- ✓ RECEPTORS
- ✓ GENERAL ASPECTS
- ✓ CYCLIC AMP
- ✓ G-PROTEIN
- ✓ INOSITOL TRIPHOSPHATE AND DIACYLGLYCEROL
- ✓ RECEPTORS COUPLED WITH G-PROTEINS
1999 Nobel Prize Awarded to: GUNTER BLOBEL, for the discovery that proteins have intrinsic signals that govern their transport and localization in the cell
RECEPTORS
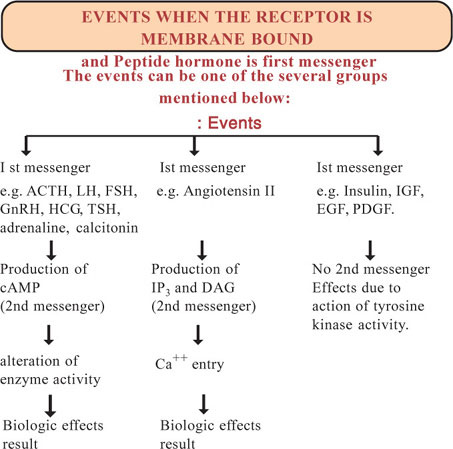
Receptors are protein macromolecules. Almost all cells of our body have receptors. There are many types of receptors. Each cell may posses one type or many types of receptors. Each receptor may have several sub-types. The chemical signals like hormones and drugs produce their effects on their target cells by binding to the receptors. The chemical signals that get bound to receptors are called ligands or first messengers. The receptors are present in the membrane, in the cytoplasm and in the nucleus.
Receptors are specific, that is, a particular type of this receptor will combine with a specific ligand. For example the ligand adrenaline combines with the specific receptors for adrenaline in the liver cell and its effect on the target hepatic cells namely glycogenolysis is caused. This ligand adrenaline is called the agonist for the receptor.
Antagonists otherwise called “blockers” are ligand molecules which have resemblance chemically to the agonist ligand molecule. These chemically similar molecules compete with agonist ligands and combine with the receptors. This combination prevents the agonist ligand to combine with the receptors. These ligands are called antagonists or blockers. For example the action of adrenaline (agonist) on β adrenaline receptors is blocked by propranolol. Hence the propranolol is an antagonist of adrenaline or β-blocker.
After producing their effects inside the targetcells, some ligands are degraded along with receptors (e.g. Insulin), whereas some other ligands after internalization, the receptor is separated from the ligand and comes back to the original membrane site. This phenomenon is called recycling.
The knowledge about receptors for neurotransmitters and other chemical messengers are due to advances in the molecular biology techniques and cloning methods.
GENERAL ASPECTS
- For each ligand there are many subtypes of receptors. Thus for example nor adrenaline acts on α1, α2, and β1, β2 receptors. This multiplies and makes more selective in any given cell the possible effects of given ligand. A ligand may be a hormone, or neurotransmitter or a drug molecule.
- There are receptors at presynaptic and postsynaptic membrane at many synapses. The presynaptic receptors are also known as autoreceptors. These often inhibit further secretion of the ligand, providing feedback control. Auto-receptors also can facilitate the release of neurotransmitters.
- The receptors on the basis of their structure and function are grouped as:
- Serpentine receptors that act via ‘G’ proteins and protein kinases to produce their effects.
- Others are ion channels.
The second messengers include cAMP, cGMP, IP3, DAG, Ca++ provide specific binding sites. eg. receptor for insulin.24
Receptor Structure
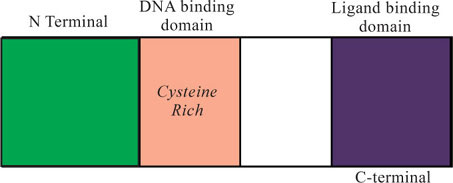
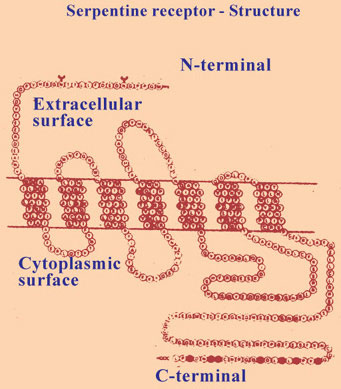
The receptors are proteins which have in common a cystein rich DNA binding domain and a ligand binding domain at or near the C terminal with a considerably variable N-terminal part of the protein (Fig. I-21). The ligand binds to the specific ligand binding site. The other domain is also called active site. This site is responsible for the post binding events. Further, this discriminates whether the ligand is an agonist or antagonist.
Post-binding events
These receptors are present in the membrane, in the cytoplasm and in the nucleus.
For the thyroid and steroids hormones, the receptors are located in the cytoplasm and nucleus, and for all other ligands the receptors are located in the membrane. Thyroid hormones T3 and T4 and estrogen bind to the receptor in the nucleus. Other steroids, retinoic acid and other lipid soluble ligands act via cytoplasmic receptor. The activated receptor binds to DNA and increase the transcription of selected m RNAs. Other ligands which get bind to the membrane receptors give rise to the release of intracellular mediators called second messengers such as cAMP, DAG and IP3 or these ligand trigger other intracellular events via GTP binding G proteins. The second messenger generally activate the protein kinases which catalyses the phosphorylation of aminoacids on proteins, changing the configuration of proteins thereby influencing the functions of the cell. In some cases the intracellular portion of the receptors themselves act as proteins kinases. More than hundred protein kinases have been identified and described.
Transcription
The thyroid and steroid hormones bind to the receptors inside the cells causing conformational changes in the receptor proteins, and the DNA binding domain in the receptor is exposed. The receptor - hormone complex moves to DNA and gets bound to enhancer elements in the untranslated 5' flanking portions of certain genes. This binding increases the transcription of the RNA's encoded by the gene to which the receptor-hormone complex binds. The mRNAs are translated in the ribosomes with more production of cellular proteins which are responsible for cell functions.
Down Regulation and Up Regulation
The receptor proteins are not static components of the cell. Their number and properties vary in response to various stimuli. When the ligand is present in excess, the number of the active receptors decreases. This is known as down regulation. When the ligand is present in less amount, the number of the active receptors increases. This is known as ‘up regulation’ Angiotensin II receptors in the adrenal cortex is an exception.
These phenomena help to explain the phenomenon of denervation hypersensitivity and tolerance to morphine.25
CYCLIC AMP
Earl Sutherland was awarded Nobel prize in 1970 for this work on cyclic AMP as a second messenger.
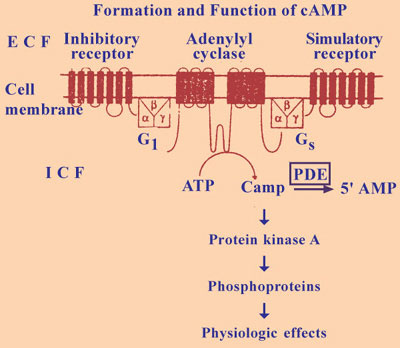
The important second messenger is cyclic AMP. This is cyclic adenosine 3 ‘5’ monophosphate. Cyclic AMP is formed from ATP by the action of the enzyme adenylcyclase and cyclic 3 ‘5’ AMP is converted to physiologically inactive ‘5’ AMP by the action of an enzyme phosphodiesterase. This enzyme is inhibited by methyl xanthines such as caffeine and theophylline thereby promoting the hormonal and transmitter effects mediated by cyclic AMP. Cyclic AMP activates a cyclic nucleotide dependent protein kinase A. Protein Kinase A like protein Kinase C catalyses the phosphorylation of proteins, changing their conformation and modifying their activity. The first messenger activates or inhibits adenyl cyclase causing increased or decreased production of cyclic AMP (Fig. I-22).
The ligands bring about changes in the concentration of intracellular CAMP by involving five different components namely:
- The catalytic unit, the enzyme adenylcyclase which catalyses the formation CAMP from ATP.
- Stimulatory receptors
- Inhibitory receptors
- Stimulatory G Proteins (Gs)
- Inhibitory G Proteins (GI)
Stimulatory ligands bind to stimulatory receptors and activate adenyl cyclase via Gs proteins. Inhibitory ligand bind to inhibitory receptor and inhibits adenyl cyclase via GI proteins.
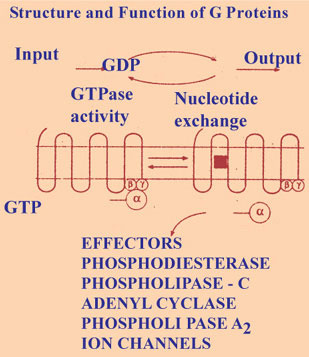
G PROTEINS (FIG. I-23)
These are nucleotide regulatory proteins. The physiologic important cyclic nucleotides are cyclic AMP and cyclic GMP. The signal by a messenger is translated into biologic effect inside the cells by the G proteins that bind to GTP. GTP is the guanisine analogue of ATP. When the signal reaches the G proteins exchanges GDP for GTP. The GTP protein complex brings about the effect. The inherent GTP - ase activity of the protein then converts GTP to GDP restoring the resting state.
Another family of G proteins are hetrotrimeric G proteins. These hetrotrimeric G proteins couple cell surface receptors to catalytic units, adenlcyclases that catalyses the intracellular formation of second messenger or couple the receptor directly to ion channels. The G proteins are made up of 3 sub units namely α, β, and γ. The α, subunit is bound to GDP, when a ligand binds to G coupled receptor, the GDP is exchanged for GTP and subunit gets separated and this separated sub unit brings about the biologic effects. The intrinsic GTPase activity of the subunit then converts the GTP to GDP and leads to reassociation of the α, unit with β, and γ subunits and termination of effector activation restoring resting state. There are large number of G proteins with more number of α, β and γ submits.
Small G proteins have a role in vesicle transport in cells, in interactions between cytoskeleton and membrane and in cell growth.26
All the hetrotrimeric G protein - coupled receptors described so far are proteins that span the cell membrane 7 times. These are known as serpentine receptors. They form a very large number and their functions are multiple and diverse. These serpentine receptors have an extracellular N terminal, and intracellular C terminal and 7 membrane spanning portions of each protein receptor. Small ligands bind to the amino acid residues in the membrane, where as large peptides and proteins ligands bind to large well developed extracellular domains, the G proteins interact with amino acid residues in the cytoplasmic loop near C terminal (Fig. I-24).
INOSITOL TRIPHOSPHATE (IP3) AND DIACYLGLYCEROL (DAG)
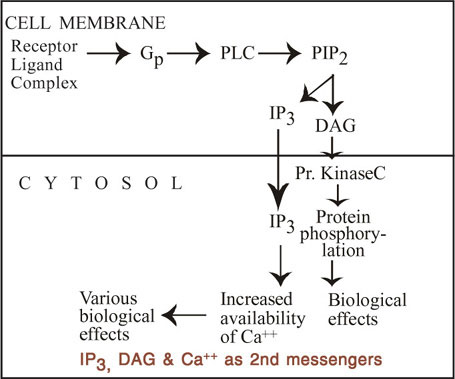
These both are second messengers. The inositol triphosphates (Inositol 1,4,5 triphosphate (or) IP3) forms the link between the membrane binding of a ligand that acts via Ca++ and prompt increase in the cytoplasmic calcium level. When one of the ligands binds to its receptor, it causes activation of phospholipase C on the inner surface of the membrane via G proteins (G or another G protein) phospholipase C (PLC) catalyse the hydrolysis of phosphatidyl inositol, 4,5 di phosphate (PIP2) to IP3 and diacyglycerol (DAG). There are several forms of phospholipase C. Eight isoforms have been isolated so far. The IP3 formed diffuses to the endoplasmic reticulum and binds to the IP3 receptor (this is only Ca++ channels) and causes release of Ca++ into the cytoplasm. IP3 is the major second messenger that causes release of calcium from internal stores. The calcium level thus increased binds with calcium binding proteins such as troponin, calmodulin and calbindin and brings about physiologic effects. Tyrosine kinase linked receptors also can produce IP3 and DAG by activating phospholipase C.
The second messenger DAG activates one of the 7 subspecies of protein kinase C.
The examples of ligands which activate phopholipase C with intracellular production of IP3 and DAG are angiotensin II, nor-epinephrine via adrenergic receptor, vasopressin via VI receptor.
Cell Function and Calcium
Calcium is one of the major cations present in the extracellular fluid. Intracellular free calcium concentration is very low 100 nmol/L. In the interstitial fluid its concentration is 1,200,00 nmol/L (12,000 times more) due to these facts there is a marked inwardly directed concentration gradient and electrical gradient. Most of the intracellular calcium is stored in the endoplasmic reticulum and in the mitochondria. Ca++ enters the cells through both voltage gated channels and ligand gated channels. In addition, Ca++ channels activated by stretch have been proposed. Four types of voltage gated channels have been identified and these are activated by depolarisation of the membrane. The ligand gated channels are activated by hormones and neurotransmitters. There are two types of calcium pump which pumps Ca++ out the cell. Ca++-H+-ATP-ase 27pumps out Ca+ in exchange for 2H+, and Ca++ is transported out of cells by an antiport driven by the Na+ gradient that exchange 3Na+ for each Ca++. The second messengers act by increasing intracellular calcium. The increase is due to release of calcium from endoplasmic and other internal storage organelles or due to entry of Ca++ into the cells or by both mechanisms. IP3 is the major second messenger which causes release of Ca++ from endoplasmic store and other internal store (Fig. 1-25).
In certain tissues for example in pancreas the cyclic adenosine diphosphate ribose (CADPR) is formed by the action of cyclic GMP on NAD+ and this CADPR act on ryanodine receptor and causes entry of Ca++ into the cell leading to increase in intracellular Ca++. Increased cytoplasmic calcium bind to and activates calcium binding proteins such as troponin, calmodulin, and calbindin and these activate protein kinases and giving rise to physiologic effects. Troponin is the calcium binding protein in skeletal muscle contraction. Calmodulin is involved in smooth muscle contraction by activating myosin light chain kinase which phosphorylates myosin and this brings about smooth muscle contraction. Another calmodulin dependent kinase is phosphorylase kinase which activates phosphorylase. Ca++/Calmodulin Kinase I and II are concerned with synaptic function and Calmodulin kinase III is concerned with protein synthesis. Principal protein kinases are Calmodulin dependent (MLCK) myosin light chain kinase, phosphorylase kinase.
- Cal/Calmodulin Kinase I, II and III
- Calcium - phospholipid dependent protein kinase C
- Cyclic AMP dependent protein kinase A
- Cyclic GMP dependent kinase
Insulin receptor, EGF receptor, PDGF receptor and MCSF receptor have tyrosine kinase activity.
EGF | = | Epidermal growth factor, |
PDGF | = | Platelet derived growth factor |
MCSF | = | Macrophage Colony - Stimulating factor. |
Clinical Aspects
Receptor diseases are caused by mutation of the genes for receptors or for G proteins and also by production of antibodies against receptors. Receptor mutation that causes diseases have been reported in the case of 1–25 dihydroxy cholecalciferol receptor
- Insulin receptor
- T3, T4 receptor and vasopressin receptor.
Diseases due to G - protein mutation are
- Pseudohypoparathyroidism
- Hyperpigmentation and hypercortisolism in McCune Albright syndrome
- Pituitary adenomas that cause acromegaly
Diseases due to antibodies against receptors.
Antibodies against Nicotinic acetylcholine receptors give rise to Myasthenia Gravis.
RECEPTORS COUPLED WITH G-PROTEINS
1994 Nobel prize was awarded to:
ALFRED G. GILMAN and MARTIN RODBELL for their discovery of G-proteins.
Receptors for the following ligands are coupled with G proteins.
Neurotransmitters
Epinephrine, nor-epinephrine, Dopamine, 5 HT, Histamine, Acetylcholine, adenosine
Tachykinins
Substance, P. Neurokinin A, and Neuropeptide K.
Other Peptides
Angiotensin II, Vasopressin, VIP, CRP, TRH & PT4
Glycoprotein hormones: TSH, FSH, LH, HCG
- Arachidonic acid derivatives:
- Thromboxane A2
- Others: Odorants, Endothelins, Platelet activating factor, cannabinoids, IL8
Some of these activate adenyl cyclase and other act via IP3 and some do both.
Summary of the Mechanisms by which the first Messengers Bring about Changes in the Cell:
- Acetylcholine on nicotinic receptor and nor-epinephrine on K+ ion channels bring about changes in the cell by opening or closing of ion channels.
- Thyroid hormones, retinoic acid and steroid hormones bring about changes in the cell by acting on cytoplasmic and nuclear receptor to increase the trnascription of RNA. - Angiotensin II, norepinephrine via adrenergic receptor and
- vasopressin via V2 receptor by the activation of phospholipase C with intracellular production of DAG, IP3 and inositol phosphates.
Norepinephrine via adrenergic receptor and by activating or inhibiting adenyl cyclase causing increase or decrease in the production of CAMP.
- ANP, NO (Nitric Oxide) (EDRF) by increasing CGMP in the cell,
- Insulin, EGF, PDGF, M-CSF by increasing tyrosine kinase activity of cytoplasmic portions of transmembrane receptors.
Apoptosis
The cell division and cell growth occur under genetic control. Similarly cell death and absorption of cells also occur normally during development and adulthood under genetic control. This process of programmed cell death is called apoptosis. This is also known as “cell suicide”, since the cell's own genes are responsible for their death. This should be differentiated from cell necrosis or “cell murder” in which healthy cells are destroyed by ischaemia and inflammation. During apoptosis there is disintegration of nucleus, chromatin and cytoplasm and subsequent absorption by phagocyte without inflammation. Necrosis is characterised by severe membrane alterations and is preceded by a period of time during which the diseased cells become permeable to immunoglobulins.
The well known examples are:
- Remodelling of CNS during development.
- Apoptosis gets rid of inappropriate clones of immunocytes responsible for the lytic effects of glucocorticoids on lymphocytes.
- Apoptosis is a normal process in the development of genitalia.
It also occurs in adult ovaries and testes and in other organs.
PGF2 produce luteolysis by apoptosis.
Failure of apoptosis may be a factor in the development of cancer.
The details of genetic control of apoptosis
The apoptosis qualifies a genetically programmed cell death due to the activation of an endogenous endonuclease. The resulting DNA fragmentation produces a characteristic “ladder” pattern when size fractionated by electrophoresis. Apoptosis is associated with expression of number of regulatory genes including b-cl-2, and an antiapoptic protooncogene, Fas, a proapoptic gene, as well as others. The first protein identified that regulates was bel-2.
Membranes associated with bel −2 prevents cell death by regulating the Ca++ homeostasis attenuating oxidative stress interacting with bax. Bax is bCI associated gene X which promotes apoptosis by binding to bCl-2 and antagonising the function of bCl-2. The ratio of bCl-2 and bax decides apoptosis. Increase in bax promotes apoptosis.
There are evidences in support of reactive oxygen species (superoxide and hydrogen peroxide) induced apoptosis occurring during hypoxia, ischaemia and overstretch. No induced apoptosis also has been described.
Apoptosis is becoming very popular in cardiology because apoptosis is easy to identify by the detection of DNA fragments and by the expression of marker genes and intact cytoplasmic structures. The main cause of cellular loss during myocardial ischaemia is not necrosis but apoptosis. During post-myocardial infarction the apoptosis is associated with necrosis. Apoptosis is a regulatory mechanism that initiates hypertrophic process in cardiac remodelling. There is increased number of apoptic cardiocytes in failing hearts.
Cancer
Cancer is caused mostly by mutation of genes or by abnormal activation of cellular genes that control cell growth and mitosis. The abnormal genes are called oncogenes. So for about 100 oncogenes have been discovered. In all our cells there are anti-oncogenes which surpress the activities of specific oncogenes. Very often the failure of inactivation of oncogenes by the anti-oncogenes lead to cancer. However only a minute fraction of cells mutate and give rise cancer.29
This is due to the following reasons:
- Most mutated cells have less survival and simply die.
- Even mutated cells are prevented from excessive growth by feed-back control mechanisms.
- Destroyed by immune mechanisms of our body.
The following agents promote mutation:
Physical agents: Ionising radiation by X-rays, Gamma Rays and radiation by radioactive substances, Radioisotopes.
Constant Physical irritation
Chemical agents: Aniline dye drivatives, Chronic Smoking.
Hereditary tendency in certain families.
Viruses: e.g. DNA and RNA viruses.
Features: Proliferation and spread to other organs. Produce and angiogenic factor and leads to enormous growth of blood vessels in the cancerous organ.