscle (tensor tarsi or Hornerʼns muscle). Okajimas Folia Anat Jpn 2001;77:225–32.
- Meyer DR, Linberg JV, Wobig JL, McCormick SA. Anatomy of the orbital septum and tissues. Implications for ptosis surgery. Ophthal Plastic Reconstr Surg 1991;7:104–13.
- Hawes MJ, Dortzbach RK. The relationship of capsulopalebral fascia with orbital septum of the lower eyelid: an anatomic study under magnification. J Craniofacial Surgery 2006;17:1118–20.
- Castanares S. Blepharoplasty for herniated intraorbital fat: anatomical basis for a new approach. Plast Reconstr Surg 1951;8:46–58.
- Kikkawa DO, Lucarelli MJ, Shovlin JP, Cook BE, Lemke BN. Ophthalmic facial anatomy and physiology. In: Levine, MR, ed. Manual of Oculoplastic Surgery, third ed. Butterworth Heinemann; New York: 2003.
- Whitnall S. On a ligament acting as a check to the action of the levator palpebrae superioris muscle. J Anat Physiol. 1910;14:131.
- Goldberg RA. Eyelid anatomy revisited: dynamic high-resolution images of Whitnallʼns ligament and upper eyelid structures with the use of a surface coil. Arch Ophthalmol. 1992;110:1598.
- Anderson R, Dixon R. The role of Whitnallʼns ligament in ptosis surgery. Arch Opthalmol 1979;97:705–07.
- Walton KA, Buono LM. Horner syndrome. Curr Opin Ophthalmol 2003;14:357–63.
- Lockwood C. the anatomy of the muscles, ligaments, and fascia of the orbit, including an account of the capsule of tenon, the check ligaments of the recti, and of the suspensory ligament of the eye. J Anat Physiol 1885;20:2–25.
- Scheie HG, Albert DM. Distichiasis and trichiasis: origin and management. Am J Ophthalmol 1966;61:718.
- Lemke BN, Lucarelli MJ. Anatomy of the ocular adnexa, orbit, and related facial structures. In: Nesi, FA, Lisman, RD, Levine, MR, (Eds). Smithʼns Ophthalmic Plastic and Reconstructive Surgery. CV Mosby; St. Louis: 1998.
- Ruskell G. The orbital branches of the pterygopalatine ganglion and their relationship with internal carotid nerve branches in primates. J Anat 1970;106:323.
- Ruskell G. The distribution of autonomic postganglionic fibers to the lacrimal gland in monkeys. J Anat 1971;109:229–42.
- Dutton J. The lacrimal systems. In: Dutton, J. Atlas of Clinical and Surgical Orbital Anatomy. WB Saunders Co; Philadelphia: 1994.
- Tucker N, Tucker S, Linberg J. The anatomy of the common canaliculus. Arch Ophthalmol 1996;114:1231–34.
- Groell R, Schaffler G, Uggowitzer M, Szolar D, Muellner K. CT: anatomy of the nasolacrimal sac and duct. Surg Radiol Anat. 1997;19:189–91.
- Drysdale D. Experimental subdural retrobulbar injection of anesthetic. Ann Ophthalmol 1984;16:716–19.
- Anderson RL, Panje WR, Gross CE. Optic nerve blindness following blunt forehead trauma. Ophthalmology 1982;89:445–55.
- Sarkies N. Traumatic optic neuropathy. Eye 2004;18:1122–25.
- Dalley R, Robertson W, Rootman J. Globe tenting: a sign of increased orbital tension. Am J Neuroradiol. 1984;10:181–86.
- Knize D. A study of the supraorbital nerve. Plast Reconstr Surg 1995;96:564–69.
- Hornblass A. Pupillary dilation in fractures of the floor of the orbit. Ophthalmic Surg 1979;10:44.
- Mansour AM, Reinecke RD. Central trochlear palsy. Surv Ophthalmol 1986;30:279–97.
- Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain 1991;114:155–80.
- Hanson RA, Ghosh S, Gonzalez-Gomez I, Levy ML, Gilles FH. Abducens length and vulnerability? Neurology 2004;62:33–36.
- Insel TR, Kalin NH, Risch SC, Cohen RM, Murphy DL. Abducens palsy after lumbar puncture. N Engl J Med 1980;303:703.
- Hayreh SS. The ophthalmic artery. I. Origin and intracranial and intracanalicular course. Br J Ophthalmol 1962;46:65–98.
- Lang J, Kageyama I. The ophthalmic artery and its branches, measurements and clinical importance. Surg Radiol Anat 1990;12:83–90.
- Ettl A, Kramer J, Daxter A, Koornneef L. High resolution magnetic resonance imaging of neurovascular orbital anatomy. Ophthalmology 1997;104:869–77.
- Zinreich SJ. Imaging of the nasal cavity and paranasal sinuses. Curr Opin Radiol 1992;4:112–16.
- Rootman J. Disease of the Orbit. JB Lippincott; Philadelphia: 1988.
- Buus D, Tse D, Farris B. Ophthalmic complications of sinus surgery. Ophthalmology 1990;97:612–19.
- Blaylock W. Moore C, Linberg J. Anterior ethmoidal anatomy facilitates dacryocystorhinostomy. Arch Ophthamol 1990;108:1774–77.
- Fujimoto N, Adachi-Usami E, Saito E, Nagata H. Optic nerve blindness due to paranasal sinus disease. Ophthalmologica 1999;213:262–64.
- Hunt JC, Pugh D. Skeletal lesions in neurofibromatosis. Radiology 1961;76:1–19.
- Mortada A: Pulsating exophthalmos with orbital neurofibromatosis, Am J Ophthalmol 1967;64:462.
- Kanter AS, Dumont AS, Asthagiri AR, Oskouian RJ, Jane JA, Laws ER. The transsphenoidal approach: a historical perspective. Neurosurg Focus 2005;18:1–4.