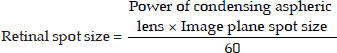Laser (an abbreviation for Light Amplification by Stimulated Emission of Radiation) is the equipment capable of emitting a powerful, highly monochromatic and coherent beam of electromagnetic radiation. Monochromatic electromagnetic radiation is meant for single frequency or single wavelength and eliminates chromatic aberration. Coherent beam means all photons produced are in phase with each other with limited divergence.
Laser Tissue Interactions
Laser interaction with various tissues of the eye may be classified into following categories (Fig. 1.1).
Photocoagulation
In photocoagulation temperature of treated tissue is increased from 37°C to at least 50°C, resulting in denaturation of tissue protein and coagulation at the absorbent tissue site. This results from conversion of light energy to heat energy.
The monochromatic light from laser is absorbed by melanin, xanthophyll present in the macula and hemoglobin.
Melanin pigment universally absorbs light spectrum between 400 and 700 nm whereas, xanthophyll and hemoglobin pigments are selective absorber. Melanin pigment is the principal absorber of light in photocoagulation of trabecular meshwork and co-absorber of light in retinal pigment epithelium (melanosomes) and choroids (melanocytes). The longer the wavelength, the deeper the chorioretinal burns. Hence, Argon laser (514.5 nm) and freq-doubled Nd: YAG (532 nm) laser are absorbed at the level of the retinal pigment epithelium (RPE) and choriocapillaries whereas, Krypton red (647 nm) and diode laser (810 nm) produce deeper lesion in the choroids.5
The appearance (ophthalmoscopic) of optimum/threshold retinal burn in argon laser and freq-doubled Nd: YAG laser (green/KTP) will be the same and quite different from the krypton red and diode laser photocoagulation burn. So, similar appearing krypton red and diode laser retinal photocoagulation burn will be markedly of higher threshold and will cause more extensive choroidal damage and pain as compared with argon laser and freq-doubled Nd: YAG laser burn.
Xanthophyll pigment is present in the inner and outer plexiform layers of retina of the macular area. They absorb blue light maximally and green light poorly. Hence, in macular photocoagulation blue light (blue-green argon laser) will cause unwanted inner retinal damage. Therefore, argon green laser (514.5 nm) and freq-doubled Nd: YAG laser (532 nm-green/KTP) are preferred over argon blue-green laser in macular photocoagulation.
Hemoglobin absorbs blue, green and yellow light considerably and red light poorly. The shorter wavelength yellow lights are more easily absorbed. The red and near infrared wavelength lights are totally unabsorbed by the hemoglobin.
Lasers Commonly Used in Photocoagulation
CW green Argon laser (514.5 nm)
- It is absorbed selectively at the retinal pigment epithelium (RPE), hemoglobin pigments, choriocapillaries, layer of rods and cones and at the outer and inner nuclear layers.
- It is readily absorbed by the melanin granules.
- It coagulates from choriocapillaries to inner nuclear layer of the retina.
- It is suitable for photocoagulation of retinal pigment epithelium (RPE), choroids and blood vessels.
Freq-doubled Nd: YAG laser (532 nm)
- It produces a pea-green beam.
- It is often termed as “green Nd: YAG laser” or “KTP laser”.
- It is more highly absorbed by hemoglobin (Hb) and the melanin present in retinal pigment epithelium (RPE) and trabecular meshwork than the argon laser beam. It coagulates from choriocapillaries to outer nuclear layer of the retina.
- It is small and portable like diode laser.
- It is a solid state and diode pumped CW laser.
- The aiming beam is usually diode laser (635 nm, max.1mW)
- It causes photocoagulation with least energy transmission and shows considerable safety in macular treatment. Hence, it is fast gaining major market share of posterior segment photocoagulator.
Krypton red laser (647 nm)
- The melanin granules also readily absorb it.
- It is not absorbed by the hemoglobin (Hb) and xanthophylls pigments present in the macular area. Hence, it is particularly suitable for macular photocoagulation and coagulation of subretinal neovascular membrane
- It coagulates deeper into the retinal pigment epithelium (RPE) and choroids. It has insignificant photocoagulation effect on the vascular system of the retina. It is less absorbed and more highly transmitted through retinal pigment epithelium (RPE). So, it is able to produce 8more extensive and deep coagulation of choriocapillaries and choroids.
Diode laser (810 nm)
- It is the most important semiconductor laser [GaAlAs (720–890 nm) GaAs (810 nm)]
- Direct photocoagulation of microaneurysm is difficult because it is poorly absorbed by hemoglobin.
- However, it is as effective as argon, freq-doubled Nd: YAG laser in reducing macular edema.
- It offers increased patient comfort due to absence of bright flash of light.
- However, due to deeper penetration in to the choroids, it may be painful if the intensity of retinal coagulation is not properly titrated /reduced.
- It is a low cost, portable, small, high powered and versatile laser.
Lasers with blue wavelength light should not be used for photocoagulation in following situations;
- In the macular area – Xanthophyll pigments absorb blue light maximally and green light poorly. Hence, in macular photocoagulation blue light (blue-green argon laser) will cause unwanted inner retinal damage
- In older patients – The ageing lens absorbs blue light much more than other light wavelengths. The shorter wavelength blue lights are also more scattered by aged crystalline lenses.
Influence of Opacities in the Ocular Media Upon Laser Parameter (Power)
Any opacity in the ocular media such as corneal edema, corneal haziness, flare and cells in the anterior chamber, lental opacity and vitreous opacity reduces energy level of the laser beam striking the retinal surface by reflection, scattering or absorption of the laser beam.9
Hence, the optimum power level should be arrived at by gradually increasing the power to cause optimum coagulation burn (Figs 1.2 and 1.3) for that procedure.
Gradation of Photocoagulation Lesions
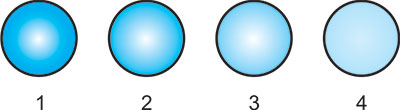
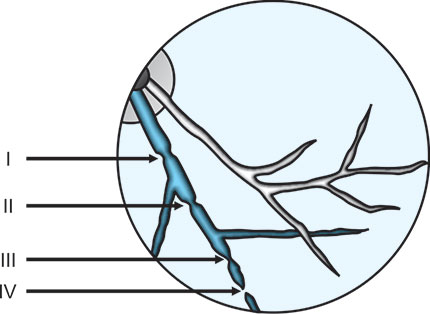
Visible photocoagulation intensity of chorioretinal tissues except retinal vessels can be graded from grade1/light to grade 4/heavy (Table 1.1, Figs 1.2 and 1.3). Similarly visible photocoagulation of retinal vessels can be graded from grade I to grade IV (Fig. 1.4).
The grading is clinically very significant to ascertain the end point /optimum intensity of photocoagulation indicated for a specific retinal lesion, e.g., Grade1/light chorioretinal coagulation is optimum intensity of photocoagulation in focal/grid laser in diabetic maculopathy whereas, Grade 3/moderate chorioretinal coagulation is optimum intensity of photocoagulation in scatter/Panretinal photocoagulation (PRP), retinal breaks and abnormal blood vessels.
Focusing of Laser Beam
All the lasers except xenon-arc emit monochromatic rays. So, energy of these lasers except xenon-arc can be focused to a fine point without significant chromatic aberration.10
Fig. 1.2: Schematic drawing of various chorioretinal coagulations (Grades 1/light to 4/heavy)
1 = Grade 1/Light, 2 = Grade 2/Mild, 3 = Grade 3/Moderate and 4 = Grade 4/Heavy
Fig. 1.3: Schematic drawing of various chorioretinal coagulations (Grades 1/light to 4/heavy)
I = Minimal visible constriction of the vessel,
II = Total constriction and spasm of the vessel,
III = Total constriction of the vessel along with coagulations of the surrounding tissue and
IV = Total constriction, charring of the vessel, coagulations of the surrounding tissue
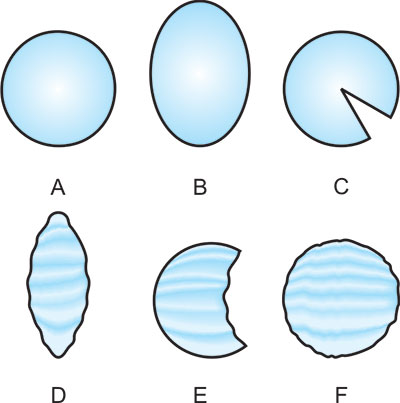
The properly focused laser beam in an eye without any opacity in the refracting mediums should be circular with clearcut margin (Fig. 1.4A). An oval beam with a blurred outline indicates incorrect focusing (Fig. 1.4B).11
Fig. 1.4: Focusing of laser beam
A = Properly focused laser beam without any opacity in the refracting mediums,
B = Oval beam with a blurred outline -incorrect focusing,
C = Large wedge shaped deficit-cortical cataracts,
D = Elongated and irregular outline-astigmatism,
E = Large irregular deficit-vitreous opacity and
F = Round hazy focus and irregular outline diffuse haziness of ocular media.
However, opacities in the ocular refracting mediums will not only block a certain percentage of laser beam energy to reach retina but also superimpose a shadow on the round laser focus. The round focused beam may take the shape of following distorted images (Figs 1.4C to F):
- Small or large wedge shaped deficit-cortical cataracts (Fig. 1.4C)
- Elongated and irregular outline-astigmatism (Fig. 1.4D)
- Large irregular deficit-vitreous opacity (Fig. 1.4E)
- Round hazy focus and irregular outline – diffuse haziness of ocular media (Fig. 1.4F)
Nd: YAG laser (1064 nm) emission rays are invisible since 1064 nm is at infrared end of the light spectrum. Single or multiple Helium-Neon/He-Ne (632.8 nm) visible red beams are usually employed for aiming of Nd: YAG laser (1064 nm). Diode red (670 nm) may be also employed as aiming beam in Nd: YAG lasers (1064 nm).
Laser Parameters
- Power = Number of”photons”emitted each second and is expressed in watts (W).
- Exposure time = The duration in second (sec.) the “photons” are emitted in each burn from the laser.
- Spot size = The diameter of the focused laser beam and is expressed in micron (µm). Spot size is usually fixed for treatment of a particular lesion. However, the energy (Power× Exposure time) parameters must be decreased or increased, with the decrease or increase inthe spot size parameter. The spot size when focused on the retina depends on; 1) Laser Spot Magnification Factor (LSMF) of the laser lens, 2) Spot size selected in the Slit-lamp and 3) Refraction ofthe eye under treatment.
- Energy = Number of”photons”emitted during an exposure of any durationand is expressed in joules (J). So, Energy (Joules) = Power (Watt) × Exposure time (Second).
Energy calculation
- 1 watt is equal to 1 Joule of energy derived in 1 sec. exposure.
- 0.5 sec exposure with 2 watt power parameter = 1 joule coagulative energy.
- 0.5 sec exposure with 1 watt power parameter = 0.5 joule coagulative energy.
Post Photocoagulation Advice
The following restrictions are advisable to continue for a period of 3 weeks postlaser. The aim is to reduce/ control the venous pressure rise in the eyes, head and neck region.
- Avoid sneezing, cough and constipation and control with medication.
- Do not lift heavy objects.
- Avoid heavy exercise and yoga.
- Avoid sudden jerky movements of the head.
- Only paracetamol can be taken orally as pain killer.
- During sleep level of head should be above the level of heart.
- Avoid medications containing ephedrine and epinephrine.
It is ideal to supply post photocoagulation advice in a printed format.
Photovaporization
In photovaporization, laser irradiation higher than photocoagulation threshold is applied to the target tissue. As a result, the tissue temperature can reach the boiling point of water and sudden fast expansion of water vapor will cause tissue disruption, i.e. photovaporization. Photovaporization, i.e. cutting is usually accompanied by photocoagulation, i.e. cautery (or hemostasis).
Photoablation
In photoablation, temperature rise does not take place in the shorter wavelengths of the ultraviolet spectrum. At the site of impact, the tissue simply disappears without any charring and temperature rise. Surface of the target tissue can be precisely removed, layer-by-layer, in photoablation. Photoablation with 193 nm argon fluoride (ArF) excimer 14laser produces superior predictable tissue ablation than longer wavelength (248 nm) krypton fluoride (KrF) excimer laser in lasik /lasek.
Photoradiation
Hematoporphyrin derivative is selectively taken up and retained by metabolically active tumor tissue. In photoradiation, this photosensitized tissue is exposed to 630 nm red lights from a dye laser, producing cytotoxic singlet oxygen and tissue destruction. Similarly, Verteporfin preferentially accumulates in choroidal neovascular membrane (CNV). In photodynamic therapy the choroidal neovascular membrane is subjected to laser emission from diode (689 nm) with resultantocclusion and thrombosis of the neovascular tissue.
Photodisruption
In photodisruption, temperature of treated localized microscopic area of tissue is increased from 37°C to 15000°C. On optical breakdown at the desired site, electrons are stripped from the atoms of target tissue resulting in development of plasma field and bubble. This leads to hydrodynamic and acoustic shock wave, which mechanically tears the tissue microscopically.
Laser delivery
Laser can be delivered through 3 types of approach;
- Slit-lamp Biomicroscope:
- The most common and popular delivery system.
- Laser parameters viz.; power, exposure time and spot size can be changed.
- Laser Indirect Ophthalmoscope (LIO):
- Argon green and diode lasers are delivered through a fiberoptic cable.
- Ideal for photocoagulation of peripheral retinal breaks and degenerations.
- Ideal for PRP/scatter photocoagulation of extreme retinal periphery in eyes with rubeosis iridis, PDR, post-CRVO, retinopathy of prematurity (ROP) etc.
- Ideal for photocoagulation in children under general anesthesia.
- Ideal for photocoagulation in eyes with small pupil, intraocular gas and lental opacities.
- Unsuitable for focal and or grid laser of macula.
- Spot size is altered by the dioptric strength of the hand held condensing lens and moving a lever on the headset.
- Spot size is also altered by the refractive status of the eye. The spot size in a hypermetropic eye is smaller than in an emmetropic eye whereas, the spot size in a myopic eye is larger than in an emmetropic eye.
- In LIO,
- Intraoperative Laser Endoscope:
- Argon green and diode lasers are delivered through Laser Endoscope during vitrectomy.
- Ideal for photocoagulation of retinal surface neovascularization (NVE), peripheral retinal breaks and degenerations after retina is attached by internal fluid-air exchange after vitrectomy.
- Since, detached retina cannot be treated, prior sub- retinal fluid (SRF) removal is essential before application of laser.
- Ideal for photocoagulation of giant retinal tear.
Indications for infiltration anesthesia
- Uncooperative patient
- Presence of significant ocular movement, e.g. nystagmus
- Presence of significant ocular pain
- Photocoagulation near the macular center
Pascal (Pattern Scan Laser) photocoagulator isrecently developed by OptiMedica corporation, USA, which is a significant improvement in laser delivery systems. Pascal photocoagulator incorporates semi-automated, multiple pattern, short pulse, multiple shot, painless and precise laser burns in a very short duration in a predetermined sequencewith Freq. doubled YAG (532 nm) laser.
Pascal photocoagulator can be used in all the retinal diseases (Proliferative and nonproliferative diabetic retinopathy, diabetic maculopathy, branch and central retinal vein occlusion, retinal tears and peripheral retinal degenerations, choroidal neovascular membrane, retinaltelangiectasia, retinopathy of prematurity, etc.) treated with conventional single spot lasers (Argon, Freq. doubled YAG, Krypton, etc.).
Advantages of pascal photocoagulator over conventional single spot lasers
- Pulse duration is very short (10–20 msec) compared to conventional single spot lasers (100–200 msec). Hence, Pascal causes less collateral damage to the eye with similar effective regression of new vessels.
- The size of the retinal burn remains relatively stable after Pascal photocoagulation due to low intensity. In conventional single spot photocoagulation the laser spot burn enlarges with time. Hence, Pascal laser burns are less destructive than conventional single spot laser spot burns.
- Pascal is as efficient as conventional single spot photocoagulators.
- The gradation of retinal burns are the same but can be titrated more easily.
- Pascal allows the laser surgeon to apply different patterns of treatment with variable retinal grades of coagulation. Since the eyeball is spherical in shape semicircular pattern is better suited for photocoagulation of retinal periphery and standard square pattern is ideal for retinal midperiphery. Circular pattern is suitable for treating retinal holes/breaks.
- Pascal allows the laser surgeon to adjust the individual spot size, adjust the distance between the spots perfectly and the pattern of the spots with much more precision than is possible with a conventional single spot photocoagulator.
- Pascal allows the laser surgeon to place multiple spots in one depression of the foot pedal.
- Pascal allows the laser surgeon to complete PRP in a regular pattern, more quickly and usually in one day. Short treatment duration leads to improved patient cooperation and fixation.
- Pascal allows the laser surgeon to place burns in distant retinal periphery in a regular pattern in PRP, peripheral retinal degenerations and retinopathy of prematurity.
- Pascal allows the laser surgeon to place burns in macular grid laser more accurately in a regular pattern compared to conventional single spot lasers.
- Increase in macular edema following confluence of retinal burns is extremely low compared to conventional single spot photocoagulator.
- Single-spot mode for conventional photocoagulation is also available.
- Using the Pascal Method, physicians can deliver up to 56 spots in approximately 0.6 seconds.
- Patients experience less pain than with traditional, single-spot laser photocoagulation.
- Reduced treatment duration.
Disadvantages of Pascal photocoagulator
- The spot size available is restricted (only 100, 200 and 400 µm). Spot sizes of 150,300 and more than 400 µm are not possible.
- Inability to design the laser patterns at the surgeon's convenience.
- Pascal photocoagulator produces some noise when activated.
- Pascal photocoagulator emits green wavelength, which is difficult to penetrate through media opacities, e.g. cataract, retinal and vitreous hemorrhages.
- Pattern burn with Pascal photocoagulator in retinal periphery is often difficult and always use lower intensity by titration in retinal periphery to avoid intense burn.
Precise, pre-determined settings
- Square arrays (2 × 2, 3 × 3, 4 × 4, 5 × 5) for proliferative diabetic retinopathy
- Triple arcs for retinal tears, lattice degeneration and proliferative diabetic retinopathy
- Modified macular grid for diffuse diabetic macular edema
- Single-spot mode for conventional photocoagulation
OptiMedica holds the exclusive license to the Pascal Photocoagulator technology, which was originally developed at Stanford University. Since its worldwide market introduction in 2006, Pascal photocoagulation procedures have been performed on tens of thousands of patients worldwide.
BIBLIOGRAPHY
- Al-Hussainy S, Dodson PM, Gibson JM. Pain response and follow-up of patients undergoing panretinal laser photocoagolation with reduced exposure times. Eye 2007;1–4.
- Bhattacharyya B. Clinical Applications: YAG Laser (Ophthalmology). Jaypee Brothers Medical Publishers (P) Ltd, New Delhi: 2005:9–19.
- David B. Karlin (ed). Lasers in Ophthalmic Surgery: Blackwell Science Inc. 1995:
- Gholam A. Peyman, Donald R. Sanders, Morton F. Goldberg (eds). Principles and Practice of Ophthalmology (1st Indian Ed.). WB Saunders company, Philadelphia: 1987:1098–1118.
- Gorisch W, Boergen KP. Heat-induced contraction of blood vessels. Lasers Surg Med 1982;2:1.
- Jain A, Blumenkranz MS, Paulus Y, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol 2008;126:78–85.
- L'esperance Jr. FA Ophthalmic Lasers. (3rd edn.). CV Mosby Co. St. Louis: 1989:96–112.
- Mainster MA. Ophthalmic applications of infrared lasers-thermal considerations. Invest Ophthalmol Vis Sci 1979; 18:414.
- Mainster MA, White TJ, Allen RG. Spectral dependence of retinal damage produced by intense light sources. J Opt Soc Am 1970;60:848.
- Steven M Bloom, Alexander J Brucker(eds). Laser Surgery of the Posterior Segment (2nd ed). Lippincott-Raven, Philadelphia: 1997.