Have you ever assembled a jigsaw puzzle? The many pieces interlock to create a picture, a work of art. Medical records resemble a jigsaw puzzle, with many pieces that build off one another to create a picture of the patient. The medical history is a large piece of the picture. The history of patient is the foundation on which your visit is built. It frequently establishes the extent of the examination and treatment. A medical history is the collection of data obtained by interviewing the patient. It sounds simple enough, but taking a medical history is truly an art. It requires a team effort by physicians and technicians. The successful history taker is compassionate, conversational, and creative. Take a moment and think about what types of questions are asked. They are very personal.
Over dependence on, and sometimes, even abuse of modern sophisticated diagnostic techniques is drawing the clinician away from bed-side diagnosis. In the field of Pulmonary Medicine, confusion exists in describing surface areas and certain typical signs and symptoms.
This chapter is described in four divisions: functional anatomy of respiratory system. history taking and symptomology, general physical examination and examination of the lungs and thorax.
FUNCTIONAL ANATOMY OF RESPIRATORY SYSTEM
Besides containing the major organs of respiration and circulation, the thorax also functions as a mechanical “bellows” responsible for movement of gas into and out of the lungs. Therefore, an overview of thorax will be discussed before going to its examination.
The thorax is that area of the body, which is formed by the rib cage, thoracic vertebrae and sternum, and contains the esophagus, trachea, lungs, heart and great vessels. Shaped somewhat like a cone, the thorax has a wide base bounded by the diaphragm below and a narrow opening at the top called the operculum. The operculum is bounded by the first ribs and the upper portion of the sternum.2
Gross Structure and Function
Mediastinum: The mediastinum is the central compartment of the chest, dividing the thorax vertically and separating the left and right pleural cavities. Functionally, the mediastinum is divided into three subcompartments. The anterior compartment, between the sternum and pericardium contains the thymus gland and the anterior mediastinal lymph nodes. The middle compartment contains the pericardium and heart, great vessels, phrenic and upper portions of the vagus nerves, the trachea, the main stem bronchi and their associated lymph nodes. The posterior compartment lies between the pericardium and the vertebral column and contains the thoracic aorta, esophagus, thoracic duct, sympathetic chains and lower portions of the vagus nerve and the posterior mediastinal lymph nodes.
Lungs and pleura: The lungs are paired conical shaped organs, lying in the pleural cavities, and separated by the mediastinum. Although the adult lungs average 800 gm in weight, by volume they consist of nearly 90% of air and only 10% tissue. Owing to the protrusion of the heart and mediastinum to the left, a concavity called the cardiac notch is formed and the left lung is somewhat narrower than the right. However, due to the displacement of the liver and the resultant elevation of the right hemidiaphragm, the right lung is somewhat shorter than the left.
The lungs extend from their apices 1 to 2 cm above the medial part of the clavicles, down till the diaphragm. The lung surfaces lying against the ribs form curved costal margins, with the medial surface being adjacent to the mediastinum. This mediastinal surface contains a vertical opening called the hilum, through which the major airways, blood vessels, lymphatics and nerves enter and exit. This grouping of pathways, bound together by connective tissue, is often referred to as the root of the lung.
Each lung is further divided into smaller anatomic units called lobes (Fig. 1.1) separated by one or more fissures. The right lung has upper, middle and lower lobes, the first two being separated by a horizontal fissure; its middle and lower lobes are set apart by an oblique fissure (Fig. 1.2). The lobes are further divided into segments according to the branching of the tracheo-bronchial tree, and ultimately into secondary lobules, the smallest gross anatomic units of lung tissue set apart by true connective tissue septa. Secondary lobules correspond to clusters of three to five terminal bronchioles and can be observed from the external and cut surface of the lung. They are polygonal in shape and range in size from 1.0 to 2.5 cm on a slide. Localized infection, hemorrhage, or aspiration is initially contained by the boundaries between these lobules.
The surface of the lungs, portions of the major interlobar fissures, the inside of the chest wall, the diaphragm, and the lateral portion of the mediastinum are covered by a thin mesothelial layer called the pleura.
The portion covering the lungs and extending on to the hilar bronchi and vessels and into the major fissures is called the visceral pleura. The deeper portions of the visceral pleura contain elastic fibers, small venules, and lymphatics. The interlobular septa are continuous with this layer; the veins and lymphatics course along these septa, starting as fine caliber vessels in the pleura. The portion of the pleura covering the inner surface of the chest wall and the mediastinum is called the parietal pleura and is named according to the structures which it encloses. Thus, the costal pleura lines the inner surface of the rib cage, the mediastinal pleura covers the mediastinum, and the diaphragmatic pleura, the diaphragm. The acute angle where the costal pleura joins the diaphragmatic pleura is known as the costophrenic angle. This area contains no lung tissue and is clearly visible on a chest radiograph. Moreover, any excess fluid in the pleural space has a tendency to gather here first, especially in the standing position, to cause the angle to appear blunted or flattened in the radiograph. The upper dome of the parietal pleura extends above the first rib and encloses the thoracic inlet. This area, called the cupula, is strengthened by a layer of connective tissue known as the suprapleural membrane or Sibson's fascia.
Between the visceral and the parietal pleura lies the pleural cavity, a potential space normally occupied by a serous fluid forming a thin film of uniform thickness that couples the visceral and parietal pleural surfaces, allowing them to slip easily one over the other. If air, blood or other fluids are introduced into this area, the two pleural surfaces separate. Under such circumstances, the parietal pleura stays relatively fixed against the inner wall of the thorax, but the lung and the visceral pleura are displaced away from the chest wall. At the root of the lung, the parietal pleura becomes continuous with the visceral pleura as it passes onto the surface of the lung. Thus although the both portions of the pleura are described by different names, they are really one continuous lining.4
The lung itself has elastic properties and tends to recoil to a smaller volume and plays an important role in the development of negative intrapleural pressure. When air or fluid is introduced into the pleural space, the lung quickly collapses into a smaller size. The most common cause of a lobar collapse is obstruction of its bronchus.
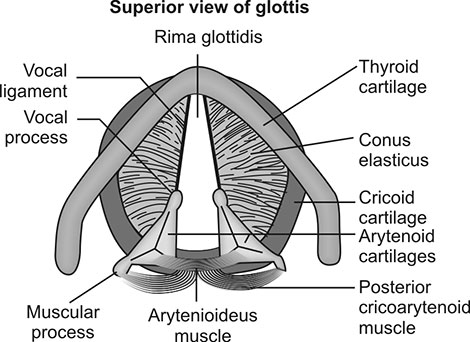
Vocal cords: The true vocal cords appear as white bordered veils, separated by a space known as glottis (Fig. 1.3). The vocal cords are composed of muscle, ligament, submucosal soft tissue and a mucous membrane covering. The loose tissue below this mucous membrane represents a potential space readily subject to fluid accumulation. Since the lymphatic drainage of this area is sparse, cord swelling resulting from such fluid accumulation resolves slowly.
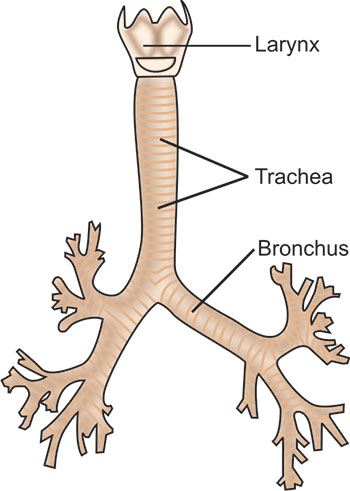
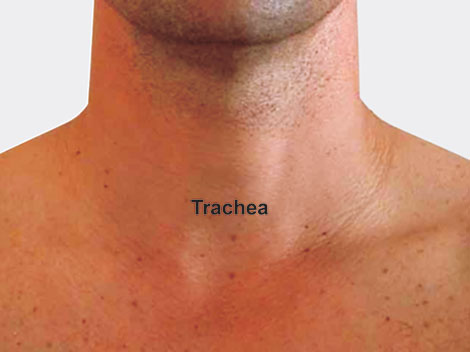
Trachea: The adult trachea is about 2 to 2.5 cm in diameter and about 10 to 12 cm long (Fig. 1.4). It is almost midline in the neck, but in the superior mediastinum it deviates slightly to the right, allowing room for the aorta to pass by on the left. At the point of tracheal bifurcation in the chest, a sharply dividing cartilage, the carina, extends up in midline to help demarcate flow into the right or left side. The right mainstem bronchus angles off at 20 to 30° from the midline, whereas the left one does so more sharply at 45 to 55°. Therefore, aspirated solid objects or fluids have a tendency to follow the straighter course of the right mainstem bronchus.
Bones of Thoracic Cage
The key thoracic bony structures include the thoracic vertebrae, the sternum, the ribs and the costal cartilages. The bony structures provide support and protection to the thoracic viscera and serve as points of origin and insertion for the respiratory muscles.
Vertebrae: The 12 thoracic vertebrae share a common structure with the rest of the vertebral column as a whole, having a body with pedicles, laminae, and a spinous and transverse processes. The bodies and transverse processes of the thoracic vertebrae have distinctive facets that serve as points of articulation for the head of each rib and tubercle of its neck. The orientation of these facets, in combination with a rounded vertebral foramen, provides for the rotation and elevation characterizing rib movements.
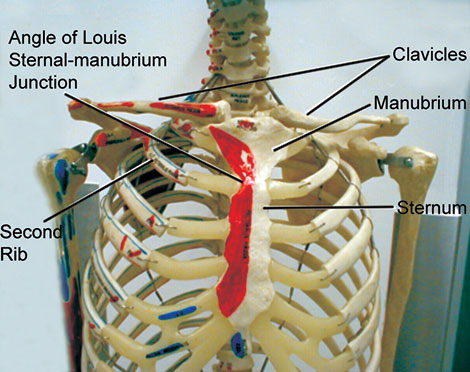
Sternum: In the adult, the sternum averages 17-18 cm in length and consists of three 5portions, the upper triangular shaped manubrium, the longer and narrower body, and the lower pointed xiphoid process. At a level equivalent to the intervertebral disc separating the fourth and fifth thoracic vertebrae, the manubrium articulates with the body of the sternum and the second costal cartilages (second ribs), forming a slightly oblique angle called the angle of Louis or sternal angle. This important external landmark, the angle of Louis demarcates the point at which the trachea divides into left and right mainstem bronchi, and the top of the heart and pericardium. The xiphoid process is located approximately at the level of the tenth thoracic vertebra and articulates with seventh rib pair.
Ribs: Corresponding to the 12 thoracic vertebrae are 12 pairs of ribs. The first seven pairs, or vertebrosternal ribs, connect directly to the sternum via bars of hyaline cartilage called the costal cartilages. The 8th through 10th pairs, or vertebrochondral ribs, connect to the ribs above and, through the costal cartilages, indirectly to the sternum. The 11th and 12th rib pairs have no connection with the other rib pairs or sternum, and for this reason, are often referred to as “floating ribs”. The floating ribs terminate in cartilaginous free ends in the wall of the abdomen.
Each rib consists of a head, a neck, and a body or shaft. With the exception of the 10th, 11th and 12th ribs, the rounded head has two facets for articulation with corresponding facets located on the bodies of thoracic vertebrae. The flattened neck is about 2.5 cm long and extends outward from the head, providing a point of attachment for the costotransverse ligaments. On the posterior surface of the neck, near its attachment to the shaft, is a tubercle, most prominent in the upper ribs. A portion of the tubercle articulates with the transverse process of the lower of the two vertebrae to which the head of rib is connected.
Rib movements: The first rib moves about the axis of its neck, raising and lowering the sternum. Although the motion is slight, it produces some increase in the anteroposterior (AP) diameter of the chest. During quiet breathing this action is not utilized, but it becomes important under conditions of stress.
The remaining six vertebrosternal ribs play an important role in ventilation (Fig. 1.5). In contrast to the first rib, these move simultaneously about the axis of the rib neck, and axis between the angle of the rib and its sternal junction.
As they rotate about the axes of their necks, their sternal ends rise and fall, thus increasing the AP thoracic diameter. This action is referred to as the “pump handle motion”. The simultaneous movement about the longer axes from the rib angles to the sternum leads to an up-and-down motion of the middle segments of the ribs. This “bucket handle” motion produces an increase or decrease in the transverse diameter of the chest. Thus the compound action of these ribs increases and decreases both AP and transverse diameters smoothly and synchronously.
The vertebrochondral ribs have rotation patterns similar to the vertebrosternal group. However, elevation of the anterior end of these ribs produces a backward movement of the lower end of sternum, with a reduction in thoracic AP diameter. Like the motion described for the vertebrosternal ribs, outward rotation of the middle portions of these ribs increases the transverse diameter of the thoracic cage. Ribs 11th and 12th do not participate in changing the contour of the chest but act as muscular insertion points only.
Muscle Action
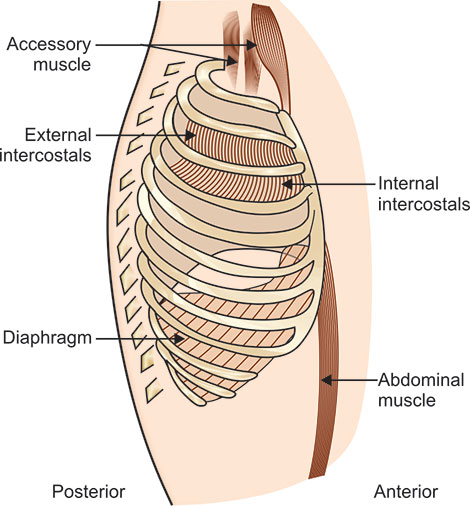
Various muscles of the thorax and abdomen contribute to the cyclical movement of gas into and out of the respiratory tract. Traditionally these muscles are divided into two groups, the primary and the accessory muscles of ventilation. The primary muscles of ventilation are active both during normal quiet breathing and exercise; and are represented by the diaphragm, intercostal muscles, and the scalene which have also been recently included under primary muscles. The accessory muscles of ventilation primarily serve other purposes but assist the primary ones under conditions of increased ventilatory demand. Although any muscles attached to ribs or sternum may qualify as accessory muscles of ventilation, the sternomastoids, abdominals and pectoralis major best represent this group.
Diaphragm: This arises from the lumbar vertebrae, the costal margin, and the xiphoid process, with its fibers converging to interlace into a broad connective tissue sheet called the central tendon. This muscle configuration is that of a tent or a dome, separating the chest from the abdomen. Although the diaphragm is a single anatomic structure, the union of its central tendon with the fibrous pericardium functionally divides its dome into leaves, often referred to as the right and left hemidiaphragms. Movements of the left and right hemidiaphragms are usually synchronous. However, dual innervations by separate phrenic nerves indicate that one hemidiaphragm can function independently of the other. The diaphragm probably accounts for some 75% of the normal changes in thoracic volumes during quiet inspiration. At rest, the normal tidal movement of the diaphragm is approximately 1.5 cm, and during deep breathing, some 6 to 10 cm. In the normal adult, each centimeter of vertical movement moves approximately 350 ml of air. The diaphragm takes no active part in exhalation and returns to its inspiratory resting position during the passive recoil of the thorax.
The mechanical action of the diaphragm is two fold (Fig. 1.6). First, contraction draws the central tendon down, flattening its contour, increasing the volume of thorax, and lowering intrapleural pressure. As the diaphragm descends, intra-abdominal pressure increases and the muscles of the abdominal wall relax, allowing the upper abdomen to balloon outward. The second mechanical action of the diaphragm is achieved through contraction of its costal fibers, raising and everting the lateral costal margins.
Increasing abdominal pressure during inspiration acts as a fulcrum against which continued contraction of the diaphragmatic fibers pull up and out on the costal margins, enlarging the thorax further. This combined vertical and transverse action of the diaphragm is easily disturbed in pulmonary disease. In advanced emphysema, the diaphragm is in an abnormally low and flat position. Under such circumstances, not only will there be a diminished vertical excursion, but contraction of the costal fibers may well pull in the lower chest boundary and narrow rather than expand the lateral dimensions of the thorax.
Diaphragm performs other important functions, such as facilitating defecation, vomiting, coughing, sneezing, and parturition. Other respiratory muscles are described (Fig. 1.7).
Intercostal muscles: The intercostal muscles consist of two sets of fibers located between each rib pair. The external intercostal muscles arise from the inferior edge of each rib, from the rib tubercle up to its costochondral junction. The fibers pass inferiorly and anteriorly to insert into the superior edge of the rib below. The muscles are thicker posteriorly than anteriorly and are also thicker than the internal intercostals.
The internal intercostal muscles are located beneath the external intercostals and arise from the inferior edge of each rib, from the anterior end of the intercostal space up to the rib angles.
The fibers pass inferiorly and posteriorly to insert into the superior edge of the rib below. This muscle group is divided into two functional parts: an interosseous portion located between the sloping parts of the ribs, and an intercartilaginous portion located where the costal cartilages slope superiorly and anteriorly, also termed as the parasternals.
During breathing, the external intercostals and parasternals are active, and contractions of these muscles during inhalation tend to elevate the ribs and thereby increase thoracic volume. These muscles also stabilize the chest wall and prevent intercostals bulging or retraction during large intrapleural pressure changes. Intercostal muscle activity continues during quiet breathing up to early exhalation. This initial expiratory activity may help to retard high airflows and facilitates a smoother and less turbulent exhalation. During respiratory distress, as the effect of vigorous respiration increases, the inspiratory activity increases in external intercostals, recruited from above downwards and also activity increases in internal intercostals, from below upwards.
Scalene muscles: The anterior, medial and posterior scalene muscles, although individual structures, are considered as single functional unit. They arise from the transverse processes of the lower five cervical vertebrae and insert into the upper surface of the first rib (anterior and medial scalenes) and the second rib (posterior scalene). Basically, they elevate and fix the first and second ribs firmly. Recently, measurements with concentric needle electrodes have demonstrated invariable inspiratory contraction of the scalene in human at rest. The inward inspiratory displacement of the upper rib cage characteristic of tetraplegia is usually not observed when the scalene function survives the cervical cord transection. There is thus no reason for using the qualifying adjective “accessory” in describing the scalenes. These muscles in humans are the primary muscles of inspiration, acting to expand the upper rib cage.
Sternomastoid muscles: As muscles of ventilation, these pull from their skull insertions and elevate the sternum, increasing the anteroposterior diameter of the chest. In chronic pulmonary disease, the sternomastoids become active in inhalation when the thorax becomes so inflated (elevated resting level) that the low placed diaphragm loses its efficiency. As these muscles contract and pull up the sternum, the ribs rotate about their neck axes but not about the rib angle-sternal junction axes. This produces an up and down motion with little side-ways expansion. In extreme cases, antero-posterior expansion of the thorax may cause the lower ribs to become indrawn, partially negating the increase in chest volume.
Pectoralis major muscle: For ventilation it pulls in a direction opposite to that of its primary function. If the arms and shoulders are fixed, as by leaning on the elbows or firmly grasping a table, the pectoralis muscle can use its insertion as an origin and pull with greater force on the anterior chest, lifting up ribs and sternum and increasing thoracic antero-posterior diameter. Patients with chronic pulmonary disease assume a characteristic posture for maximum use of pectoralis muscles. In advanced cases most of the air moved, may be the result of the action of this powerful muscle. But it only aids inhalation, taking no part in exhalation.
Abdominal muscles: As accessory muscles of ventilation, the abdominals function mainly to aid in forced expiratory activity. This action is achieved both, by increasing 9intra-abdominal pressure and by drawing the lower ribs downwards and medially. In both the relaxed supine and standing positions, these muscles are normally inactive during quiet breathing. They come into play only when the normal elastic recoil of the thorax provides insufficient force to achieve the needed expiration. In such circumstances, contraction of the powerful abdominals builds up strong intra-abdominal pressure and drives the diaphragm, like a piston, into exhalation. In emphysema effective use of the abdominals is often lost, and without these powerful generators of force to push the diaphragm into expiratory action, the patient is at a great disadvantage.
Innervation of the Lung and Thoracic Musculature
The lung is innervated by elements of both the autonomic and somatic divisions of the nervous system. Autonomic innervation is via branches of the paired vagus nerves and the upper four or five thoracic sympathetic ganglia. Both contribute to the anterior and posterior pulmonary plexuses at the roots of the lung. This provides both motor and sensory pathways to the lung.
Somatic innervation of the respiratory system is mainly by way of efferent motor stimulation of the primary muscles of ventilation, the diaphragm and intercostals. The diaphragm is innervated by paired phrenic nerves (C3-C5). The intercostal muscles receive their motor innervation via intercostal nerves (T2-T11).
Vascular Supply
The vascular supply of the lungs is composed of two separate systems, the pulmonary and the bronchial circulations. Pulmonary circulation routes the venous blood coming from the tissues of the lungs for purposes of restoring its oxygen content and removing carbon dioxide the gaseous product of metabolism. Bronchial circulation provides freshly arterialized blood back to the lungs to meet its own metabolic requirements.
Lymphatics
Lymph nodes are organized in groups that drain specific regions of the body. This knowledge guides the clinician to inspect particular areas of anatomy when lymphadenopathy occurs.
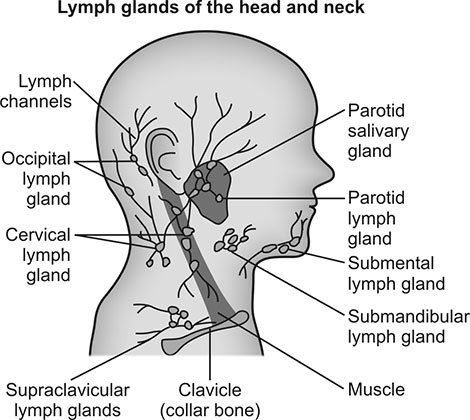
Lymphatic drainage of the head and neck is traditionally divided into 6 regions (Fig. 1.8). The most important nodes in this grouping are around the internal jugular lymph nodes. The superior aspect is termed region II; it receives lymph from the supraglottic larynx, anterior nasopharynx, and oropharynx via submental and submandibular lymph nodes (region I). The middle portion of the internal jugular chain is region III; it collects drainage from the superior hypopharynx and superior larynx via direct drainage through lymphatic capillaries.
The inferior part of the internal jugular chain is region IV; it collects drainage from the inferior hypopharynx, inferior larynx, and thyroid and supraclavicular regions. Region VI sits in the anterior aspect of the neck; it contains supraclavicular, pretracheal, and thyroid nodes, which drain into region IV. Region IV of the internal jugular chain is the common collecting point for regions I-III and VI. Region V collects lymph from the scalp and posterior nasopharynx. All lymphatic drainage from region V and region IV on the internal jugular chain collect into the jugular trunk (a group of nodes positioned at the internal jugular anterior brachiocephalic veins) and then subsequently into the thoracic duct on the left or directly into the brachiocephalic vein on the right.
The thoracic cavity maintains a distinct collection of lymph nodes, with a slightly complex drainage route that parallels bronchi, arteries, and veins. Each major bronchi division has a collection of nodes called the intrapulmonary lymph nodes, which lie within the lungs and drain each of the lung's corresponding segments. The intrapulmonary nodes drain into a set of nodes, the left and right bronchopulmonary (hilar) lymph nodes, which are located at the junction of each lung and its main bronchi. These nodes collect the lymphatic drainage from the segments of their respective lung. At the bifurcation of the trachea and beginning of each bronchus, 3 sets of nodes reside, the right and left tracheobronchial lymph nodes and the inferior tracheobronchial lymph nodes. An unusual feature of this anatomy is that the inferior tracheobronchial nodes, also known as the carinal nodes, collect lymph from the left lower lobe but drain that fluid into the right tracheobronchial lymph nodes. This is significant because a suspicious-appearing lymph node in the right hilar region should prompt evaluation of the left lower lobe and the right lung.
Aligned with the sides of the trachea are groups of nodes known as the right and left paratracheal lymph nodes, which collect lymphatic fluid from the right and left tracheobronchial nodes, respectively. The posterior thoracic cavity is drained via the intercostal lymph nodes and into the posterior mediastinal lymph nodes. The anterior thoracic cavity is drained through the parasternal lymph nodes, which are located next to the sternum in the intercostal space. The parasternal lymph nodes collect lymph from the anterior mediastinum and communicate with the medial aspect of the anterior chest wall. The common drainage site for all of the aforementioned lymph nodes is into the jugular trunk and then into the thoracic duct on the left or directly into the brachiocephalic vein on the right.
HISTORY TAKING AND SYMPTOMATOLOGY
The principles of medicine represent the application of the fruits of clinical experience, superimposed on knowledge acquired through study. These principles can be acquired only if a properly organized approach to a clinical problem is used. This approach to the patient and disease is what, is meant by clinical methods. The underlying diagnosis is often of more interest to the doctor than to the patient, although it is, of course, of fundamental importance in determining the clinical problem presented by the patient. First the clinical database should be collected by history-taking, physical examination and ancillary investigations. Then this data should be interpreted in terms of disordered function and structure.
The aim is to elicit an accurate account of the symptoms. While speaking out the symptoms, patient should not be interrupted unnecessarily. He must be allowed to tell his 11story in his own way. The patient should be given full attention while he or she is describing the symptoms. Gazing out of window or continually writing notes will put him off (students commonly make this mistake). While telling the history, the patient may appear to be evasive; this is, seldom, if ever, deliberate. For example, he/she may attempt to hide the history of tuberculosis. In such situations, the patient should be made more comfortable by one's talk and gestures.
The following may be the presenting symptoms in a patient suffering from the lung disease.
Cough
Cough is a powerful physiological mechanism that causes the central airways to be cleared of foreign material and excess secretions. It may be a voluntary act or a reflex response to stimulation of vagal afferent endings in the larynx, trachea or bronchi. It consists of a sudden explosive release of air following forceful expiration against a closed glottis. Cough provides the high flow rates that are required to shear away mucus and remove foreign particles from the larynx, trachea and large bronchi. Of particular relevance to diagnosis is the sound of cough, the circumstances in which it occurs and the nature of expectorated material. Cough itself may give rise to other symptoms and signs.
A cough may sound dry or moist and be short or prolonged. A short, dry cough is characteristic of upper respiratory tract infections and early stages of pneumonia. A moist productive cough, often prolonged and repetitive, occurs in chronic bronchitis and bronchiectasis. Cough may bring out wheeze in patients with airway obstruction, and when expiratory airflow is severely reduced, it may have a muffled quality. Violent fits of coughing followed by inspiratory stridor or whoop due to laryngeal spasm suggest pertussis or the inhalation of a foreign body. A feeble non-explosive bovine cough is heard when there is vocal cord paralysis or a more widespread respiratory muscle weakness from any cause. The cough of laryngeal inflammation or tumour tends to be harsh and hoarse while the cough associated with tracheal compression is said to have a curious metallic brassy quality.
The circumstances in which cough occurs may give clues to its cause. Nocturnal cough is a common presenting symptom of asthma in children, while among older patients, postnasal drip, esophageal reflux of gastric contents and pulmonary congestion from left heart failure should be considered. Patients with chronic bronchitis and asthma commonly complain of cough which is worse in winters, on rising in the morning, in a smoky atmosphere and during change of temperature. Cough during swallowing is an important symptom of neurogenic dysphagia suggesting inhalation of food or fluid; patients with otherwise unexplained cough should invariably be observed while drinking from glass of water, especially those with known neurological conditions or when tracheoesophageal fistula is suspected. In a persistent cough, a history of medication with angiotensin-converting enzyme inhibitors treatment should be sought.
Chronic cough predisposes to inguinal and femoral herniation, postoperative wound dehiscence and uterine prolapse with stress incontinence. Acute paroxysms of coughing can result in stress fracture of the ribs, cough syncope due to impaired venous filling of heart, and ocular hemorrhages.
Sputum
The character of secretions expectorated from the tracheobronchial tree is of fundamental importance in the diagnosis and management of respiratory disease. One should first 12ensure that the material has originated from the lower respiratory tract. The best way of establishing this is to have the patient produce a sample in the presence of the doctor who can easily check whether it is coughed up, simply spit from the mouth, hawked from the nose or throat, or regurgitated from the esophagus. Patients may say that they vomit phlegm when a productive bout of cough concludes with retching and indeed sputum is sometimes swallowed and subsequently vomited. The sputum is collected in a sterile screw-top container in the presence of a physician or nurse. If the patient is unable to produce a specimen, he should be instructed to return with one expectorated early in the morning. When a measure of quantity is required, sputum is collected over a period of 24 hours. The macroscopic examination of the sputum includes quantity, colour, consistency, shape of any solid constituent and smell.
- The regular production of purulent sputum in large amounts is characteristic of bronchiectasis and chronic bronchitis; when it happens as a sudden event on a single occasion, the rupture of a lung abscess or rupture of empyema into the bronchial tree is the most likely cause. The continuous expectoration of a great quantity of thin watery sputum, sometimes with a salty taste, is an occasional symptom of rare alveolar cell carcinoma. The profuse expectoration of pink frothy sputum in an acutely breathless patient suggests pulmonary edema.
- The colour of the sputum is also of diagnostic value. A green colour imparted by the pigment verdeperoxidase, indicates pus usually arising from a bacterial infection. Yellow sputum may also be due to pus or the presence of eosinophils in allergic states such as asthma. A brown coloured sputum may result from altered blood or from fungal infections and may rarely be seen when an amebic liver abscess ruptures into the lung. A greenish yellow tinge suggests a bronchobiliary fistula. The sputum of coalminers may be black due to breaking down and expectoration of a pneumoconiotic nodule. The presence of blood in sputum is considered separately (see below).
- The consistency of sputum has great therapeutic significance. Thick, viscid, tenacious sputum is the most typical and dangerous feature of acute severe asthma; it is difficult to expel and the patient may asphyxiate from plugging of the smaller airways. The thin frothy sputum of acute pulmonary edema may drown the patient in his own secretions.
- The shape and general appearance of any solid expectorated material should be noted. Viscid secretions sometimes assume the shape of the airway from which they are expectorated. These bronchial casts may appear as string-like strands or short elliptical plugs; they are a characteristic feature of bronchopulmonary aspergillosis in which they are often golden-brown in colour. Other solid matter which may be recognized in the sputum includes blood clot, necrotic tumour, inhaled foreign material and parasitic worms.
- An offensive odor or taste suggests infection by anaerobic organisms as encountered in some cases of bronchiectasis, lung abscess and empyema.
Hemoptysis
The coughing up of blood is one of the most alarming and potentially significant of all respiratory symptoms. Prior to embarking on an extensive work-up of hemoptysis, it is essential to confirm that the blood is in fact 13coming from the respiratory tract, and not from the nasopharynx or gastrointestinal tract. Distinguishing hemoptysis from hemetemesis is difficult at times. In hemoptysis, the prodrome is usually a tingling in the throat or a desire to cough, and the blood when coughed up, is usually bright red and frothy. In hemetemesis, the prodrome includes nausea and abdominal discomfort, and the blood vomited out, is usually dark and brownish in colour. Once symptom of hemoptysis is established, it is important to ensure that the blood has come from the lower respiratory tract and not from the nose or mouth. In recurrent hemoptysis, every effort should be made to examine the patient at the time of hemoptysis.
- A rough assessment of the amount of lost blood should be made. Whether this consists streaks or clots in the sputum, or a more diffuse staining or pure blood. Massive hemoptysis is defined as a loss greater than 600-800 ml of the blood, is also important, because the presence of blood in a single sample is much less likely to result from serious organic disease than staining of successive samples. Presence of any accompanying symptoms, notably dyspnea and pleuritic pain should be recorded.
- The patient with hemoptysis tends to keep the bleeding side dependent. He may also be able to give history of a burning or deep pain which may localize the side of bleeding. In many cases, no serious cause for hemoptysis may be found other than an acute exacerbation of chronic bronchitis but a focal organic source for the bleeding must always be excluded. The most important of these are bronchial carcinoma, carcinoid, tuberculosis, bronchiectasis, mitral valve disease and pulmonary infarction; pneumonia can also produce hemoptysis in the form of rusty sputum, while acute left ventricular failure results in pink frothy sputum. Hemoptysis may be the sole presenting symptom of bronchial carcinoma, carcinoid tumour and tuberculosis but it is usually associated with purulent sputum in bronchiectasis, with dyspnea in mitral valve disease and with sudden dyspnea or pleuritic pain in pulmonary infarction. Rare causes for hemoptysis are mycetoma, vascular malformations, hemorrhagic disorders and Good pasture's syndrome. A recent history of blunt trauma to chest suggests a lung contusion. Hemoptysis is rare in metastatic carcinoma to the lung.
Chest pain
The lung itself is insensitive to pain. Hence chest pain arises either in the pleura, chest wall or tracheobronchial tree and must be distinguished from the pain of esophageal, cardiac or musculoskeletal disorders.
- Pleuritic pain: It is typically sharp, stabbing and related to inspiration. Inflammation of the upper part of the parietal pleura causes pain localized to the chest itself. The lower portion including the outer segment of the diaphragmatic pleura, is innervated by the lower six intercostal nerves which also supply the abdominal wall; pleuritis at this site may give rise to pain in the upper abdomen or loin. The central part of the diaphragmatic pleura is innervated by the phrenic nerve (C3 and C4); so that pain from this site is felt in the neck and tip of the shoulder. Pleural pain associated with pneumonia causes rapid, shallow, grunting respiration. When pleural effusion forms, the pain of pleurisy abates to a dull discomfort. The most important causes of pleuritic pain are pneumonia, cancer, tuberculosis, 14pulmonary infarction, and pneumothorax and, less commonly, autoimmune disorders such as systemic lupus erythematosus.
- Chest wall pain: It results from respiratory diseases as well as from primary musculoskeletal disorders. Patients with chronic cough and dyspnea from any cause often complain of chest pain. This may vary from the vague discomfort or feeling of tightness commonly among asthmatic subjects, to the acute pain of rib fracture, torn muscle or disc injury induced by violent coughing. A local traumatic lesion in the chest wall causes pain very similar to pleuritic pain. The main distinguishing features of the former are the sudden onset following violent cough or other trauma and the presence of tenderness localized to the site of pain. The invasion of the chest wall by an intrathoracic neoplasm, metastasis of lung or malignant mesothelioma of pleura causes dull, aching or boring pain which is unrelated to respiratory or other movements. It tends gradually to become more persistent and disturb the patient's sleep. The Pancoast's tumor may infiltrate the brachial plexus and causes pain which radiates to the arm. The lung tumor usually causes deep seated vague chest pain due to involvement of bronchial nerve plexus.
- Tracheobronchial pain: Pain of tracheobronchial origin may accompany acute inflammation due to infections or from inhalation of irritant fumes. It is usually described as a raw retrosternal discomfort and is distinguished from esophageal and cardiac pain by its relationship to cough rather than to meals or exertion.
- Non-respiratory pain: Many chest pains are of non-respiratory origin. The retrosternal burning pain of acid reflux into the esophagus occurs after meals or on stooping or lying down. The retrosternal gripping pain of angina which occurs typically on effort may radiate to the neck, jaw, arm or back. The pain of pericarditis is usually retrosternal but can sometimes be pleuritic in character. Neuromusculoskeletal chest discomfort can be caused by cervical disc disease (because of compression of nerve roots) by arthritis of the shoulder or spine or by costochondritis, which is an inflammation of costochondral junctions. Intercostal muscle cramps may occur throughout the chest.
Dyspnea
The term ‘dyspnea’ refers to an undue awareness of breathing, or of the need to breathe more. It can result from an increased demand for breathing, impaired performance, or a combination of the two. The most important causes of dyspnea are listed in Table 1.1.
The sensation of dyspnea may be described in different ways by the patient, as shortness of breath, feeling puffed, and difficulty with breathing in or out, inability to get enough air, suffocation or sometimes just a sense of fatigue on effort. Patients, whose dyspnea is psychogenic, feel the need to take occasional deep sighing breaths and often complain of dyspnea at rest or while talking rather than on effort of greater clinical importance is the circumstances in which dyspnea occurs and other symptoms with which it is may be associated. The most important distinction is between sudden onset dyspnea and gradual onset dyspnea which occurs only on effort. The sudden onset of dyspnea suggests pulmonary embolism, spontaneous pneumothorax, and acute left heart failure, airway occlusion from a foreign body or bronchial asthma.
The dyspnea of anemia, emphysema, pleural effusion, pulmonary fibrosis and collapse from bronchial carcinoma is usually of more gradual onset and noticed first on effort. But these conditions may present suddenly when a sedentary patient engages in a rare stint of exercise e.g. running after a bus, climbing hills. Orthopnea is a characteristic feature of left heart failure and also of diaphragmatic paralysis, but can occur in patients with respiratory dysfunction from any cause. Patients of acute severe asthma are not able to lie down, a distinguishing feature. Paroxysmal nocturnal dyspnea, although a common symptom of left heart failure may be wrongly attributed to this cause when an inadequate history is taken. Patients, who complain of attacks of breathlessness in bed at night, fall into one of the following three categories:
- Those who are breathless on first going to bed: This is a common complaint among patients with multiple causes for dyspnea, e.g. the effort of going to bed due to climbing the stairs, undressing, washing etc.
- Those who awake breathless during night: This is not only an important symptom of left heart failure but also of asthmatic and bronchitic patients. Intense non-wheezing dyspnea which forces the patients to sit up or get up suggests a cardiac cause. Bronchitic subjects are often awakened by violent coughing, which is not followed by dyspnea; while in the nocturnal dyspnea of asthma, wheezing is a dominant feature.
- Those who are troubled by dyspnea on first waking up in the morning: This symptom is characteristic of both chronic bronchitis and asthma. In the former, it is probably due to overnight retention of secretions and abates when these are expelled. Asthmatic patients get worse early in the morning due to low peak expiratory flow and other causes.
- The term trepopnea is used to describe the unusual circumstances in which dyspnea occurs only in the left or right lateral decubitus positions, most often in patients with heart disease; while platypnea is dyspnea which occurs only in the upright position. Both of these patterns remain to be fully explained but may be related to positional alternations in ventilation perfusion relationship.
- The symptoms which accompany dyspnea may give clues to the cause. Dyspnea of sudden onset with pleuritic chest pain suggests 16pulmonary infarction or pneumothorax, and the former is often associated with hemoptysis. When the accompanying pain is retrosternal, myocardial infarction or massive pulmonary embolism should be considered as possible causes for acute dyspnea, and in these two conditions, syncope may also occur. Wheezing is of course a characteristic feature of asthma, chronic bronchitis and emphysema. The wheezing dyspnea occurring some 10 to 15 minutes after exertion is characteristic of exercise-induced asthma.
- The dyspnea of hyperventilation syndrome (HVS) is non-organic. Such patients complain of breathlessness occurring at rest for no apparent reason e.g. sitting, reading or watching television. They also have light headedness or dizziness, which is often associated with sensation of pins and needles in the fingers. Their breathlessness is unrelated to the degree of exertion; when breathless it is harder for them to breathe in than out (although this is also an occasional feature in severe airways obstruction).
- The subjective severity of effort dyspnea can be gauged roughly from the amount of exercise needed to induce it. The most widely used scale is the one devised by the Medical Research Council (MRC) in which dyspnea is divided into three grades:
- Shortness of breath hurrying on level ground or walking up a slight incline.
- Shortness of breath keeping up with others of similar age on level ground.
- Shortness of breath forcing stops when walking at own pace on level ground.
But this grading is good only for epidemiological and research purposes. The dyspnea should be quantified according to the activities of an individual. For example, if a sedentary person becomes breathless on climbing stairs, it is not of concern but on the other hand dyspnea developing while climbing stairs by an athlete is of relevance. Therefore the severity of dyspnea should be assessed as per the routine activities of an individual.
Hoarseness of Voice
Involvement of the recurrent laryngeal nerve may make the voice hoarse and patient may lose voice power. The long intrathoracic course of recurrent laryngeal nerve makes it vulnerable to involvement by pulmonary and other neoplasms at around the aortic arch, aortic aneurysm, mediastinal fibrosis, tuberculosis, and radiotherapy and thyroid surgery.
Patients of superior vena cava syndrome have heaviness of voice due to edema of vocal cords. It is more severe during morning rather than the later part of the day due to greater edema of vocal cords on rising.
Hysterical aphonia may also be seen at times. In such cases, the cords appear unable to adduct; and because the pure adductor paralysis does not occur as a neurological lesion, if the patient is persuaded to cough, the cord can be seen to approximate with each other.
Excessive Daytime Sleepiness
The sleep apnea syndrome is a common clinical problem with main symptom of hyper-somnolence during day time which will continue to be missed until physician include two additional questions in standard 17history taking: ‘How often do you fall asleep when not in bed’ and ‘Do you snore’.
GENERAL PHYSICAL EXAMINATION
An initial impression is formed by observing the patient's level of consciousness and general state of health. If the patient demonstrates signs of acute, severe illness, the remainder of physical examination may have to be limited to gathering only essential information. When the patient's condition is more stable, a thorough examination can be performed to identify all abnormalities. The alert patient who is well oriented in time, place and person, is said to be “oriented × 3”, and sensorium is considered normal, and the history narrated by patient is considered reliable. Obvious indicators of the patient's general state of health are usually recorded as part of the initial impression. Comments on the patient's height, weight, apparent versus actual age, and obvious degree of illness may be included.
Assessment of vital signs represents the most frequently made clinical measurement as they provide important diagnostic information. The four basic vital signs are body temperature, pulse rate, respiratory rate and blood pressure.
Body Temperature
The normal body temperature for most individuals is approximately 98.6°F with a range from 97°F to 99.5°F and with daily variation of 1°F to 2°F. The body temperature is usually lower in the early morning and higher in the late afternoon. Temperature elevation associated with disease is called fever and the patient is said to be febrile. For every 1°F elevation of body temperature, oxygen consumption and carbon dioxide production increase approximately 10%. Examination of febrile patients often reveals an increasing heart rate and breathing rate. When the body temperature is below normal, hypothermia is said to exist and it reduces oxygen consumption and carbon dioxide production by body tissues. The patient with hypothermia may exhibit slow, shallow breathing and a reduced pulse rate.
- The body temperature is most often measured at one of the three sites; mouth, axilla or rectum. Rectal temperature most accurately reflects the actual body-core temperature. Oral temperature measurement is the most acceptable for an awake, adult patient, but this method cannot be used with infants, comatose patients, or orally intubated patients. After the patient has ingested hot or cold drink or has been smoking, a 10-15 minute waiting period is necessary. Before inserting the thermometer, it should invariably be washed in an antiseptic or cold water, and further ensured that mercury is well shaken down. The axillary temperatures may be taken safely in case of infants and small children who do not tolerate rectal thermometers.
- The oral temperature is not affected significantly by simple oxygen administration via nasal cannula or mask. Therefore it is not necessary to remove oxygen or take rectal temperature of patients receiving simple oxygen therapy. But the oral temperature may not be a valid measure in patients breathing heated or cooled aerosol via face masks. There is tendency for oral temperature to be increased slightly with the application of heated aerosol and decreased slightly with cool aerosol inhalation. In these cases, if absolute accuracy is essential, the rectal route should be employed.
- Three classical types of fever are described—continuous, remittent and intermittent. When fever fluctuates within 1°F to 2°F during 24 hours, but at no time touches the normal, it is termed as continuous. When the daily fluctuations exceed 2°F, it is called remittent, and when fever is present only for several hours during the day it is called intermittent. When a paroxysm of intermittent fever occurs daily, the type is quotidian; when on alternate days, tertian; when two days intervene between consecutive attacks, quartan. Pel-Elbstein fever is described as one lasting for a few days alternating with a number of afebrile days (cyclical fever) and often occurs in patients of lymphoma.
Pulse Rate
The peripheral pulse should be evaluated for rate, rhythm and strength. The normal pulse rate for adult is 60 to 100 beats per minute and it is regular in rhythm. A pulse rate exceeding 100 beats per minute is termed tachycardia and below 60 beats per minute, is termed bradycardia. When the oxygen content of arterial blood falls below normal, usually due to lung disease, the heart tries to compensate by increasing the cardiac output to maintain an adequate oxygen delivery to tissues partly through an increase in heart rate.
- The most common site for evaluation of the pulse is the radial artery. The examiner's second and third finger pads are used in assessment of the radial pulse. Ideally the pulse rate should be counted for one minute. The following characteristics of the pulse should be noted and documented; rate—is it normal, high, or low; rhythm— is it regular, consistently irregular, or irregularly irregular; amplitude— are there any changes in the amplitude of the pulse in relation to ventilation or are these changes from one beat to another; the presence of thrills or bruits. If the patient's wrist is held too far above the level of his heart, the pulse may be difficult to obtain. When the patient's pulse strength decreases with spontaneous inhalation, it is referred to as pulsus paradoxus.
- Other common sites for assessment of pulse include the carotid, brachial, femoral, temporal, popliteal, posterior tibial and dorsalis pedis arteries. When the blood pressure is abnormally low, the more centrally located pulses such as carotid and femoral pulses are identified more easily than the peripheral pulse. If the carotid site is used, great care must be taken to avoid the carotid sinus area, as mechanical stimulation of the latter can evoke a strong parasympathetic response and may cause bradycardia or even asystole.
Respiratory Rate
The normal resting adult rate of breathing is 12 to 20 breaths per minute. Tachypnea is the term used to describe respiratory rates above normal. A slow rate, referred to as bradypnea, is uncommon but may occur in patients with head injury, hypothermia, or as a side effect of narcotics. The respiratory rate is counted by watching the abdomen or chest wall movement with breathing. In some patients, the examiner may need to place a hand on the patient's abdomen to confirm the breathing rate. Ideally the patient should be unaware that the respiratory rate is being counted. One successful means of accomplishing this is to count the respiratory rate immediately after evaluating the pulse, while retaining the fingers on the radial artery.19
Blood Pressure
The arterial blood pressure is the force exerted against the wall of the arteries as the blood moves through them. Arterial systolic blood pressure is the peak force exerted during contraction of the left ventricle. Diastolic pressure is the force occurring when the heart is relaxed. Pulse pressure is the difference between systolic and diastolic pressures. Normally, it is 35 to 40 mm Hg. When less than 30 mm Hg, the peripheral pulse is difficult to detect. Normal systolic pressure ranges from 95 to 140 mm Hg with an average of 120 mm Hg and diastolic from 60 to 90 mm Hg with an average of 80 mm Hg.
- The most common technique for measuring arterial blood pressure is the auscultatory method, which uses a blood pressure cuff (sphygmomanometer) and a stethoscope. When the cuff is applied to the upper arm and pressurised to exceed the systolic blood pressure, the brachial blood flow is stopped. As the pressure in the cuff is released slowly to a point just below systolic pressure, blood intermittently overcomes the obstruction. Partial obstruction of the arterial blood flow creates a turbulent flow, producing the vibrations called Korotkoff sounds. Korotkoff sounds can be heard over the artery distal to the obstruction with the aid of a stethoscope. To measure the blood pressure, a deflated cuff is wrapped snugly around the upper arm with its lower edge 2.5 cm above the antecubital fossa. The brachial pulse is palpated, and the cuff is inflated to a pressure 30 mm Hg higher than the point at which the pulse is obliterated. The bell of the stethoscope is placed over the site of brachial artery. Then the cuff is deflated at a rate of 2 to 3 mm Hg per second while observing the manometer. The systolic pressure is recorded at the point at which the initial Korotkoff sounds are heard. The point at which the sounds become muffled is recorded as the diastolic pressure. This muffling sound is the final change in the Korotkoff sounds just before they disappear. At this point, the cuff pressure is equal to the diastolic pressure, and no turbulent sounds are created. The examiner must be careful to perform the procedure rapidly, since the pressurized cuff impairs circulation to the forearm and hand. Common mistakes that can result in erroneously high measurements include too narrow a cuff, cuff applied too tightly or loosely, and excessive pressure in the cuff during measurement, inflation pressure held too long in the cuff, and incomplete deflation of cuff between measurements.
- The systolic pressure usually decreases with normal inhalation. This decrease in systolic pressure is more significant during a forced maximal inhalation. If the drop is more than 6-8 mm Hg during inhalation at rest, a definite abnormality exists, termed as paradoxical pulse or pulsus paradoxus. The mechanism responsible for this fluctuation in blood pressure centers on the negative intrathoracic pressure created by the respiratory muscles during inhalation. The paradoxical pulse can be identified most accurately with a sphygmomanometer. However if the pulse can be felt to wane with inspiration in several accessible arteries, paradoxical pulse is present. To confirm and quantify its presence, the blood pressure cuff is inflated until no sounds are heard with the stethoscope bell over the brachial artery, and then it 20is gradually deflated until sounds are heard on exhalation only. The cuff pressure then is released even more slowly until sounds are heard throughout the respiratory cycle. The difference between these two pressure readings indicates the degree of paradoxical pulse. A reading in excess of 6 to 8 mm Hg is significant. A paradoxical pulse may occur with acute airway obstruction such as asthma or constrictive pericarditis.
Cyanosis
Cyanosis is one of the most commonly described clinical signs of hypoxia. It imparts a blue-grey colour to skin, mucous membranes, and nail beds (Fig. 1.9) and is caused by the presence of abnormally high amounts of reduced or unsaturated hemoglobin in the blood. Cyanosis may be categorised as central or peripheral. Central cyanosis, observed best in the capillary beds of lips or buccal membranes, is caused by a reduction in the hemoglobin saturation of arterial blood; here the extremities are warm. Peripheral cyanosis, on the other hand, results from an excessive amount of reduced hemoglobin in the venous blood and the extremities remain cold; this occurs, when oxygen extraction by the tissues is abnormally high, e.g. with poor perfusion or blood stasis.
- Normally capillary blood has about 2.5 gm/100 ml reduced hemoglobin (Hb). For cyanosis to be detected, the capillaries in general must contain at least 5 gm/100 ml Hb of reduced. This occurs when oxygen saturation (SaO2) drops below 80%, and corresponds to a PaO2 of about 45 mm Hg. In anemia, there may not be enough unsaturated hemoglobin to produce central cyanosis until the arterial saturation drops well below 80%. Conversely in polycythemia or erythrocytosis, the amount of reduced hemoglobin may be sufficient to produce central cyanosis even when there is adequate oxygen delivery to tissues.
- The evaluation of the presence and degree of cyanosis depends upon the examiner's perception and is modified by factors such as the ambient lighting, the colour of the skin, and the presence of abnormal blood pigments (e.g. methemoglobin or sulfhemoglobin).
Clubbing and Hypertrophic Pulmonary Osteoarthropathy
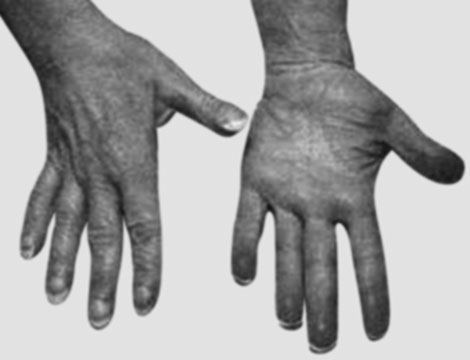
Clubbing is manifested by a painless bulbous swelling of terminal phalanges of the fingers (Figs. 1.10 and 1.11) and toes. The mechanism responsible for clubbing is not known, but it is often associated with a chronic decrease in oxygen supply to the body tissues in general. A characteristic feature of clubbing is the contour of the nail which becomes rounded both longitudinally and transversely. Although such curvature may be seen in healthy individuals also, the distortion of the nail in clubbing is marked by increased hyponychial angle, which is the angle of the fingernail to the nail base, and is normally 160°. In clubbing, it may advance to 200°.
Associated with an increase in the angle in the early stages of clubbing, is floating nail base, or sponginess under the base of the nail, which allows it to move up and down with compression. Then, as the clubbing progresses from mild to severe, the degree of nail curvature also gradually increases. Depth of finger at base of nail (DPD) is greater than depth of interphalangeal joint (IPD) in clubbing. Both DPD and IPD are derived from the shadowgram of finger, which is obtained by throwing light on the finger in question and getting a shadow on the screen or wall in a dark room.
- In adults, approximately 75% to 85% of all clubbing is associated with respiratory diseases, such as lung tumours, bronchiectasis, pulmonary fibrosis, empyema and cystic fibrosis. Only 10% to 15% have an underlying disease and remaining 5% are associated with miscellaneous conditions.
- Clubbing may be associated with a more generalised condition that affects the bones and joints and is known as hypertrophic pulmonary osteoarthropathy (HPOA see chapter 42). HPOA is a chronic inflammatory process that results in thickening of the periosteum, as evidenced on X-ray examination. Joints may also be swollen and inflamed. In a well-developed case, there may be pain and disabling limitations of motion. Clubbing may occur early in the development of HPOA.
Pedal Edema
Patients with chronic respiratory disease may have pedal edema as a manifestation of their chronic disease due to right ventricular hypertrophy and failure. The peripheral blood vessels engorge, resulting in an accumulation of fluid in the subcutaneous tissues of the ankles, referred to as pedal edema. The ankles are most often affected, since they are naturally maintained in a gravity-dependent position throughout the day. The edematous tissues pit (indent) when pressed firmly with the fingertips for 20 to 30 seconds and the resultant indent sustains for one and half times the pressing period. The level of pitting edema should be evaluated above the ankle in an effort to quantify the degree of right ventricular failure. For example, pitting edema occurring at a level well above the knee is more significant than that around the ankles only.
Flapping Tremors (Asterixis)
Flapping tremors indicate metabolic encephalopathy, including carbon dioxide narcosis. 22These are the up and down movements (like bird's wings) at metacarpophalangeal joints of the hands.
To elicit the flapping tremors, the examiner has to fix the wrist joint by dorsiflexing it through pushing the hand against the patient's palm, at the same time keeping the metacarpophalangeal joint free for movement. Sometimes asterixis may be present only on one side; therefore an attempt should always be made on both hands to demonstrate.
Head examination
Abnormalities to be identified on inspection of the face and which are produced by respiratory disease include nasal flaring, cyanosis (discussed above) and pursed-lip breathing. Nasal flaring is identified by observing the external nares flare outward during inhalation. This occurs especially in neonates with respiratory distress and indicates an increase in the work of breathing. Patients with chronic obstructive lung disease may use pursed lip breathing during exhalation. This technique is often taught to patients and may even be used by some who have not had instruction on its benefits. They naturally begin to pucker their lips during exhalation to provide a slight resistance to the exhaled breath. This resistance theoretically provides a slight back pressure in the small airways during exhalation and prevents their premature collapse.
In emphysema, due to elevation of the sternum, the distance between suprasternal notch and the cricoid cartilage (normally 3-4 fingers breadths) may be reduced; and a finger on the thyroid cartilage may detect an inspiratory tracheal tug attributed to the contraction of a low, flat diaphragm due to hyperinflated lungs.
Jugular Venous Pressure/Distension (JVP/JVD)
Assessment for distention of the right Internal Jugular vein (IJ) is a difficult skill. Its importance lies in the fact that the IJ is in straight-line communication with the right atrium. The IJ can therefore function as a manometer, with distention indicating elevation of Central Venous Pressure (CVP). This in turn is an important marker of intravascular volume status and related cardiac function. The focus here is on simply determining whether or not Jugular Venous Distention (JVD) is present. A discussion of a, c and v waves that make up the jugular venous pulsations shall not be stressed. These are quite difficult to detect for even by the most seasoned physician.
Why is JVD so hard to assess? The IJ lies deep to skin and soft tissues. Besides, this blood vessel is under much lower pressure than the adjacent pulsating carotid artery. It therefore takes a sharp eye to identify the relatively weak, transmitted venous impulses. A few things to remember:
- Think anatomically. The right IJ runs between the two heads (sternal and clavicular) of the sternocleidomastoid muscle (Fig. 1.12) and up in front of the ear. This muscle can be identified by asking the patient to turn their head to the left and into your hand while you provide resistance to the movement. The two heads form the sides of a small triangle, with the clavicle making up the bottom edge. You should be able to feel a shallow fossa formed by the borders of these landmarks. Note, you are trying to identify impulses originating from the IJ and transmitted to the overlying skin in this area. You can not actually see the IJ.The External Jugular (EJ) runs in an oblique direction across the sternocleidomastoid and, in contrast to the IJ, can usually be directly visualized (Fig. 1.12). If the EJ is not readily apparent, have the patient look to the left and attempt valsalva manouvre. This usually makes it quite obvious. EJ distention is not always a reliable indicator of elevated CVP as valves, designed to prevent the retrograde flow of blood, can exist within this vessel causing it to appear engorged even when CVP is normal. It also makes several turns prior to connecting with the central venous system and is thus not in a direct line with the right atrium.
- Take your time. Look at the area in question for several minutes while the patient's head is turned to the left. The carotid artery is adjacent to the IJ, lying just medial to it. If you are unsure whether a pulsation is caused by the carotid or the IJ, place your hand on the patient's radial artery and use this as a reference. The carotid impulse coincides with the palpated radial artery pulsation and is characterized by a single upstroke timed with systole. The venous impulse (at least when the patient is in sinus rhythm and there is no tricuspid regurgitation) has three components, each associated with the aforementioned a, c and v waves. When these are transmitted to the skin, they create a series of flickers that are visible diffusely within the overlying skin. In contrast, the carotid causes a single up and down pulsation. Furthermore, the carotid is palpable. The IJ is not and can, in fact, be obliterated by applying pressure in the area where it emerges above the clavicle.
- Search along the entire projected course of the IJ as the top of the pressure wave (which is the point that you are trying to identify) may be higher than where you are looking. In fact, if the patient's CVP is markedly elevated, you may not be able to identify the top of the wave unless they are positioned with their trunk elevated at 45° or more (else there will be no identifiable “top” of the column as the entire IJ will be engorged). After you have found the top of the wave, see what effect sitting straight up and lying down flat have on the height of the column. Sitting should cause it to appear at a lower point in the neck, while lying has the opposite effect. Realise that these maneuvers do not change the actual value of the central venous pressure. They simply alter the position 24of the top of the pulsations in relation to other structures in the neck and chest.
- Shine a light tangentially across the neck. This sometimes helps to accentuate the pulsations. If you are still uncertain, apply gentle pressure to the right upper quadrant of the abdomen for 5 to 10 seconds. This elicits Hepato-Jugular Reflux which, in pathologic states, will cause blood that has pooled in the liver to flow in a retrograde fashion and fill out the IJ, making the transmitted pulsations more apparent. Make sure that you are looking in the right area when you push as the best time to detect any change in the height of this column of blood is immediately after you apply hepatic pressure.
- Once you identify JVD, try to estimate how high in centimeters the top of the column is above the Angle of Louis. The angle is the site of the joint which connects the manubrium with the rest of the sternum. First identify the suprasternal notch, a concavity at the top of the manubrium. Then walk your fingers downward until you detect a subtle change in the angle of the bone, which is approximately 4 to 5 cm below the notch. This is roughly at the level of the 2nd intercostal space. The vertical distance from the top of the column to this angle is added to 5 cm, the rough vertical distance from the angle to the right atrium with the patient lying at a 45° angle. The sum is an estimate of the CVP. However, if you can simply determine with some accuracy whether JVD is present or not, you will be way ahead of the game. Normal JVD is 7-9 cm.
- The level of jugular venous distension may vary with breathing. During inhalation the level of the blood column may descend towards the thorax; and return to the previous position with exhalation. For this reason, JVP should always be estimated at the end of exhalation.
- The examination of the waves of jugular vein should be carried out while the patient is in sitting position, with light thrown tangentially on the neck; the examiner should sit by the side of the patient, to palpate the carotid artery of opposite side simultaneously to time and recognise the waves. The a wave precedes and v wave follows the artery pulse.
- Neck veins are distended with absent pulsations in superior vena cava syndrome. In the absence of distended neck veins, the earliest sign of superior vena cava obstruction is the flushing of face and dizziness when the patient stoops to touch his feet.
Lymph Nodes
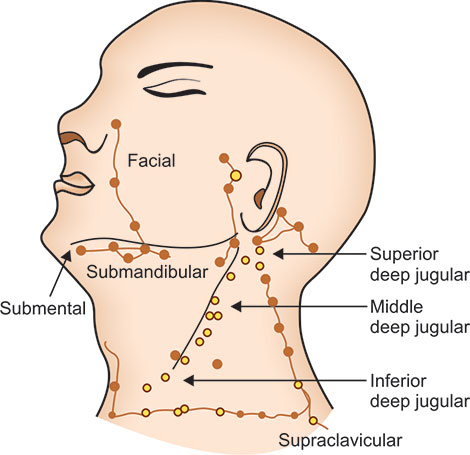
The lymph nodes of neck region (Fig. 1.13) and axilla are relevant in respiratory diseases. The various groups of lymph nodes in neck region are preauricular, post auricular, submental, sub-maxillary, superficial cervical and deep cervical (both superior and inferior). Scalene lymph nodes lie between the sternal and clavicular heads of sternocleidomastoid muscle, and this should be palpated preferably with the index finger.
If lymph nodes are palpable, the following points should be considered
- How many are palpable?
- What is their approximate diameter in centimeters?
- What is the consistency?
- Are these discrete or confluent (matted)?
- Are these mobile or fixed?
- Is the skin in the vicinity of nodes abnormal?
The lymph nodes at sites other than the ones described above are also not less important and should be examined. One should also look for sinus formation or old scars in the neck region often seen with tubercular lymphadenitis.
EXAMINATION OF THE THORAX AND LUNGS
To perform an accurate physical assessment of the respiratory system, the examiner must understand how the lungs are placed within the chest. Topographic (surface) landmarks of the chest are helpful in identifying the location of underlying structures and in describing the location of abnormalities.
Imaginary lines
On the anterior chest the midsternal line divides the chest into two equal halves. The left and right midclavicular lines parallel the midsternal line and are drawn through the midpoints of the left and right clavicles. The midaxillary line divides the lateral chest into two equal halves. The anterior axillary line parallels the midaxillary line and is situated along the anterolateral chest. The posterior axillary line is also parallel to the midaxillary line and is located in the posterolateral chest.
Three imaginary vertical lines are described on the posterior chest. The midspinal line divides the posterior chest into two equal halves. The left and right midscapular lines parallel the midspinal line and pass through the inferior angles of the scapulae in the relaxed upright individual.
Imaginary Thoracic Areas
For description of the findings, the surface of thorax is divided arbitrarily into eight areas on each side (Table 1.2).
Thus, the examination findings should be mentioned as per areas described in Table 1.2 and not according to intercostal spaces. These imaginary areas identify the underlying anatomical part of the lungs, whereas the intercostal spaces or ribs may be mentioned to describe the findings only if they are related to the particular abnormality.
Thoracic Cage Landmarks
On the anterior chest, the suprasternal notch is located at the top of the manubrium and can be located by palpating the depression at the base of the neck. Directly below this notch is the sternal angle, which is also referred to as the angle of Louis. Identification of the sternal angle can be achieved by palpating down from the suprasternal notch until the ridge between the body of the sternum and the manubrium is identified. This important landmark is visible in most individuals. The second rib articulates with the top of the corpus sternum at this point (Fig. 1.14). Rib identification on the anterior chest can be accomplished with this landmark as a reference point.
|
It is recommended that ribs be counted to the side of the sternum, since individual costal cartilages that attach the ribs to the sternum are not identified as easily near the sternum.
On the posterior chest, the spinous processes of the vertebrae are useful landmarks. The spinous process of the seventh cervical vertebra (C7) can usually be identified by having the patient flex the head and neck forward and slightly down. At the base of the neck, it is the most prominent spinous process that can be visualised and palpated. The spinous process just below C7 belongs to the first thoracic vertebra (T1). The scapular borders can also be useful landmarks on the posterior chest. When the patient's arms are raised above the head, the inferior border of the scapula approximately overlies the oblique fissure that separates the upper lobe of each lung from the lower lobe posteriorly.
Lung Fissures
Between the lobes of the lung are the interlobar fissures. Both lungs have an oblique fissure that begins on the anterior chest approximately at the level of the sixth rib in the midclavicular line. This fissure extends laterally and upward until it crosses the fifth rib on the lateral chest in the midaxillary line and continues on the posterior chest approximately at T3 (Fig. 1.2). The right lung, in addition, also has a horizontal fissure that separates the upper lobe from the middle lobe. The horizontal fissure extends from the fourth rib at the sternal border to the fifth rib in the mid axillary line. In rare cases the left lung may also have a horizontal fissure.
Tracheal Bifurcation
The carina is approximately located beneath the angle of Louis on the anterior chest and approximately at T4 on the posterior chest.
Diaphragm
The diaphragm is a dome-shaped muscle that lies between the thoracic and abdominal cavities and moves up and down during normal ventilation. At the end of the tidal expiration, the right dome of the diaphragm is normally located at the level of T9 posteriorly and fifth rib anteriorly. On the left side, the diaphragm normally comes to rest at end expiration at T10 posteriorly and sixth rib anteriorly. The right hemidiaphragm is anatomically a little higher than the left because of the placement of the liver.
Lung Borders
Superiorly on the anterior chest, the lungs extend 1 to 2 cm above the medial third of the clavicles. At end expiration, the inferior borders extend to approximately the sixth rib at the mid-clavicular line and to the eighth rib on the lateral chest wall (Fig. 1.2). On the posterior chest the superior border extends to T1 and inferiorly varies with ventilation between approximately T9 and T12.
Chest examination should be done strictly in the following sequence—inspection, palpation, percussion, auscultation. Diversion from this pattern may lead to lung abnormalities getting overlooked.
INSPECTION
Visual examination of the chest is of value in assessing the thoracic configuration and the pattern and effort of breathing. For adequate inspection, the room must be well-lit and the patient should be sitting upright. If the patient is too ill to sit up, the examiner must carefully roll the patient to one side in an effort to examine the posterior chest. Male patients should be stripped to the waist. Female patients should be given some type of drape to prevent embarrassing exposure of the breasts.
Chest Shape
The normal adult thorax has an anteroposterior diameter less than the transverse diameter. Both these diameters can be measured by using two cardboards or books by keeping one on the anterior chest and the other on the back; the distance between the two is the anteroposterior diameter. Similarly the transverse diameter is measured as the distance between the two cardboards kept by the sides of the chest.
Normally the anteroposterior diameter increases gradually with age but may increase prematurely in patients with certain types of chronic obstructive lung disease. Chest with abnormal increase in anteroposterior diameter is called barrel chest. When the anteroposterior diameter increases, the ribs lose their normal 45° angle in relation to the spine and become more horizontal, with the intercostal spaces becoming widened.
Other abnormalities of the thoracic configuration include the following.
- Scoliosis: Scoliosis is the most important of all chest deformities, because of its potential effect on the cardiorespiratory function. This lateral curvature of the spine is almost invariably accompanied by the rotation of the spine and only rarely by significant kyphosis, so that the popular term ‘kyphoscoliosis’ is often inappropriate. It is the rotation of the spine which causes a posterior hump comprising ribs and the scapula on one side (simulating a kyphosis) and an anterior hump on the other. The rotation is also largely responsible for the reduction in lung volume and impaired mechanical function, which in severe cases, leads to respiratory and then 28cardiac failure even in early middle life. This complication is more likely to occur if scoliosis affects the upper part of the spine. Postural scoliosis can be differentiated from pathological scoliosis, by asking the patient to stoop forward. In case of former, the lateral curvature is rectified while in latter case, it persists.
- Kyphosis: Kyphosis is the most common cause of barrel chest which is therefore not a specific sign of emphysema alone. It is a deformity in which the spine has an abnormal anteroposterior diameter. Kyphosis unaccompanied by scoliosis does not cause any significant impairment of lung function except in extreme cases.
- Pectus carinatum: Pectus carinatum is pigeon chest, with a sternal protrusion anteriorly. There is prominence of the sternum and/or anterior ends of the ribs. Pigeon chest may be congenital or a consequence of airflow obstruction in childhood as in Harrison's sulcus; but it does not by itself cause any respiratory dysfunction.
- Pectus excavatum: Pectus excavatum is also known as funnel sternum. It is a congenital saucer shaped depression of the sternum and is only of cosmetic significance.
- Rib deformity: Abnormalities of the ribs altering the configuration of the chest include those associated with scoliosis, local flattening of the chest resulting from surgical excision of ribs for tuberculosis (thoracoplasty) or empyema and the phenomenon of Harrison's sulcus. The latter consists of a bilateral groove in the rib cage, which has been attributed to the effect of diaphragmatic contraction in patients with airways obstruction (from adenoids or asthma) during early childhood when the bones are still malleable. It is particularly common among rachitic children in whom enlargement of the rib epiphyses may also be seen (‘rickety rosary’).
- Soft tissue deformity: Asymmetry of the chest with either prominence or flattening may result from abnormalities of soft tissues, such as a diffuse lipoma, congenital absence of the pectoralis major or wasting of the scapular muscles. Pleural or pulmonary fibrosis can lead to flattening, contraction and impaired expansion of one hemithorax.
Breathing Pattern, Effort and Movements
One is concerned here not only with the chest itself, but also with respiratory movements of nose, mouth, neck and abdomen. A record of the breathing movements of chest itself should include the rate, depth and rhythm of breathing, the equality of expansion and movement, and the presence of paradox.
- At rest, the normal adult has a consistent rate and rhythm of ventilation. Breathing effort is minimal on inhalation and exhalation is passive. Men typically breathe using their diaphragm, causing the abdomen to move slightly outward during inhalation. Women tend to use the combination of intercostal muscles and diaphragm, thus producing comparatively more chest wall movement than men.
- A healthy subject breathes at a rate of 12-20 respirations per minute at rest. Rapid, shallow breathing suggests pulmonary consolidation, edema, diffuse fibrosis or other conditions associated with reduced compliance; but it may also occur when inspiration is painful. Abnormal slowing of respiration is invariably central in origin. Psychogenic causes account for most instances of 29irregular breathing, especially when this is punctuated by deep sigh breaths.
- Individuals with disorders characterised by an increased elastic work of breathing, such as pulmonary fibrosis, tend to assume a rapid and shallow breathing pattern. For these patients, such a pattern results in the minimum mechanical work necessary to effectively ventilate the lungs. On the other hand, patients with obstructive lung disorders tend to assume the pattern best able to reduce the frictional work of breathing, and the breathing tends to be slow and deep. Table 1.3 describes the abnormal patterns of breathing.
- The symmetry of movement of either side of the chest is observed from the foot end of bed of the supine patient. Of greater significance is the inequality of movement, as this may occur with any predominantly unilateral disease of the underlying pleura or lung. Inspiratory indrawing of intercostal spaces is commonly seen in children with respiratory distress from any cause. The distorted action of a depressed and flattened diaphragm in patients with chronic airflow obstruction, especially emphysema is probably responsible for the indrawing motion of lower ribs during inspiration and this paradoxical costal margin movement is known as Hoover's sign. The movements of the mouth are characteristic of severe airflow obstruction, especially in patients with emphysema.
- In patients with airways obstruction, ‘Match test’ is applied at bed side. The subject is directed to blow out a lit-match held 15 cm from the open mouth. Inability to perform this test indicates severe airway obstruction with FEV1 less than 1.6 liters. The ‘20 deep breath test’ is conveniently performed in patients of hyperventilation syndrome (HVS). The subject is asked to take 20 deep breaths continuously; positive test produces the symptoms of dizziness and paraethesiae in the extremities and around the mouth, and is not only diagnostic of HVS but also therapeutic. Patients with HVS are more sensitive to the effects of hypocapnia.
|
Listening to Breathing Sounds
It is worth listening to the sounds of breathing before reaching for the stethoscope. The breathing of a normal person is inaudible beyond about 12 inches from the mouth. Breathing sounds which can be heard at the bedside are abnormal, and occur due to the turbulent flow in proximal airways, indicating either airway narrowing or a flow rate greater than 20 liters per minute. The following breathing noises should be identified.
- Gasps: These are the noises of sudden, rapid, deep inspirations. These are physiological response to fright; and pathological response to severe expiratory airflow obstruction, where rapid deep inspiration compensates for slow prolonged expiration. Infrequent, irregular, gasping inspirations are also seen as a terminal event when the respiratory center is deranged.
- Pants: These are noises of rapid shallow breathing, with inspiration and expiration being equal in duration and intensity of sound is also equal during both phases. These are the normal physiological response to exercise and a pathological response to decreased compliance of lungs in conditions such as interstitial pulmonary fibrosis, edema and consolidation.
- Sighs and yawns: Both these consist of a slow deep inspiration to vital capacity, followed by an expiration which may be prolonged and intensified in sound because of apposition of the vocal cords. There is frequently a large psychogenic and voluntary component in proportion to the loudness of the sound. To prevent alveolar collapse from surface tension forces, even in healthy persons, the occasional deep breaths of sighs or yawns are required.
- Hisses: Hissing or Kussmaul's breathing is the noise resulting from hyperventilation through apposed teeth and is characteristic of acidosis in patients of uremia, diabetic ketoacidosis and salicylate poisoning. These patients, in the absence of lung disease, hyperventilate without dyspnea and therefore without reflex opening of the mouth.
- Sniffles: It is a noise from a sniffly nose either due to nasal obstruction, or discharge due to chronic sinus infection commonly accompanied by bronchiectasis, hay fever or nasal granulomas.
- Snores: Snoring is mainly an inspiratory noise arising from vibrations of the soft tissues of the pharynx, tongue, palate and cheeks during sleep. It is quite a common condition; but in severe cases, may lead to obstructive sleep apnea syndrome.
- Whistles and grunts: These are expiratory noises heard in patients with emphysema and especially important as these may be the only sounds heard in such patients, even with a stethoscope. There are several reasons for breathing being otherwise silent in emphysema; firstly, airway sounds are filtered out by the overinflated lung; secondly, expiratory airflow may be too slow to generate a wheeze; and thirdly, unlike asthma, emphysema is not associated with intrinsic airway narrowing. The wheeze of emphysema, which is often heard only on forced expiration, is due to passive collapse of proximal airways induced by the positive pressure needed to expel air from the inelastic lungs. Passive airway collapse can be reduced if the intrabronchial pressure is raised by expiratory apposition (pursing) of lips or larynx. Lip pursing gives rise to the characteristic expiratory whistle of emphysema. 31Laryngeal pursing is less common and consists of intermittent cogwheel like grunts during expiration, often thought to be attention-seeking device.
- Whoops, wheezes and stridor: The inspiratory component of an asthmatic wheeze can be difficult to distinguish from ‘whoop’ or stridor of main airway narrowing. Stridor is of a higher pitch than the inspiratory wheeze of asthma and unlike wheeze may seem to continue even after airflow at the mouth has stopped. Stridor is a crowing sound due to obstruction in the larynx or trachea. When the diameter at site of lesion in either larynx or trachea is reduced to 5 mm, then inspiratory obstruction as well as stridor results at rest. Laryngeal stridor is high pitched while tracheal stridor is lower in pitch. Breathlessness is always associated with tracheal stridor; latter tends to be biphasic and is increased on coughing.
- Rattles: This is the sound of air passing through fluid, or of mucus being set into vibrations in the main airways both during inspiration and expiration. It is usually a sign of failed cough reflex either because of decreased efficacy or excessive demand. The reflex may be impaired as a result of narcotics or extreme weakness, or it may be overwhelmed by a massive transudate into the airways in acute pneumonia or by aspiration of swallowed or regurgitated fluid.
- Crackles: The early inspiratory crackles of chronic airways obstruction can sometimes be heard at the mouth.
PALPATION
Palpation is the art of touching the chest wall in an effort to evaluate under-lying structure and function. It is performed to feel for lymph nodes in the neck, subcutaneous emphysema, prominent veins, chest swelling, position of trachea and apex beat, thoracic expansion and movements, and vocal fremitus.
Cervical and Axillary Lymph Nodes
These may be involved in diseases which also affect the lung, such as tuberculosis, sarcoidosis and lymphoma. In these conditions, any group of lymph nodes may be enlarged; their consistency tends to be firm and rubbery but not hard and the individual group nodes are discrete and unattached to the surrounding tissues except in advanced cases of tuberculosis. Bronchial carcinoma usually reaches the supraclavicular lymph nodes and it is especially important to palpate the scalene nodes with finger and thumb deep to sternal head of the sternomastoid. The affected nodes are hard and may become fused to form a mass which may be fixed to adjacent structures.
Subcutaneous (Surgical) Emphysema
When the air leaks from the lung into the subcutaneous tissue, fine beads of air produce a crackling sound and sensation when palpated. The neck especially, but also the chest, should be lightly palpated for the characteristic crackly feeling. The crackling can be heard as well as felt and may be confused with lung crackles on auscultation if not already detected by palpation. A special search should be made for this sign following chest trauma, an episode of acute airflow obstruction or violent vomiting. In case it is present in chest trauma it may signify lung injury. Acute asthma or a difficult intubation can cause intrapulmonary rupture of air spaces with tracking of air in the peribronchial tissues to the mediastinum and neck. Subcutaneous emphysema in the neck is also an important sign of ruptured esophagus, 32and should be sought in every patient in whom violent vomiting is accompanied by severe chest pain or followed by a left pleural effusion.
Prominent Veins
In patients with superior vena cava obstruction, the veins of chest wall become prominent. It is important to distinguish the pre-azygos superior vena cava obstruction from post-azygos obstruction, as the prognosis in the latter is generally poor. In pre-azygos superior vena cava obstruction, the veins become prominent on the upper part of chest wall and arms. As blood routes via external jugular vein to superficial venous plexus on anterior chest wall, which in turn connects via perforating branches to the internal mammary and intercostal veins, finally joining the azygos venous system to superior vena cava below the site of obstruction, the blood flow therefore is from above downwards. On the other hand, in post-azygos superior vena cava obstruction, the prominent dilated veins are found on anterior and lateral chest wall, arms, abdominal wall, back and groin; because four venous collecting systems including internal mammary, vertebral, azygos and lateral thoracic are utilised for purpose of carrying blood to inferior vena cava via femoral and iliac veins. However the direction of blood flow remains the same, from above downwards.
Swelling
Swelling on the chest wall should be examined just like any other swelling on the body.
Rib Fracture
Chest pain arising from rib fracture or localised muscle trauma is important to differentiate. Both sites of rib fracture and muscle injury are tender on palpation, but crepitus can be felt only at the site of rib fracture. Apply gentle pressure on sternum with one hand and on vertebral column on back with the other hand simultaneously to push both inwards; severe pain is felt at the site of rib fracture, while at the site of chest trauma there is no pain.
Trachea and Apex Beat of Heart
This may provide information about the position of the mediastinum. But sometimes the trachea may be displaced by neck tumours such as goiter. The apex beat also shifts due to cardiac enlargement. Gross deformity of the spine may also alter the position of trachea or heart apex. When these causes are excluded, displacement of the trachea from mid-sternal line will usually signify disease of the pleura or lung. Deviation away from the abnormal side (as identified also by percussion and auscultation) suggests pleural effusion, pneumothorax, bullous emphysema or, rarely, a massive tumour. Deviation towards the abnormal side occurs when there is collapse of the upper lobe or the whole lung, pleural or pulmonary fibrosis, advanced mesothelioma or lung collapse with effusion. Trachea may even remain in a central position when the carcinoma has caused a combination of collapse and effusion or has infiltrated and fixed the mediastinum.
- The trachea should be palpated with the patient's head fully extended and the chin in mid-line (Fig. 1.15). A finger is gently slid deep into the suprasternal notch, where in a normal person it should meet the center of the trachea; or the observer should attempt gently to slide the tip of index finger between the trachea and the lower part of the belly of sternocleidomastoid muscle and feel for the resistance; the side to which the resistance felt is more, the trachea is considered shifted to the same side.
- The site of apex beat is a less useful indicator of the position of the mediastinum, not only because it may be displaced by cardiac enlargement but also because it is more difficult to locate than the cervical trachea. It is mainly of value in detecting the lower lobe collapse where it shifts, while trachea remains central; and also for picking up the occasional case of dextrocardia in patients with bronchiectasis (Kartagener's syndrome).
Thoracic Expansion and Movements
The normal chest expands symmetrically during deep inhalation. This is judged well by inspection from the foot end of the bed of patient. However, palpation may help to confirm a suspicion during inspection where the movement is diminished or unequal. Apical expansion is examined by resting the fingers on the clavicles and drawing the thumbs together in mid-line; lower down anteriorly, the examiner's hands are placed over the anterolateral chest with the thumbs extended along the costal margin toward the xiphoid process. On the posterior chest, the hands are positioned over the posterolateral chest with the thumbs meeting at approximately the eight thoracic vertebra (Fig. 1.16). The patient is instructed to exhale slowly and completely while the examiner's hands are positioned as described. When the patient has exhaled maximally, the examiner gently secures the tip of fingers against the sides of chest and extends the thumbs towards the midline until the tip of each thumb meets at the midline. The patient is then instructed to take a full, deep breath. The examiner should make note of the distance each thumb moves from the midline. Normally it moves an equal distance of approximately 3 to 5 cm. The side, on which the thumb moves less, is indicative of reduced expansion or movement of the same hemithorax.
- The chest expansion or movement should be examined during tidal breathing as well. If the reduced movement of hemithorax seen during tidal inspiration improves on deep and maximal inhalation, it is indicative of partial obstruction of the whole lung or its part.This is due to the fact that incomplete bronchial obstruction causes partial collapse and reduced air entry. The latter improves on deep inhalation, thereby improving the expansion of hemithorax.
- A unilateral reduction in chest movement occurs with respiratory diseases that reduce the expansion of the lung or a major part of one lung. This may occur with lobar consolidation, atelectasis, pleural effusion and pneumothorax. Diseases that affect expansion of both lungs cause bilateral reduction in chest expansion, commonly seen with neuromuscular diseases and chronic obstructive pulmonary disease.
- The overall expansion of the chest should be measured with a tape at the level of the nipples (or beneath the breasts in women). The circumference of chest should increase by 6 to 9 cm from full expiration to full inspiration. This measurement, though still required for job fitness, is of little clinical value except to demonstrate fusion of costovertebral joints in patients with ankylosing spondylitis.
Vocal Fremitus
The term vocal fremitus refers to the vibrations created by the vocal cords during phonation. These vibrations transmit down the tracheobronchial tree and through the alveoli to the chest wall. When these vibrations are felt on the chest wall, is called tactile or vocal fremitus. During the assessment of tactile fremitus, the patient is directed to repeat the words ek do teen or ninety-nine while the examiner systematically palpates the chest. The examiner can use the palmar aspect of the fingers or the ulnar aspect of the hand as illustrated in Fig. 1.17). If one hand is used, it should be moved from one side of the chest to the corresponding area on the other side. All the areas already described in Table 1.2 should be evaluated.
- The vibrations of tactile fremitus felt may be increased, decreased, or absent. Increased fremitus results from the transmission of vibrations through a more solid medium. The normal lung structure is a combination of solid and air-filled tissue. Any condition that tends to increase the density of the lung, such as consolidation, results in an increased intensity of fremitus. If the area of consolidation is not in connection with a patent bronchus, fremitus will not be increased; in fact it may be decreased. A reduced tactile fremitus is often present in patients who are obese or overly muscular. Also, when the pleural space lining the lung gets filled with air (pneumothorax) or fluid (pleural effusion), the vocal fremitus is reduced significantly or may even be absent.
- In patients with emphysema, the lungs become hyperinflated with a significant reduction in the density of lung tissue. In this situation, the vibrations transmit poorly through the lung tissue, resulting in a bilateral reduction in tactile fremitus.This bilateral reduction in tactile fremitus is more difficult to detect than the unilateral decrease or increase in fremitus.
- The passage of air through airway(s) containing thick secretions may produce palpable vibrations referred to as rhonchial fremitus. Rhonchial fremitus is often identified during inhalation as well as exhalation and may clear if the patient produces an effective cough.
PERCUSSION
Percussion is the art of tapping on a surface in an effort to evaluate the underlying structures. Percussion of the chest wall produces a sound and a palpable vibration useful in evaluating the underlying lung tissue. The vibration created by percussion penetrates the lung 5 to 7 cm beneath the chest wall. The technique most often used in percussion of the chest wall is termed indirect percussion. The right handed examiner places the middle finger (pleximeter) of the left hand firmly against the chest wall parallel to the ribs, with the palm and other fingers held off the chest. The tips of the middle (percussing) finger of the right hand or the lateral aspect of the right thumb strikes the pleximeter finger against the chest near the base of the terminal phalanx with a sharp quick blow. Movement of the hand striking the chest should be generated at the wrist and not at the elbow or shoulder (Fig. 1.18).
The percussion note is clear if the examiner remembers to keep the finger on the chest firmly against the chest wall and to strike this finger and immediately withdraw it. The two fingers should be in contact only for an instant. As one gains experience in chest percussion, the feel of the vibration created becomes as familiar as the sound in the evaluation of lung structures.
- Percussion over lung fields: Percussion of the lung fields should be done systematically, consecutively testing comparable areas on both sides of the chest. Percussion over the bony structures and breasts of the female is not of value and should be avoided. Percussion over clavicle is performed with the striking finger directly, after stretching the overlying skin. Asking the patient to raise arms above shoulders will help to move the scapula laterally and minimise their interference during percussion of the posterior chest wall. The sounds generated during percussion of the chest are evaluated for intensity (loudness) and pitch. Percussion over normal lung fields produces a sound moderately low in pitch that can be heard easily. This sound is best described as normal resonance. When the percussion note is louder and lower in pitch than normal, the resonance is said to be increased or hyperresonant. Percussion may produce a sound with characteristics just the opposite of resonance, referred to as dull or flat. This sound is high pitched, brief and not loud.
- Clinical implications: Percussion of the chest, by itself is of little value in making a diagnosis. When the percussion note is considered along with history and other physical findings, it may contribute significantly.
- Any abnormality that tends to increase the density of lung tissue, such as consolidation of pneumonia, lung tumours, or alveolar collapse (atelectasis) results in a loss of resonance and a dull percussion note over the affected area. Percussion over pleural spaces filled with fluid, such as blood or water, also results in a dull or flat percussion note. An increase in resonance is detected in patients with hyperinflated lungs, which can occur as a result of acute bronchial obstruction (asthma), or emphysema, or when the pleural space contains air (pneumothorax).
- Unilateral abnormalities are easier to be detected than bilateral ones because the normal side provides an immediate comparison. The dullness heard during percussion over consolidation is a distinct sound that is easier to detect than the subtle increase in resonance associated with hyperinflation or pneumothorax.
- Limitations of percussion: Percussion of the chest has limitations that are often clinically important. Abnormalities that are small or more than 5 cm below the surface are likely to be missed (see auscultatory percussion below).
- Diaphragmatic excursion (tidal percussion): The range of diaphragmatic movement may be estimated by percussion and is conducted best on the posterior chest wall. To estimate diaphragmatic movement, the patient is instructed to take first a deep, full breath and hold it. Then the examiner determines the lowest margin of resonance by percussion over the basal lung area and moving downward in small increments until a definite change in percussion note is detected. The patient is then instructed to exhale maximally, holding this position, while the percussion procedure is repeated. The examiner should work rapidly to prevent the patient from becoming short of breath. The normal diaphragmatic excursion during a deep breath is about 5 to 7 cm but it is reduced in patients with certain neuromuscular diseases or severe pulmonary hyperinflation.
- Percussion of mediastinum: Over sternum and mediastinum, it is accomplished by light percussion if patient lying down and heavy percussion if patient sit up. The mediastinum is usually percussed to detect its widening by eliciting dullness on either side of the sternum.
- Shifting dullness: The shifting dullness is elicited in hydropneumothorax, pyopneumothorax and moderate pleural effusion, because a shift or change in position of the fluid occurs on changing the posture of the patient. While patient sitting up, the upper border of dullness is delineated by percussion over anterior chest, the pleximeter finger is then kept at the same upper border of dull area and ask the patient to lie supine for 10-15 seconds (during this period the fluid flows to the dependent area) and dull area will become resonant on repercussion. Fluid can not shift in loculated or interlobar pleural effusions thus shifting dullness is absent. In case, the patient is not able to sit upright, shifting dullness may be demonstrated by rolling the patient to keep the dull area uppermost and repeating percussion after 10-15 seconds.
- Shifting dullness in case of infrapulmonary effusion can be elicited by repeating the percussion over dull area with patient bent forward. The dull area at the base then changes to less dull or even normal resonant.
AUSCULTATION
Auscultation is the process of listening for sounds with an external aid. Over the thorax it is performed to identify lung sounds. A stethoscope is used to promote better transmission of sounds to the examiner. Whenever auscultation is performed, the room must be as quiet as possible.
- Stethoscope: A stethoscope possesses four basic parts: a bell, a diaphragm, tubing and earpieces.
- The bell detects a broad spectrum of sounds and is of particular value when listening to low pitched sounds, such as those produced by the heart. It is also valuable in the auscultation of lungs in certain situations, as in the emaciated patient where rib protrusion restricts placement of the diaphragm flat against the chest. The bell should be pressed lightly against the chest when attempting to auscultate low-frequency lung sounds. If the bell is pressed too firmly against the chest wall, the skin will be stretched under the bell and may act as a diaphragm, filtering out certain low-frequency sounds.
- The diaphragm is used more often for auscultation of the lungs, since most lung sounds are of relatively high frequency. It is also useful in listening to high-frequency heart sounds. The diaphragm should be pressed firmly against the chest so that the external sounds are not heard. The ideal tubing should be thick enough to exclude external noises and measure approximately 25 to 35 cm. If longer it may compromise sound transmission, and shorter tubing is often inconvenient for reaching the patient's chest. The stethoscope should be examined regularly for cracks in the diaphragm, wax or dirt in the earpieces, and other defects that may interfere with the transmission of the sound. It should be wiped off with alcohol on a regular basis to prevent a build up of microorganisms.
- Technique: Whenever possible, the patient should be sitting upright in a relaxed position, and instructed to breathe a little deeper than normal with the mouth open. Inhalation should be active and exhalation passive. The bell or diaphragm must be placed directly against the chest wall, since clothing may alter lung sounds or produce distorted sounds.
- Hair on chest wall can also alter the sounds; therefore if present these should be made wet to avoid the distortion of sounds. Also, the tubing should not rub against any object, since this may produce extraneous sounds. Auscultation should be systematic, and include all lobes and areas on the anterior, lateral and posterior chest (See Table 1.2). It is recommended that the examiner should start auscultating at the lung base, because certain abnormal lung sounds (e.g. fine end-inspiratory crackles) that primarily occur in the dependent lung areas may be altered by several deep breaths. At least one full ventilatory cycle should be evaluated at each stethoscope position. Television or radio should be turned off and the room should be calm and quiet. If alert, the patient should be asked to sit up; a comatose patient should be rolled onto side to auscultate posterior chest.
- Four characteristics of breath sounds should be specifically looked for by the examiner; the pitch (vibration frequency), the amplitude or intensity (loudness), the distinctive characteristics (vesicular, bronchovesicular, bronchial) and finally the duration of inspiratory sound are compared with those of expiration sound. The acoustic characteristics of breath sounds can be illustrated in a breath sound diagram. The characteristics of normal breath sounds are described in Table 1. 4. The examiner must first be familiar with the characteristics of normal breath sounds before he can expect to identify the subtle changes that signify respiratory disease. Also, one should be familiar with the added (adventitious) sounds.
Normal Breath Sounds
Laënnec believed that normal breath sounds were caused by friction of air against the lining of airways. In 1884, Bullar investigated the site of origin of vesicular sounds by using a sheep lung preparation and concluded that the sound was generated within the lung. In 1927, Bushnell observed that the vesicular breath sounds were generated at the larynx and transmitted to the chest wall through the airways. McKushik and associates (1955) used a sound spectrograph to analyse lung sounds and found that the inspiratory vesicular sound was maximal in intensity at approximately 200 Hz and decreased rapidly with increasing frequency and no sound energy was detected at more than 500 Hz. Kraman studied the normal subject by subtraction phonopneumography. With the support of other studies, he concluded that the multicentric sources of inspiratory sounds are only confined to the larger airways (more than 2 mm) where turbulence is known to exist, and contribution by the mainstem bronchi and trachea was excluded. The expiratory component also seems to be multicentric in origin, but includes the mainstem bronchi and trachea as well. The larynx, while certainly producing considerable noise, seems to take no part in the production of either component of the vesicular lung sounds.
- Measurements of the inspiratory breath sounds through the chest wall demonstrated regional difference between the apex and the base of the lung. Comparison of regional ventilation measured with 133Xenon and the loudness of the breath sounds over the corresponding areas of the chest wall showed that the sounds are faint where ventilation is impaired.
- Transmission of breath sounds: Sound transmits in air within large tubes like the trachea and main bronchi to reach the mouth almost un-attenuated.
Table 1.4 Characteristics of breath sounds Breath soundPitchIntensityLocationDiagram of sound *Vesicular or normal breath soundsLowSoftPeripheral lung areas BronchovesicularModerateModerateAround upper part of sternum, between scapulae
BronchovesicularModerateModerateAround upper part of sternum, between scapulae BronchialHighLoudOver trachea
BronchialHighLoudOver trachea *Upstroke represents inhalation, and downstroke exhalation; length of upstroke represents duration.The small bronchi offer greater resistance to the oscillations of air at acoustic frequencies, so that beyond segmental bronchi, the breath sounds travel mainly across the lung. The unattenuated breath sounds at the mouth contain a wide range of evenly distributed frequencies between 200 and 2000 Hz, while those recorded from the chest wall are at a relatively narrow range of low frequencies, with a steep fall in amplitude above 200 Hz, as chest wall acts like a low pass filter. When the examiner auscultates over the lung parenchyma of a healthy individual, soft and muffled sounds are heard. These sounds are referred to as vesicular or normal breath sounds, and is lower in pitch and intensity (loudness) than bronchial breath sounds. Vesicular sounds are most difficult to hear, and are heard primarily during inhalation with only a minimal exhalation component. The breath sounds transmitted through the chest fade out during expiration. This is due to the fact that during inspiration the turbulence generated is carried further towards periphery, while during expiration, flow rate falls and direction of flow is reversed.
*Upstroke represents inhalation, and downstroke exhalation; length of upstroke represents duration.The small bronchi offer greater resistance to the oscillations of air at acoustic frequencies, so that beyond segmental bronchi, the breath sounds travel mainly across the lung. The unattenuated breath sounds at the mouth contain a wide range of evenly distributed frequencies between 200 and 2000 Hz, while those recorded from the chest wall are at a relatively narrow range of low frequencies, with a steep fall in amplitude above 200 Hz, as chest wall acts like a low pass filter. When the examiner auscultates over the lung parenchyma of a healthy individual, soft and muffled sounds are heard. These sounds are referred to as vesicular or normal breath sounds, and is lower in pitch and intensity (loudness) than bronchial breath sounds. Vesicular sounds are most difficult to hear, and are heard primarily during inhalation with only a minimal exhalation component. The breath sounds transmitted through the chest fade out during expiration. This is due to the fact that during inspiration the turbulence generated is carried further towards periphery, while during expiration, flow rate falls and direction of flow is reversed.
Bronchial Breath Sounds
When the lung tissue between central airways and chest wall is airless as a result of consolidation, atelectasis or fibrosis, the breath sounds are transmitted to the stethoscope with relatively no loss by attenuation or filtration. These resemble the sounds normally heard over the trachea. It has been graphically demonstrated that bronchial sounds contain a higher frequency component than vesicular sounds. Martini and Mueller provided evidence that the site of bronchial breath sounds was in airway 4 mm or more in diameter. It was demonstrated that the presence of larynx is not necessary for the generation of the bronchial sounds. Consolidated lung acts like an acoustically continuous conduction medium that does not attenuate the transmission of sounds as normal inflated lung does. Bronchial sounds are high pitched or low pitched sounds with an expiratory component equal to or slightly longer than the inspiratory component and there is a gap between both phases (Table 1.4). In healthy individuals the sounds heard over the trachea have a loud, tubular quality of bronchial breath sounds.
- Cavernous (low pitched) bronchial breathing can be normally heard over the occipital region of the skull. It has its peculiar hollow character. When it is heard over lung fields, it is suggestive of cavity of the lung, an open pneumothorax or when trachea shifted to one or other side by fibrosis or collapse of the lung (pulled trachea syndrome). The pulled trachea syndrome may thus lead to an erroneous diagnosis of cavitation of the lung apices. Tubular (high pitched) bronchial breathing, with its characteristic tubular quality is suggestive of consolidation of lung. Latter results from inflammation as in lobar pneumonia, engorgement of blood in pulmonary infarction or collapse of alveoli in atelectasis in presence of partial open bronchi. In certain cases of massive pleural effusion, breath sounds instead of being diminished or absent, paradoxically become high pitched bronchial breathing. It is due to the fact that collapsed lung under large pleural effusion transmits the sound from some large bronchus or bronchi to the fluid 40collected. Amphoric breathing is a special variety of high pitched bronchial breathing with a distinctive echo like or metallic quality that can be imitated artificially by blowing intensely across the mouth of a bottle. It is usually suggestive of large smooth walled cavity of the lung or bronchopleural fistula. This is due to the amplification of certain vibrations or high pitched overtones in the original bronchial sound. The d'Espine's sign is high pitched tubular bronchial breathing heard over the fourth or lower thoracic vertebrae. This is due to the transmission of tracheal breath sounds through a mass in the middle or posterior mediastinum between the trachea and the stethoscope. Similarly, tubular breath sounds can be transmitted from the trachea to anterior chest wall when there is a large mass in the anterior mediastinum.
- Bronchial breath sounds, to be present on any part of lung, generally, need a partial or fully patent bronchus supplying it. Bronchial breathing may be heard over an airless upper lobe, irrespective of whether the lobar bronchus is patent or not; this is due to the fact that the mediastinal surface of the upper lobes is in direct contact with the trachea, and the sound from trachea is transmitted to solid lung directly. There is no such direct path of transmission to the lower lobes and thus sounds do not reach them unless the intervening bronchi are patent. Bronchial breathing is usually absent when the lower lobe is both consolidated and atelectatic as a result of bronchial obstruction.
Bronchovesicular Breath Sounds
Breath sounds intermediate in characteristics between vesicular and bronchial, are referred to as bronchovesicular. The mechanisms responsible for their production represent a combination of the mechanisms discussed already. In healthy persons, these represent a combination of sounds arising at the site of auscultation from different sources. In disease, partial changes in filtration characteristics may result in sounds with a frequency intermediate between bronchial and vesicular. These sounds are not as loud as bronchial, slightly lower in pitch, and have equal inspiratory and expiratory components without any gap in between (Table 1.4). In healthy individuals, bronchovesicular breath sounds may be heard around the upper half of sternum anteriorly and over the interscapular area on the posterior chest.
Voice Sounds
Speech contains a fundamental note of low frequency, generated by oscillations of the vocal cords, and several partials or overtones amplified by resonance of the mouth, pharynx and the paranasal sinuses. The frequency of the fundamental note is about 150 Hz in men and 230 Hz in women. Consonants are articulated by momentary interruption of flow (k, t, p) by turbulent flow in the mouth (s, sh) or vibrations of the tongue (r). Vowels are produced by attaining the shape and resonance of the mouth and pharynx such that two particular higher frequency partials are amplified; e.g. the vowel ‘e’ in word ‘team’ has strong partials of 375 Hz and 2400 Hz respectively, while the vowel ‘a’ in word ‘father’ has two strong partials at 825 Hz and 1200 Hz. Each vowel is characterized by a pair of such partial tones called formants, which are the same in both men and women unlike that of the fundamental note. If the formants are lost during transmission, speech becomes incomprehensible.
- Bronchophony: Voice sounds heard through the normal lung and chest wall are attenuated and filtered. The low 41frequencies upto 200 Hz are well transmitted while those above 200 Hz are attenuated. Due to this selective filtration, most of the vowel formants are lost and speech heard through the stethoscope over normal lung becomes a meaningless, low pitched rumble. When the lung between the trachea and point of auscultation is airless, the higher frequencies, including the formants of vowels are transmitted and speech becomes intelligible, known as bronchophony. The acoustic basis of bronchophony and bronchial sounds is identical, both being signs of unfiltered sound transmission through solid lung. Therefore bronchophony is an increase in intensity and clarity of vocal resonance resulting from enhanced transmission of vocal vibrations. It indicates an increase in lung tissue density, and can occur in the consolidation phase of pneumonia. This sign is easier to detect when unilateral and is often accompanied with bronchial breath sounds.
- Whispering Pectoriloquy: During whispering, the vocal cords get abducted and do not oscillate and voice sounds are generated only by turbulent flow of air through the trachea, glottis and pharynx. This white noise is inaudible over the normal air-containing lung because it lacks the powerful low frequency component (fundamental note) which is selectively transmitted through the normal chest. On the other hand the high frequency component of this white noise of the turbulence and the formants of the vowels are transmitted through airless lung, with the result the whispered speech becomes intelligible. This sign known as whispering pectoriloquy occurs in the same conditions in which bronchial breathing and bronchophony are present; and thus completes the triad of auscultatory signs of consolidation. The patient is instructed to whisper ek do teen or one two three, while the examiner listens over the chest with a stethoscope, comparing both the sides. Through normal lung, muffled and low pitched sounds are heard. The high pitched sounds heard over the consolidated lung are clear and comprehensible.
- Egophony: Like breath sounds, the voice sounds are reflected by fluid or air in the pleural cavity, so that in pleural effusion and pneumothorax, speech is inaudible over the chest wall. When the consolidated lung is separated from the chest wall only by a thin layer of fluid, the attenuation at the interfaces between fluid and the two layers of the pleura is selective. Low frequency sounds below 1000 Hz are lost, so that only the high frequency formants of the vowels survive. Thus speech remains intelligible, but the removal of the lower frequencies of vowel explains the nasal bleating quality of egophony. It is identified most easily when the patient says ‘e-e-e’. If egophony is present, the ‘e-e-e’ will be heard over the chest wall with the stethoscope as ‘a-a-a’. Egophony is usually detected only over an area of upper area of pleural effusion (where fluid layer is thin) and underlying consolidation.
- Vocal resonance: If inspection, palpation, percussion and auscultation of the patients' chest suggest any respiratory abnormality, vocal resonance is assessed. It is produced by the same mechanism as that of vocal fremitus described earlier. The vibrations created by the vocal cords during phonation travel down the tracheobronchial tree and 42through the peripheral lung units to the chest wall. The patient is instructed to repeat the words ‘ek do teen’ or ‘one two three’ while the examiner listens over the chest wall with a stethoscope, comparing the sides. The normal, air filled lung tissue filters the voice sounds significantly, reducing intensity as well as clarity. Pathological abnormalities in lung tissue alter the transmission of voice sounds, resulting in either increased or decreased vocal resonance. Vocal resonance is reduced in same lung abnormalities as those resulting in reduced breath sounds and decreased vocal fremitus. Hyperinflation of lung parenchyma, pneumothorax, bronchial obstruction, and pleural effusion all reduce the vocal resonance through the lung or chest wall. An increase in intensity and clarity of vocal resonance resulting from enhanced transmission of vocal vibrations is referred to as bronchophony, and often present in the consolidation phase of pneumonia.
Nomenclature of Adventitious Sounds
In ancient times, only limited observations could be made on lung sounds by listening to such phenomena as wheezing or stridor from a distance. Direct application of the ear to the chest further increased the knowledge of number of sounds that could be related to diseases. The science of auscultation, however, began with the invention of the stethoscope by Laënnec.
Examination of current medical literature reveals that different terms are used for the same lung sounds. A total of 663 case reports in 7 English language journals were reviewed, and it was found that the word crepitations in British Journals seemed to be virtually equivalent to the American ‘rales’. To reduce this confusing ‘Tower of Babel’ a committee of the International Lung Sounds Association met in 1976. By acoustic analysis of sound from tape recordings made by different investigators, the new nomenclature of adventitious sounds was adopted by the American Thoracic Society in 1977. The word ‘crackle’ was recommended in place of ‘rale’. The work of Holford provided an objective basis for describing them by using such criteria as whether fine or coarse, their duration, and their form as measured on time expanded waveforms of individual crackles.
Continuous Adventitious Lung Sounds (Wheezes and Rhonchi)
These lung sounds are superimposed on the breath sounds in certain circumstances and usually indicate disease. They are usually louder than the underlying breath sounds. The word ‘continuous’ in this context implies a duration of more than 250 ms rather than meaning a sound that continuous throughout the respiratory cycle (Table 1.5); and several such continuous sounds may be present simultaneously. The term wheezes is used for high-pitched continuous lung sounds with dominant frequency of 400 Hz or more; and the term rhonchi is used for low-pitched continuous lung sounds with dominant frequency of 200 Hz or less.
- The mechanism for producing wheezes in the intrathoracic airways appears to involve the airway walls interacting with the air moving through them. As the airway is narrowed, the air flow velocity through it increases to cause fall in lateral pressure due to the venturi effect, which results in further narrowing or closing of airway. As soon as the airway is closed, the venturi effect ceases and the lumen of the airway reopens, and thus setting the cycle into operation again. This sequence of self perpetuating pressure changes results in rapid oscillations of the airway wall, to generate a musical sound (wheezing).
Table 1.5 Classification of common adventitious lung sounds Acoustic characteristicsAmerican Thoracic Society NomenclatureCommon SynonymsLaennec's originalDiscontinuous, interrupted explosive sounds. Sounds low in pitch, Average values IDW=1.25 ms, 2CD=9.Course cracklesCourse raleRale muquexou grargouillementDiscontinuous interrupted explosive sounds. Sounds louder and higher in pitch than coarse crackles. Average valuesFine crackleFine rale, crepitationRale humide ou crepitation*IDW = 0.92 ms,**2CD = 6.02 msContinuous sounds lasting longer than 250 ms, high Rale sibilant longer than 250 ms, high pitched; dominant frequency of 400 Hz or more, a hissing sound.WheezeSibilant rhonchusRale sibilantsec ou sifflementContinuous sounds lasting longer than 250 ms, low pitched; dominant frequency about 200 Hz or less, a snoring soundRhonchusSonorous rhonchusRale sec sonorous nonflement*IDW = Initial deflection width ms = millisecond**2 CD = Two cycle durationA fall in lateral pressure during inspiration can produce stridor in stenosis of cervical portion of trachea where the outside pressure is atmospheric. - In the presence of hyperinflated lungs, high pitched wheezes may be heard more easily over the trachea than lung. Tracheal auscultation has therefore been advocated as part of the routine clinical assessment in asthma.
- Patients with chronic airflow obstruction who do not wheeze, are not likely to show significant improvement in expiratory flow rates, whereas those who wheeze are more likely to show a significant improvement after bronchodilators.
- A single musical note of constant pitch is a characteristic sign of incomplete occlusion of a main or lobar bronchus is termed as fixed monophonic wheeze. In widespread airflow obstruction especially in bronchial asthma monophonic wheezes arise from airways narrowed to the point of closure by swelling of the mucosa. When there is more than one wheeze, they occur at random, vary in duration and often overlap in time, are called as random monophonic wheezes. A characteristic feature of polyphonic wheezing is that all its component notes begin at the same time and continue together to the end of expiration. Polyphonic wheezing at rest is a reliable sign of widespread airflow obstruction.
Discontinuous Adventitious Lung Sounds
These are a series of brief explosive sounds with individual components less than 20 ms (Table 1.5). Theories explaining the 44mechanism of production of these sounds have so far been supported by indirect evidence only. These sounds are probably produced by more than one mechanism and two probable ones are discussed here.
- One, crackles may be due to the sudden opening of a succession of small airways, with rapid equalisation of pressure between the collapsed airways below and patent ones above, thus causing a sequence of implosive sound waves; the other mechanism could be the bubbling of air through secretions. The first of these is the likely mechanism for crackles heard in patients with interstitial lung disease and congestive heart failure.
- Crackles are categorised into fine and coarse based on waveform analysis of tracings of time expanded crackles (Fig. 1.19). The fine crackles have been defined as those having an initial deflection width (IDW) of less than 0.9 ms and a two cycle duration (2CD) shorter than 6.0 ms. And those with IDW more than 0.9 ms and 2 CD longer than 6.0 ms are termed as coarse.
- Pleural rub: The striking feature in pleuritis is the pleural rub or pleural crackles. The smooth well lubricated layers of the normal pleura move silently over one another. When the surface is roughened by fibrin deposits or infiltrated by inflammatory or malignant cells, the sliding motion is momentarily interrupted due to frictional resistance. The lung then acts on the chest wall like the bow of a string instrument. If a large area of the chest wall is set into resonant oscillation, the resulting sound is musical. More commonly, the jerky sliding movement of the lung produces a series of non-musical sounds which are usually longer lasting and of a lower pitch than crackles arising from the lung; and these non-musical sounds are termed as pleural rub. Sound of pleural rub heard during inspiration often recurs in reversed sequence during expiration giving a mirror image effect. Pleural friction rub often sounds similar to coarse crackles but is not affected by coughing. The intensity of pleural rub may increase with deep breathing and on pressing the chest wall with the chest piece of the stethoscope, while there is no effect on intensity of coarse crackles.
Sequential Inspiratory Wheezes (Squawks)
These are a series of sequential (not overlapping) inspiratory sound or sometimes a single sound, due to the opening of airways which had become abnormally apposed during the previous expiration. These tend to occur in deflated areas of lung and are therefore heard in various forms of pulmonary fibrosis, especially hypersensitivity pneumonitis and rheumatoid bronchiolitis. Squawk often follows the late inspiratory crackle and is probably caused by vibrations in the wall of bronchiole as it opens following abnormal closure.
‘Scratch Sign’ Test
The ‘scratch sign’ test is sometimes useful in diagnosing pneumothorax. In this test, the stethoscope is placed at the mid-point of the sternum and the surface of the chest wall is scratched with a finger at points equidistant from the stethoscope, both on its left and right sides. The sound heard is louder, when the side of the pneumothorax is scratched.
Auscultatory Percussion of Chest
A technique that combines auscultation and percussion is known as auscultatory percussion. The procedure is simple to perform and requires only a stethoscope. The examiner percusses on the patient's manubrium with the index finger of one hand while auscultating over the posterior lung fields, scanning and comparing both sides. This method is used for lesions which are 5 to 7 cm deep. The auscultatory percussion is very useful in the hands of experts.
Findings of auscultation in some respiratory diseases are discussed below (Table 1.6)
- Chronic bronchitis: Crackles during expiration and at the beginning of inspiration are common in widespread airflow obstruction (Table 1.6). These sounds are low pitched, scanty, and loud and widely conducted through the lower lobes, transmitted through the large airways, and are often audible as a series of clicks through the stethoscope held near the patient's mouth. Crackles of chronic bronchitis are even-spaced and correspond to the passage of a bolus of gas through lightly closed central airways, which open intermittently when the upstream gas pressure rises above a critical level. The cause of intermittent obstruction is uncertain and could be due to the sputum lying in the large bronchi.
- Emphysema: Patients of emphysema were studied both for production as well as transmission of sounds and compared with normal subjects. It was concluded that transmission of sounds varies from breath to breath. The study revealed areas of normal, decreased and increased sound transmission comprising areas of relatively normal lung parenchyma, areas of hyperinflation with airway obstruction and less ventilation, and areas of focal atelectasis or consolidation respectively. Sound heard through stethoscope at mouth has been reported to be decreased in emphysema whereas it is increased in bronchitis and asthma.
Table 1.6 Lung crackles in obstructive chronic bronchitis, bronchiectasis and interstitial fibrosis FeaturesObstructive chronic bronchitisBronchiectasisInterstitial fibrosisTiming of inspiratory cracklesEarly phaseEarly and mid-phaseEnd phaseNumber of cracklesFewerModerateCan be profuseEffect of coughNo changeTemporarily reducedNo changeEffect of positionNo changeNo changeModified/abolishedIntesityFaintLoudModerately loudPitchCoarseCourseFineExpiratory cracklesMay be presentTypically presentMay be presentTransmission to mouthTransmittedtransmittedNot transmitted - Bronchial asthma: The most prominent feature on auscultation of bronchial asthma patient is a high-pitched continuous sound. In mild cases, wheezing may be heard over the central airways only during expiration and becomes evident over the entire chest during both phases of respiration as asthma becomes more severe. The absence of wheezing in acute severe asthma is regarded as an ominous sign, because wheezes are not generated due to resulted low flow. Wheezing may occur in apparently normal persons during maneuver of forced expiration. With improvement in flow rate after bronchodilators or spontaneously, the frequency of wheezing also decreases.
- Bronchiectasis: The dominant auscultatory feature of bronchiectasis is crackles. The crackles in patients of bronchiectasis can be distinguished from those of chronic bronchitis and interstitial fibrosis (Table 1.6). In bronchiectasis, inspiratory crackles start early in inspiration, continue to mid inspiration and fade by the end of it. Expiratory crackles are also present. Therefore the crackles of bronchiectasis are present in both phases of the respiratory cycle and may be made less abundant after coughing.
- Interstitial fibrosis: The most striking feature in patients with interstitial fibrosis is the presence of fine crackles. These have been described as close to the ear cellophane or velcro crackles. In mild forms of the disease, these are usually confined to end-inspiration and are gravity dependent. These are best heard at the lung bases when the patient is upright, and their distribution can vary with the change in position (Table 1.6). They may disappear or reduce from lung bases as the patient bends forward. In advanced stages of illness, these crackles persist despite positional changes and are heard at higher levels above the base of lungs.
- Fine crackles in interstitial fibrosis may become pan-inspiratory, often with an end-inspiratory accentuation as the disease progresses. Expiratory crackles can also be heard in this disease and their mechanism is explained as follows: the airways initially patent at the end of inspiration, close early in expiration, and then reopen with the building of pressure due to the air trapped within them; when pressure exceeds that of the adjacent airways during expiratory phase, it results in crackles.
- In pulmonary fibrosis caused by asbestos exposure, crackles are end-inspiratory, present first at the inferior axillary area in the midaxillary lines, and tend to spread posteriorly at the bases of lungs. Some believe it to be an early sign of asbestosis.
- Pneumothorax: The breath sounds are absent when there is a pneumothorax of either side. If left sided, a loud crackling sound may be heard; it is generated either by a sudden displacement of the small collection of air trapped in the mediastinal pleural space or by direct impact of the heart on the mediastinal surface of the lung. Shallow pneumothorax is associated with clicking, as the heart and lungs are separated only by a narrow air space. The clicking disappears when the lung reexpands or when the pneumothorax deepens.
Notation of Lung Sounds
A system of notation easily reproducible by hand, typescript and in print is more valuable in clinical practice. A suitable framework consists of a stave of two parallel horizontal lines divided by a vertical line into inspiratory and expiratory halves on which wheezes are represented by horizontal lines and crackles are shown as dots. The vertical position of these symbols on the stave indicates their pitch and their relation to the dividing mark corresponds to their timing. The use of this notation is illustrated in the (Fig. 1.20) for the interpretation of adventitious lung sounds.
How to Present Diagnosis
While stating the diagnosis in a patient of respiratory disease, one should adopt a systematic approach.
Fig 1.20: Notation for recording the timing and pitch of adventitious lung sounds. A, fixed monophonic wheeze; random monophonic wheezes; sequential monophonic wheezes (squawk); and expiratory polyphonic wheezes. B, late inspiratory crackles; early inspiratory and late expiratory crackles.
|
The following should be sequentially mentioned in a diagnosis: pathology of disease, anatomical part or parts of lung involved and finally the etiology of the disease e.g. consolidation of right lower lobe due to pyogenic bacteria;or collapse of right upper lobe due to intrabronchial obstruction (malignancy); or pleural effusion of right side due to tuberculosis. The terms like consolidation or collapse of upper zone should be avoided, as the term ‘zones’ is used to describe radiological findings only.
Table 1.7 shows the physical signs of abnormal pulmonary pathology.
Reliability of Physical Signs
The inter-observer reliability of respiratory physical signs falls midway between chance and total agreement. It is relatively independent of the experience of the observer. It is found that clubbing, wheezes, pleural rub, crackles and percussion note are the most reliably elicited signs.
SUGGESTED READING
- Anderson CL, Chanker PS, Scott JH. Physiological significance of sternomastiod muscle contraction in chronic obstructive pulmonary disease. Resp Care 1980;25:937.
- Brewis RAL, Gibson GJ, Geddes DM. Respiratory Medicine, ed. 1, 1990, Bailliere Tindall. London,
- Bullar JP. Experiments to determine the origin of respiratory sounds. Proc R Soc London 1884;37:411.
- Bushnell GE. The mode of production of the so-called vesicular murmur of respiration. JAMA 1921; 77:2104.
- Diagnostic standards and classification of tuberculosis - ATS. Am Rev Respir Dis. 1990; 142:725.
- Earis JE. Marsh K, Pearson MG, Ogilive CM. The inspiratory ‘sqawk’ in extrinsic allergic alveolitis and other pulmonary fibroses. Thorax 1982;37:923.
- Forgacs P. Lung sounds, ed 2, 1986, Jaypee Brothers. New Delhi,
- Guarino JR. Auscultatory percussion of chest, Lancet 1980;2:1332.
- Hira HS. Lung Sounds. JAPI 1993;4:33.
- International Symposium on Lung Sounds. Chest 1987;92:342.
- Kraman SS. Determination of the site of production of respiratory sounds by subtraction phonopneumography. Am Rev Respir Dis 1980;122:303.
- Kraman SS. Does Laryngeal Noise Contribute to the Vesicular Lung Sound? Am Rev Respir Dis 1981;124:292.
- Lawson JD. The scratch sign: a valuable aid in the diagnosis of pneumothorax. New Eng J Med 1961;264:88.
- Lewis RA, Howell JBL. Defination of the Hyperventilation syndrome. Bull Eur Physiopathol Respir 1986;22:201.
- Loudon R, Murphy RLH Jr. State of the art: lung sounds. Am Rev Repir Dis 1984;130:663.
- McKusick VA, Jenkins JT, Webb GH. The acoustic basis of the chest examination: Studies by means of sound spectrography. Am Rev Tuberc 1966;72:12.
- Murray JF. The normal lung: the basis for diagnosis and treatment of pulmonary disease, ed. 2, 1986, WB Saunders Co. Philadelphia,
- Nath AR, Capel LH. Lung Crackles in bronchiectasis. Thorax 1980;35:694.
- Olsen CR. The match test: A measure of ventilatory function. Am Rev Respir Dis 1962;86:37.
- Rippe JM. Manual of intensive care medicine, ed. 2, 1989, Little, Brown and Company. Boston,
- Scanlan CL, Spearman CB, Sheldon RL. Egan's Fundamentals of Respiratory Care, ed. 5, 1990. The C.V. Mosby Company. ST Louis,
- Seaton A, Seaton D, Leitch AG. Respiratory Diseases, ed, 4, 1989, Oxford University Press. Delhi
- Sokolowski JW. Guidelines for thoracentesis and needle biopsy of the pleura. Am Rev Respir Dis 1989;140:257.
- Spiteri MA, Cook DG, Clark SW. Reliability of eliciting physical signs in examination of the chest. Lancet 1988;1:873.
- Troyer AD, Estenne M. Functional anatomy of the respiratory muscles. Clinics in Chest Med 1988;9:175.