Introduction
The structural characteristics of the cornea facilitate its essential functions, specifically to serve as both a transparent barrier and the predominant refractive element of the eye. Given the integral relationship between form and visual function, the biological and mechanical responses of the cornea to surgical interventions impact its optical performance. While major advances have occurred in the refinement and standardization of corneal surgical techniques, our ability to predict individual biological responses to surgery remains limited and can influence the predictability and stability of visual outcomes after corneal surgery. Some of these biomechanical responses are seen immediately, e.g., following lamellar keratectomy and/or laser ablation.1 Others may manifest in shape instability over time or with further surgery, or result in serious complications such as wound dehiscence, scarring, haze formation, and induction of irregular astigmatism. Corneal biomechanical characteristics often change with wound healing, and also may be better understood in the context of whole eye rigidity.2 In this chapter, we highlight the biochemical and ultrastructural features that most strongly contribute to the biomechanical properties of the normal cornea. Further understanding of these factors provide the basis for improving outcomes and reducing complications of corneal surgery, by identifying individual response outliers and developing strategies for regulating or compensating for these biomechanical features.3 While more familiar to engineers than to most ophthalmologists, we also review the definition of corneal hysteresis and other metrics that have been applied to studies of corneal biomechanics — a subject that encompasses the effects of corneal hydration, regional pachymetry, viscoelasticity and other inherent corneal characteristics that may not yet be fully defined.
Collagen Structure of the Cornea
The tensile integrity and refractive curvature of the cornea is determined in large part by the stroma, which represent the bulk of the corneal thickness. On a weight basis, the stroma is approximately 78% water, 15% collagen and 7% non-collagenous proteins and proteoglycans.4 Approximately 300 collagen lamellae, spanning from limbus to limbus, comprise the center of the cornea.5 This number increases to about 500 as the cornea thickens toward the periphery.4,5 Presumably, this occurs from branching of the lamellae, with some lamellae branches merging with others.6 Branching is seen more extensively in the corneal periphery, where there is primarily a circumferential orientation of the fibrils.7,8 Branching and interlacing of lamellae has been implicated to play an important role in corneal tensile strength.9
The orientation and spacing of the collagen fibrils appears to be controlled by stromal proteoglycans. Swelling studies have shown that the interlamellar adhesive strength of the central cornea depends upon proteoglycan bonding, whereas branching and interlacing of lamellae provides additional adhesive strength peripherally.7 Changes in the proteoglycan matrix may explain the increased pliability of the central cornea in keratoconus and may potentially impact the corneal response to keratorefractive surgery, contact lens wear and tonometric testing.10
The anterior-most stromal lamellae have oblique branching and interweaving fibers that insert into Bowman's layer.11 Because of these features, the anterior stroma swells less and is about 25% stiffer than its posterior counterpart.12 Similarly, as there is greater interlacing of peripheral fibers, swelling of the peripheral cornea is usually less than in the central stroma.13 These findings suggest that peripheral and/or posterior incisional surgery may have less of a profound impact on corneal biomechanics than anterior, central surgery (Table 1-1).
It appears that corneal shape is not determined on a random basis, but results from a steady state balance between the biomechanical properties of the cornea and intraocular pressure (IOP).14 The cornea assumes the shape for which its potential energy content is minimal and for which its stromal fibrils are in a relatively relaxed state, as a function of variables such as tissue elasticity, thickness, fibril length, rate of change of IOP, among others. External physiologic corneal stresses, such as from normal blinking or diurnal variation in IOP, and non-physiologic corneal stresses, caused by increases in IOP from forceful lid closure or rubbing, may potentially impact the corneal shape. However, normal corneas have been found to show low extensibility, measured by changes in anterior surface sagittal height, for a wide range of physiologic conditions and even with marked elevations in IOP in order to maintain refractive stability.15 Conversely, when the corneal biomechanical properties are altered via incisional surgery such as radial keratotomy, diurnal variation in IOP can lead to fluctuation in corneal refractive power by greater than one diopter (D).15
Metrics of Corneal Biomechanical Properties
In the terminology of material science, the cornea is a complex composite (of collagen, other proteins, proteoglycans, water, and salts) with non-linear elastic and viscoelastic properties characterized by important local variation in organization in central versus peripheral and anterior versus posterior dimensions. Mathematical modeling of such a complex system is therefore quite difficult, but begins with identification of intrinsic properties of corneal tissue, as described below (Table 1-2).
|
Elasticity (Young's modulus, E) is an indicator of material stiffness, with a higher modulus corresponding to a stiffer material. For example, a metallic rod would have a higher modulus than a wood rod (Figure 1-1). A perfectly elastic material returns to its original form, when an external stress is withdrawn, in a completely reversible and symmetric manner, i.e., along the same stress-strain pathway.16 Elasticity is traditionally measured ex vivo with an extensiometer that records the force generation required during steady axial elongation of a tissue sample. The slope of stress (force per unit area) over strain (the current length divided by the starting length) is calculated for a representative portion of the curve. A linear approximation can be obtained from the instantaneous slope of the stress-strain curve (tangent modulus) or as a chord between two points on the curve (secant modulus).
A limitation of extensiometer measurement of elasticity is that the range of in vivo corneal elasticity modulus in healthy or diseased tissue is unknown,17 and reports on animal and human tissues can span orders of magnitude.15,18 Additionally, while most biological soft tissues approximate linear elastic behavior when a small range of stresses are introduced, their overall elastic behavior is highly non-linear. Nevertheless, understanding elastic properties is important for evaluating the instantaneous response of the cornea to surgery and can affect its subsequent viscous behavior. Some studies have demonstrated, for example, a decrease in stiffness and increase in extensibility of keratoconic tissue relative to normal tissue.19 The elastic modulus was identified by Guirao20 in mathematical modeling as the most influential risk factor for posterior corneal steepening after keratorefractive surgery.
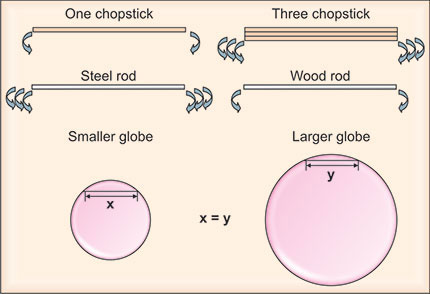
Figure1-1: The influence of structural and material properties upon the ability to deform the cornea. Bending a single chopstick is usually easy. However, bending three of the same type of chopsticks at once is much more difficult (top row). Hence, a larger deformation will be generated for thinner corneas given the same applied force. This partially explains the underestimation of IOP in eyes with thinner corneas. In contrast, it requires greater pressure to applanate or indent a thicker cornea, which contributes to overestimation of IOP in eyes with thicker corneas. Similarly, much more force is required to bend a steel rod than a wood rod of the same dimensions (middle row). The difference in this case is the elastic properties of the material, specifically Young's modulus. Steel has a much higher Young's modulus (w200 000 MPa) than wood (w10 000 MPa); therefore, if all other parameters are the same, it is much harder to deform a steel structure than a wood structure. Corneal curvature is another variable that can affect the accuracy of IOP measurement, possibly because of the difference in the volume of the displaced fluid after a given area is flattened (bottom row).17
Viscoelasticity extends the biomechanical response of biological tissues into complex mathematical descriptions of viscous fluids, where elastic responses are time and rate dependant.21 Viscoelastic materials return to their pre-stress shape via different stress-strain pathways that depend upon loading rates. Viscoelastic properties can be described through metrics of hysteresis, stress relaxation and creep. Viscoelastic creep is a time-dependent elongation of tissue (or increasing strain) that occurs under a sustained or constant stress (such as IOP).21 The effect of creep is a reduction in effective tissue stiffness, which can lead to a decrease in resistance to stretch. Creep may be a precursor to ectasia, where stressed collagen fibrils undergo a pathologic weakening without an initial change in length.6
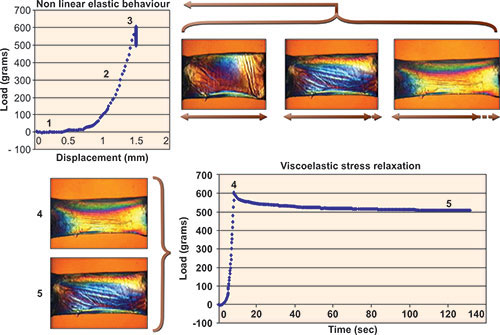
Figure 1-2: Experiments illustrating elastic and viscoelastic properties in a 7 mm, full-thickness horizontal corneal strip from a 63-year-old donor. Elliptical polarization allows visualization of non-homogeneous internal stresses. Progressive stretching of the sample (1, 2 and 3) and measurement of the induced load (stress) allows calculation of the elastic (Young's) modulus from the slope of the stress-strain relationship. The relationship is non-linear. A second experiment in which a constant displacement is imposed in the same sample demonstrates time-dependent stress relaxation, a viscoelastic property of biological soft tissues (4 and 5).(Courtesy of W.J. Dupps, Jr., MD, PhD and T. Doehring, PhD.16)
Once the collagen fibrils are weakened, a gradual stretching then occurs under constant stress or IOP. Viscoelastic stress relaxation refers to a situation where strain is increased then held constant (no more tissue elongation) while a slow but quantifiable time-dependent relaxation of the load is observed (Figure 1-2).
Hysteresis, in general, is a property of physical and biological systems that do not instantly follow the forces applied to them but react slowly or do not return completely to their original state.21 Hysteresis describes a lag between making a change, such as increasing or decreasing power, and the response or effect of that change. Whereas a rubber band can be described as elastic because is springs back to its original shape at the same rate as when it is stretched, a putty exhibiting viscoelastic behavior quickly assumes a new shape when pushed upon but will not immediately return to its original shape when the mechanical pressure is released. Another example of hysteresis is a thermostat set at 80 degrees, which actually regulates the room temperature between 78 and 82 degrees. In broad terms, corneal hysteresis can be thought of as a metric of the ability of the cornea to absorb energy.
Shear strength describes stromal resistance to lamellar sliding and bending. The shear resistance provided by collagen interweaving and other matrix forces has been estimated from metrics such as the interlamellar cohesive strength.7 Corneal shear strength is low relative to its tensile strength, but provides a mechanism for tensile load transfer between lamellae.9
Volumetric distension experiments provide a measure of whole globe stiffness, or ocular rigidity. The slope of a pressure-volume curve can be recorded during such experiments. Ocular rigidity is non-linearly dependent upon IOP and has been shown to increase with age.2 The utility of metrics describing ocular rigidity may be limited with respect to their impact on the understanding of corneal surgery, given the contributory role of the scleral and uveal tissue to ocular rigidity.
New Techniques for in vivo Measurement of Corneal Biomechanical Properties
While ex vivo diagnostic techniques, such as extensiometry, have provided valuable information on the biomechanical nature of normal and pathological corneas,22 a new era is dawning in biomechanical research with the development of techniques to measure structural and biomechanical properties in vivo. Imaging of the cornea can now be performed by confocal microscopy,23 very high frequency,24 7and optical coherence technology (See Section 2: Corneal Imaging). Holographic interferometry uses optical comparison to evaluate corneal elasticity.25,26 Another method, dynamic corneal imaging, uses stepwise central indentation of the cornea and computer analysis of videokeratography images during indentation to assess corneal elastic properties in vivo.27 A commercially available device that uses a dynamic, bidirectional, air-puff applanation to measure in vivo IOP and corneal viscoelastic properties is the Ocular Response Analyzer ([ORA], Reichert Ophthalmic Instruments, Depew, New York, USA).28
Biomechanics and Intraocular Pressure
Increasing attention has focused on the impact of corneal parameters, particularly central corneal thickness (CCT), on the measurement of IOP.29 IOP measurements have been demonstrated to vary with CCT using the Goldmann applanation,30 pneumotonometry,31 and non-contact tonometry.32 The deformation of the cornea during applanation is determined by an interaction of the external applied force with the intrinsic properties of the cornea. With the same applied force, a larger deformation will be produced for less rigid corneas. This partially explains the underestimation of IOP in eyes with thinner corneas. In contrast, it requires greater force to applanate a more rigid cornea, partially explaining the overestimation of IOP in eyes with thicker corneas. Additionally, alteration of corneal biomechanics by LASIK flap creation and excimer laser ablation affects the postoperative measurement of intraocular pressure (IOP) using Goldmann applanation tonometry (GAT).33–35 The impact of pachymetry on intraocular pressure readings has been recently highlighted by the Ocular Hypertension Treatment Study,36 which demonstrated an inverse relationship between CCT and the risk of developing glaucoma.
Goldmann tonometry is a static measurement, calculating IOP from the force applied during a steady state applanation of the cornea.37 Its design is based upon a number of assumptions, including that all corneas were of uniform thickness (i.e., 500 microns), that the eye's volume was spherical and that the cornea behaved biomechanically as an infinitely thin and perfectly flexible membrane. Corneal biomechanics embody far more than central pachymetry alone, and include viscosity, elasticity, hydration, regional pachymetry and other factors.17 As an example, whereas corneas from patients with keratoconus are generally thinner than average and biomechanically “floppy”, corneas from patients with Fuchs’ dystrophy are thicker than normal while they are also biomechanically similar to keratoconus.28 Other illustrative examples are the decrease in corneal rigidity following radial keratometry29 and hyperopic LASIK33 with subsequent drop in static applanation tonometry readings, yet little or no change in central corneal pachymetry. These examples highlight the complexity and potential flaws of simplistic attempts to linearly offset IOP based upon pachymetry alone.
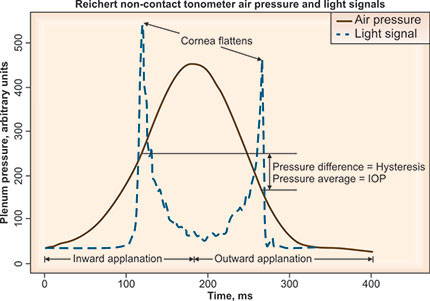
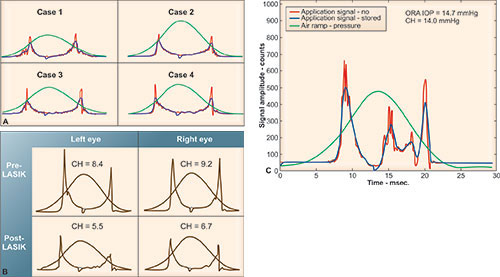
Alternative techniques that dynamically derive IOP from the corneal movement in response to a rapid air pulse simultaneously assess and compensate (to varying degrees) for the effect of the cornea's viscous and elastic qualities on IOP measurement. Reichert's ORA 28 utilizes a metered collimated air pulse to applanate the cornea and an infrared electro-optical system to record inward and outward applanation events. The air-pulse deforms the cornea through an initial applanation event (peak 1), then beyond into concavity and then gradually subsides, allowing the cornea to rebound through a second applanation (peak 2) (Figure 1-3). Corneal hysteresis (CH) is defined as the difference between the applanation pressure at peak 1 (P1) and peak 2 (P2), so that CH = P1-P2. This dynamic assessment of corneal biomechanical properties yields metrics of both the cornea's viscous and elastic qualities. Whereas corneal hysteresis may reflect mostly corneal viscosity, corneal resistance factor (CRF, defined by a linear function of P1 and P2) may predominantly quantify corneal rigidity. The equation used to determine CRF is: CRF = P1-0.7 × P2.
Pascal Dynamic Contour Tonometry (PDCT, Swiss Microtechnology AG; Port, Switzerland) employs a concave tip to “contour match,” rather than applanate, a convex segment of the central cornea and, thus, may be relatively independent of the effects of CCT or surgical intervention on IOP assessment.38–40
Figure 1-3: The waveform generated from the ocular response analyzer identifies the pressure difference, or hysteresis, during inward (peak 1) and outward (peak 2) applanation events during non-contact tonometry.43
Figure 1-4: Diurnal measurement of corneal hysteresis (left) and intraocular pressure (right) with the ocular response analyzer. While there was a statistically significant reduction in ORA IOP during the course of the day, diurnal measurements of hysteresis did not statistically differ.42
The instrument dynamically records over 100 IOP measurements per second, measuring IOP fluctuations throughout the cardiac cycle and digitally displaying the average diastolic IOP. Ocular Pulse Amplitude (OPA), the difference in IOP between systole and diastole (IOP systolic – IOP diastolic), is also reported and may be a marker for overall ocular rigidity,2 although it is also affected by ocular blood flow.
Clinical Applications of Hysteresis and Corneal Biomechanics
Corneal hysteresis in normal eyes has been reported to range from 5.0 to 18.7, with a mean hysteresis of 9.6 to 12.7 mm Hg.28,41,42 Hysteresis values did not show a statistical difference in a cohort of 21 normal patients between right and left eyes, with a mean difference of 0.4 mm Hg (p>0.08).42 Hysteresis values appear to be relatively insensitive to diurnal effects (Figure 1-4), although intrasubject variations have been observed. The correlation of CCT with CH and CRF was 0.59 and 0.62, respectively.41 Lower hysteresis was associated with visual field progression in a glaucomatous population.43
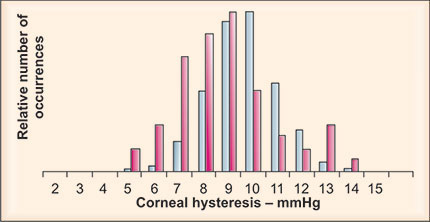
Corneas with keratoconus (Figure 1-5), Fuchs’ dystrophy and post-refractive surgery demonstrate a general decrease in corneal hysteresis compared to corneas in normal eyes.28 The low corneal hysteresis in the Fuchs’ eyes is seen despite unusually thick, but edematous, corneas. However, the large 99% confidence interval of corneal hysteresis seen in normal controls has considerable overlap with diseased and post-surgical corneas, limiting its diagnostic value as a single metric in individual cases. What may turn out to allow better diagnostic differentiation are the significant changes seen in applanation waveform in diseased or post-surgical corneas. Keratoconic and post-LASIK corneas appear to have similar applanation signal morphology, indicating reduced or low corneal viscoelastic properties in both cases (Figure 1-6). Investigations to quantify morphologic characteristics of the ORA waveform are now underway that attempt to extract additional corneal biomechanical information. Corneal hysteresis may be useful as a qualification factor for LASIK in corneas that have similar CCT but display significantly different waveform properties. Thus, the ORA waveform, along with the derived biomechanical metrics of corneal hysteresis and resistance factor, may provide a more complete characterization of corneal biomechanical properties than corneal thickness alone and is perhaps a better tool for assessing refractive surgery qualification and outcomes.
Figure 1-5: Distribution of hysteresis values in 339 normal (blue) and 60 keratoconic (red) eyes. Mean hysteresis for normal and keratoconic eyes was 9.6 mm Hg and 8.1 mm Hg, respectively.28
Figure 1-6: ORA applanation signal in keratoconic and post-LASIK eyes shows depressed applanation peak amplitudes and altered applanation peak widths. A: Case 1–4 are from keratoconic eyes. B: pre- and post-LASIK waveforms show a decrease in hysteresis postoperatively, along with reduced applanation peak amplitudes. C: waveform post penetrating keratoplasty shows marked alternation in the applanation signal, with increased noise during the applanation events.28
Modeling Based Upon Biomechanical Metrics: Implication for Surgical Planning
Models of the cornea have taken many forms, including complex computational models that integrate structural, biomechanical and optical corneal properties.44 Dupps and Wilson16 have proposed a strategy (Figure 1-7) for such modeling. By comparing these models to clinical experiments, useful models can be created, which can significantly improve our understanding of corneal biomechanics and allow us to better predict refractive effect after corneal transplant procedures.45 Results of finite element simulations indicate that significant changes in corneal refractive power could be introduced if refractive procedures (Figure 1-8) are combined with corneal transplants.
Figure 1-7: An approach to biomechanical modeling of surgery and disease in the cornea. Disease is simulated by alteration of the substructural components or their material properties. Surgery is simulated by imposing an ablation profile or incisions. The model is optimized retrospectively by comparing model simulations to analogous experiments in tissue or clinical models. A model optimized with clinical data can then be used prospectively to design and evaluate patient-specific treatment algorithms.16
Figure 1-8: Major biomechanical loading forces in the cornea and a model of biomechanical central flattening associated with disruption of central lamellar segments. A reduction in lamellar tension in the peripheral stroma reduces resistance to swelling and an acute expansion of peripheral stromal volume results. Interlamellar cohesive forces and collagen interweaving, whose distribution is greater in the anterior and peripheral stroma and is indicated by grey shading, provide a means of transmitting centripetal forces to underlying lamellae. Because the central portions of these lamellae constitute the immediate postoperative surface, flattening of the optical surface occurs, resulting in hyperopic shift. The degree of flattening is associated with the amount of peripheral thickening. This phenomenon is exemplified clinically by PTK-induced hyperopic shift but is important in any central keratectomy, including PRK and LASIK. Simultaneous elastic weakening of the residual stromal bed may occur, and the threshold for inducing irreversible (plastic) or progressive (viscoelastic) steepening (or ectasia) is a matter of great clinical concern.16
This requires high precision resections of corneal grafts, which may improve with the application of femtosecond laser technology.46 Treatment of the cornea with riboflavin and UVA to increase collagen cross-linking 47 may also allow us to modulate the stiffness of the cornea before or after corneal transplant procedures.
Conclusion
A review of the histology of corneal fibrils indicates evidence for inextensibility, under a wide range of physiologic conditions, which appears to be the basis for stability of refraction and corneal curvature. Given the major biomechanical loading forces of the normal cornea, surgical disruption of the central corneal lamellae may lead to central corneal flattening and peripheral thickening.1 Studying the dynamics of corneal shape changes that occur in response to collimated air pulses (via the Ocular Response Analyzer) may provide a basis for understanding the biomechanical effects of incisional and lamellar corneal surgery. The in vitro interactions of corneal fibroblasts and a fibrillar collagen substrate48 can be combined with advanced structural and functional in vivo diagnostic imaging techniques to develop mathematical models of the wound healing and viscoelastic features of the normal and post-surgical cornea. As our understanding of these processes improves, so will our ability to offer rational interventions and strategies for further improving the predictability of keratorefractive surgery and minimizing its complications.
References
- Roberts C. The cornea is not a piece of plastic. J Refract Surg 2000;16:407–13.
- Pallikaris IG, Kymionis GD, Ginis HS, Kounis GA, Tsilimbaris MK. Ocular rigidity in living human eyes. Invest Ophthalmol Vis Sci 2005;46:409–14.
- Reinstein DZ, Roberts C. Biomechanics of corneal refractive surgery. J Refract Surg 2006;22:285.
- Maurice DM. The cornea and sclera. In: Davson, H. (Ed.), The Eye. Academic Press, Orlando, FL, 1984;1–158.
- Meek KM, Boote C. The organization of collagen in the corneal stroma. Exp Eye Res 2004;78:503–12.
- Radner W, Zehetmayer M, Aufreiter R, Mallinger R. Interlacing and crossangle distribution of collagen lamellae in the human cornea. Cornea 1998;17:537–43.
- Smolek MK, McCarey BE. Interlamellar adhesive strength in human eyebank corneas. Invest Ophthalmol Vis Sci 1990;31: 1087–95.
- Meek KM, Newton RH. Organization of collagen fibrils in the corneal stroma in relation to mechanical properties and surgical practice. J Refract Surg 1999;15:695–9.
- Dupps WJ, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg 2001;17:658–69.
- McMonnies CW, Schief WK. Biomechanically coupled curvature transfer in normal and keratoconus corneal collagen. Eye Contact Lens 2006;32:51–62.
- Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci 1991;32:2244–58.
- Muller LJ, Pels E, Vrensen JM. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Ophthalmology 2001;85:437–43.
- Doughty MJ, Bergmanson JPG. Collagen fibril characteristics at the corneo-scleral boundary and rabbit corneal swelling. Clin Exp Optom 2004;87:81–92.
- Sjontoft E, Edmund C. In vivo determination of Young's modulus for the human cornea. Bull Math Biol 1987;49:217–32.
- Jue B, Maurice DM. The mechanical properties of the rabbit and human cornea. J Biomech 1986;19:847–53.
- Dupps WJ Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–20.
- Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement Quantitative analysis. J Cataract Refract Surg 2005;31:146–55.
- Hoeltzel DA, Altman P, Buzard K, Choe K. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng 2002;114:202–15.
- Edmund C. Corneal topography and elasticity in normal and keratoconic eyes. A methodological study concerning the pathogenesis of keratoconus. Acta Ophthalmol Suppl 1989; 193:1–36.
- Guirao A. Theoretical elastic response of the cornea to refractive surgery: risk factors for keratectasia. J Refract Surg 2005; 21:176–85.
- Dupps WJ. Biomechanical modeling of corneal ectasia. J Refract Surg 2005;21:186–90.
- Sherwin T, Brookes NH. Morphological changes in keratoconus: pathology or pathogenesis. Clin Exp Ophthalmol 2004;32:211–7
- Reinstein DZ, Silverman RH, Raevsky T, Simoni GJ, Lloyd HO, Najafi DJ, Rondeau MJ, Coleman DJ. Arc-scanning very high frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16:414–30
- Smolek MK. Holographic interferometry of intact and radially incised human eye-bank corneas. J Cataract Refract Surg 1994; 20:277–86.
- Jaycock PD, Lobo L, Ibrahim J, Tyrer J, Marshall J. Interferometric technique to measure biomechanical changes in the cornea induced by refractive surgery. J Cataract Refract Surg 2005;31:175–84.
- Grabner G, Eilmsteiner R, Steindl C, Ruckhofer J, Mattioli R, Husinsky W. Dynamic corneal imaging. J Cataract Refract Surg 2005;31:163–74.
- Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg 2005:31:156–62.
- Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol 2000;44:367–408.
- Lleo A, Marcos A, Calatayud M, Alonso L, Rahhal SM, Sanchis-Gimeno JA. The relationship between central corneal thickness and Goldmann applanation tonometry. Clin Exp Optom 2003;86:104–8.
- Morgan AJ, Harper J, Hosking SL, Gilmartin B. The effect of corneal thickness and corneal curvature on pneumotonometer measurements. Curr Eye Res 2002;25:107–12.
- Stabuc SM, Hawlina M. Influence of corneal thickness on comparative intra-ocular pressure measurements with Goldmann and non-contact tonometers in keratoconus. Klin Montasbl Augenheilkd 2003;220:843–7.
- Jarade EF, Abi Nader FC, Tabbara KF. Intraocular pressure measurement after hyperopic and myopic LASIK. J Refract Surg 2005; 21:408–10.
- Svedberg H, Chen E, Hamberg-Nystrom H. Changes in corneal thickness and curvature after different excimer laser photorefractive procedures and their impact on intraocular pressure measurements. Graefes Arch Clinic Exp Ophthalmol 2005; 243:1218–20.
- Chang DH, Stulting RD. Change in intraocular pressure measurement after LASIK: the effect of the refractive correction and the lamellar flap. Ophthalmology 2005:112:1009–16.
- Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 2001;108:1779–88.
- Goldmann H, Schmidt T. On applanation tonography. Ophthalmologica 1965;150:65–75.
- Siganos DS, Papastergiou GI, Moedas C. Assessment of the Pascal dynamic contour tonometer in monitoring intraocular pressure in unoperated eyes and operated eyes after LASIK. J Cataract Refract Surg 2005;31:458–9.
- Duba I, Wirthlin AC. Dynamic contour tonometry for post-LASIK intraocular pressure measurements. Klin Monatsbl Augenheilkd 2004:22:347–50.
- Kaufman C, Bachmann LM, Thiel MA. Intraocular pressure measurements using dynamic contour tonometry after laser in situ keratomileusis. Invest Ophthalmol Vis Sci 2003;44:3790–4.
- Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CA. Changes in corneal biomechanics and in intraocular pressure pre- and post-LASIK using static, dynamic and non-contact tonometry. Am J Ophthalmol 2006; accepted.
- Laiquzzaman M, Bhojwani R, Cunliffe I, Shah S. Diurnal variation of ocular hysteresis in normal subjects: relevance in clinical context. Clin Experiment Ophthalmol 2006;34:114–8.
- Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 2006;141:868–75.
- Buzard KA. Introduction to biomechanics of the cornea. Refract Corneal Surg 1992;8:127–38.
- Cabrera Fernandez D, Niazy AM, Kurtz RM, Djotyan GP, Juhasz T. Biomechanical model of corneal transplantation. J Refract Surg 2006;22:293–302.
- Sikder S, Snyder RW. Femtosecond laser preparation of donor tissue from the endothelial side. Cornea 2006;25:416–22.
- Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg 2006;32:279–83.
- Petroll WM, Cavanagh HD, Jester JV. Dynamic three-dimensional visualization of collagen matrix remodeling and cytoskeletal organization in living corneal fibroblasts. Scanning 2004;26:1–10.