1A. REACTIONS OF MONOSACCHARIDES
INTRODUCTION
Carbohydrates are aldehyde or ketone derivatives of polyhydric alcohols. They are widely distributed in plants and animals. Plants synthesize glucose by photosynthesis and it is converted mainly to storage form, the starch and structural frame work form, the cellulose.
Animals largely depend on plant source to obtain carbohydrates though they can synthesize carbohydrates from non carbohydrates sources like glycerol and amino acids in their body (gluconeogenesis).
The glucose is the major form of carbohydrate absorbed from the gut in humans.
According to the metabolic status it has different fates–
- catabolized to release energy
- polymerized to form the storage fuel—the glycogen
- sometimes converted to other sugars like fructose and galactose.
Different carbohydrates are present in intracellular and extracellular fluids and are excreted in urine when the concentration of them rises in the blood as in certain diseases (glucose in urine in diabetes mellitus, fructose in urine in fructosuria, galactose in urine in galactosemia). Hence, it is essential to understand the tests for their detection.
The classification of carbohydrates will be useful for the detection of various types of carbohydrates by different chemical tests.
CLASSIFICATION
- Monosaccharides: Cannot be hydrolyzed into simpler carbohydrates. They are classified into trioses, tetroses, pentoses, hexoses, heptoses based on the number of carbon atoms present in them. They are again divided into aldoses and ketoses based on the functional group present in them (see Table 1A-1).
|
- Disaccharides: Give rise to two monosaccharide units upon hydrolysis
E.g.: | Sucrose (glucose + fructose) |
Lactose (glucose + galactose) | |
Maltose (glucose + glucose) |
- Oligosaccharides: yields less than ten monosaccharides.
E.g.: | Maltotriose (3 glucose units), |
Raffinose (glucose + fructose + galactose) |
- Polysaccharides: Contain more than ten monosaccharide units
- Homopolysaccharides (consisting of same type of monomeric units)Polymer of glucose: Starch, glycogen, cellulosePolymer of fructose: Inulin
- Heteropolysaccharides (consisting of different types of monomeric units)
Proteoglycans, e.g. Heparin (D-glucosamine sulfate + D-sulfated iduronic acid)
Hyaluronic acid (D-β glucuronic acid + N-acetylglucosamine).
REACTIONS OF MONOSACCHARIDES
Monosaccharides possess one or more hydroxyl groups and an aldehyde or keto group. Therefore many reactions of monosaccharides are the known reactions of alcohols, aldehydes or ketones. Many of the reactions shown by monosaccharides are exhibited by higher carbohydrates also. Differences in the structures of sugars often affect the rate of a reaction and sometimes the ability to react.
The reactions described below, are applied in the identification of sugars.
The reactions due to hydroxyl group:
- Dehydration (e.g. Molisch test, Rapid furfural test, Seliwanoff's test)
The reactions due to carbonyl group:
- Reduction (e.g. Benedict's test, Barfoed's test)
- Condensation (e.g. Osazone test)
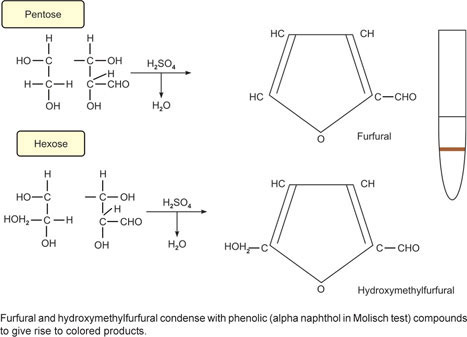
1. Molisch Test (α-Naphthol Reaction) (Fig. 1A-1)
Procedure: To 5 ml of sugar solution in a test tube add two drops of Molisch reagent. Mix thoroughly. Add 3 ml of concentrated sulphuric acid along the sides of the test tube by slightly inclining the tube, thus forming a layer of acid (acid being heavier goes down beneath the sugar solution) in the lower part.
Observation: A reddish violet ring appears at the junction of two liquids.
Inference: Indicates presence of a carbohydrate and hence the presence of monosaccharide.
Principle: Concentrated acid dehydrates the sugar to form furfural (in the case of pentoses) or furfural derivatives (hexoses and heptoses) which then condense with α-naphthol to give a reddish violet colored complex
Application of the test: Used as a general test to detect carbohydrates.
Aberrant Observations
- Instead of a violet ring in the Molisch test, appearance of dark brown color indicates charring of sugar due to the heat generated during the addition of acid (acid water interaction generates heat). It will become obvious when the concentration of the sugar solution is high. To avoid charring, dilute the sugar sample solution with water as depicted in Figure 1A-2 and repeat the Molisch test.
- Appearance of a green color while doing the test, which persist even after completion of the test suggest excess use of Molisch reagent than required or due to the presence impurities in the reagent.
2. Benedict's Test (Fig. 1A-3)
Procedure: To 5 ml of Benedict's reagent in a test tube add exactly 8 drops of the sugar solution. Mix well. Boil the solution vigorously for two minutes or place in a boiling water bath for three minutes. Allow the contents to cool by keeping in a test tube rack. Do not hasten cooling by immersion in cold water.
Observation: The entire body of the solution will be filled with a precipitate, the color of which varies with the concentration of the sugar solution—green, yellow, orange or red.
In the absence of reducing substance, blue color of the Benedict's reagent remains as such. The test is sensitive up to 0.1-0.15 gm% of sugar solution (that is Benedict's test will not be 6positive with solutions containing less than 0.1-0.15 gm% of sugar).
Inference: Reducing monosaccharides, glucose, fructose, galactose and mannose give a positive reaction with Benedict's reagent.
The color of the precipitate give an idea about the concentration of the sugar solution as shown below.
- Blue – absence of reducing sugar
- Green – up to 0.5 gm%
- Yellow – > 0.5 to 1.0 gm%
- Orange – > 1.0 to 2.0 gm%
- Brick red – ≥ 2 gm%
Thus, Benedict's test is described as a semi-quantitative test.
Principle: (see Fig. 1A-4) Carbohydrates with a free aldehyde or keto group have the ability to reduce various metallic ions. In this test cupric ions are reduced to cuprous ions by the enediols formed from sugars in the alkaline medium of Benedict's reagent.
Benedict's reagent contains copper sulphate, sodium citrate and sodium carbonate.
Copper sulphate dissociate to give sufficient cupric ions (in the form of cupric hydroxide) for the reduction reactions to occur.
Sodium citrate keeps the cupric hydroxide in solution without getting precipitated.
Sodium carbonate (Na2CO3) make the pH of the medium alkaline.
In the alkaline medium sugars form enediols which are powerful reducing agents. They reduce blue cupric hydroxide to insoluble yellow to red cuprous oxide.
Application of the test: To detect reducing sugars. It is widely used in detecting glucose in urine even though not specific for glucose.
3. Barfoed's Test (Fig. 1A-5)
Procedure: To 5 ml of Barfoed's reagent in a test tube add 0.5 ml of sugar solution. Mix well. Keep in a boiling water bath for 2 minutes. Keep the tube in a test tube rack and examine for precipitate after 10–15 minutes.
Observation: A red precipitate clinging to the bottom most part of the test tube forms, in the presence of a monosaccharide.
Inference: The test is answered by monosaccharides only, e.g. glucose, fructose, galactose, mannose.
Principle: It is a reduction test. Reducing property owes to the carbonyl group (aldehyde or keto group). Barfoed's reagent is copper acetate in acetic acid.
Difference between Barfoed's test and Benedict's test: Barfoed's test differs from Benedict's test with respect to the pH of the medium. It is alkaline in the case of Benedict's and acidic in the case of Barfoed's test. In the acid medium monosaccharides enolize much more readily than disaccharides and these enediols reduce cupric ions released by copper acetate of Barfoed's reagent to produce a red precipitate.
Points to Ponder
- It is important to keep the time limit (2 minutes) prescribed for Barfoed's test otherwise disaccharides will also respond to the test positively.
- Disaccharides when present in high concentrations (> 5 gm%) also will give positive response
- Unlike the Benedict's test, Barfoed's test is unsuitable for testing sugars in urine or any fluids containing chloride.
- The red precipitate is formed at the bottom of the tube. To see the precipitate, lift the tube to the eye level, otherwise the precipitate formed adhering to the bottom most part of the tube may escape notice.
Application of the test: Useful to distinguish between monosaccharides and disaccharides.
Chemistry of the test: Reduction reaction as shown under Benedict's test.
4. Rapid Furfural Test
Procedure: To 2 ml of sugar solution add 6 drops of Molisch reagent and 3 ml of concentrated HCl. Boil for 30 seconds only.
Inference: Development of violet color within 30 seconds of boiling indicates presence of a keto sugar, e.g. fructose.
Principle: A dehydration reaction which owe to the hydroxyl groups of the sugar. Concentrated HCl being weaker than concentrated sulphuric acid, dehydrate ketoses (e.g. fructose) more readily than aldoses to form hydroxymethyl furfural, which then condenses with α-naphthol to form a violet colored complex.
Chemistry of the test: Dehydration reaction as shown under Molisch test.
Aberrant reaction: If red color develops instead of violet color due to charring action of acid, dilute the sugar sample with water and conduct the test with diluted sugar solution (Fig. 1A-7).
Application of the Test
- For the detection of ketoses.
- Useful for differentiating ketoses from aldoses.
5. Seliwanoff's Test
Procedure: To 5 ml of Seliwanoff's reagent in a test tube add 5 drops of fructose solution and heat the contents to just boiling.
Inference: This test is given by ketoses. e.g. fructose.
Principle: A dehydration reaction due to the hydroxyl groups of the sugar. Selivanoff's reagent is resorcinol in dilute hydrochloric acid. Ketoses (e.g. fructose) are more readily dehydrated by HCl than the aldoses to form hydroxymethyl furfural which then condenses with resorcinol of Seliwanoff's reagent to form a red colored complex.
Points to Ponder
- The test is sensitive up to 0.1 gm% of fructose in the absence of glucose.
- In the presence of glucose, the test becomes less sensitive to fructose.
- Large amounts of glucose gives the same color.
- If the boiling is prolonged a positive reaction may occur with glucose because of Lobry de Bruyn-van Ekenstein transformation of glucose into fructose in the presence of acid.
The precautions to be followed to get a positive test for fructose are given below:
- Concentration of HCl used must be less than 12%.
- The reaction must be observed within 20 to 30 seconds of performing the test.
- Those reactions occurring after 20–30 seconds, must not be taken into account.
- Glucose must not be present in amounts more than 2% or else it will interfere with the test.
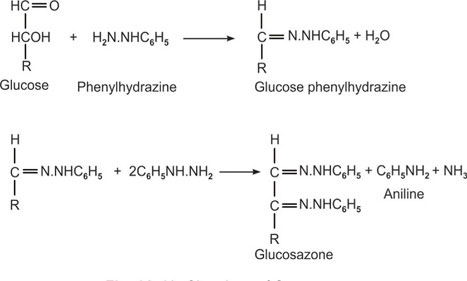
6. Osazone Test
Procedure: To 5 ml of sugar solution in a test tube add 300 mg (one or two scoopfuls) of phenyl hydrazine mixture. Shake well. Heat in a boiling water bath for 15 minutes. Then take the tube out of the water bath and allow cooling at room temperature by placing it in the test tube rack.
Avoid showing under the tap water because rapid cooling disturbs crystallization where as slow cooling ensures crystallization (ideally within the water bath itself).
Observation: Crystals are formed readily (within 1–5 minutes) at the room temperature in the case of mannose. For other sugars minimum time required in minutes in the water bath for the formation of insoluble yellow osazone is given in the Table 1A-2.
Look under the microscope to view the crystals (see Fig. 1A-9).
|
Inference: Glucose, fructose, mannose yield the same shaped phenyl osazone crystals because of the elimination of differences in configuration about the carbon atoms 1 and 2 during osazone formation.
Principle: The reaction involves the carbonyl carbon (either aldehyde or ketone as the case may be) and the adjacent carbon. One molecule of sugar reacts with one molecule of phenyl-hydrazine to form phenylhydrazone which then reacts with two additional phenyl hydrazine molecules to form the osazones as shown in the figure 1A-10.
Points to Ponder
If the solution appears red after heating process, it indicates that the solution has become concentrated in the boiling process and no crystals will separate in the concentrated form. So dilute with water for the separation of crystals.
1B. REACTIONS OF DISACCHARIDES
INTRODUCTION
Disaccharides are glycosides in which both components are monosaccharides. The general formula of common disaccharides is C12H22O 11. The common disaccharides studied are detailed below.
Maltose (α-D-glucopyranosyl-(1→4) α-D-glucopyranose) (Fig. 1B-1): Maltose yield 2 glucose molecules upon hydrolysis. Maltose is formed from the hydrolysis of starch by the action of the enzyme maltase. It is also produced as an intermediate product of mineral acid hydrolysis of starch. It is dextrorotatory, exhibits mutarotation, reduces metallic ions in alkaline solutions. Like other disaccharides maltose is hydrolyzed by dilute acid leading to the formation of two molecules of glucose. With phenyl hydrazine maltose forms maltosazone.
Examples for other disaccharides that produce only glucose upon hydrolysis:
- Cellobiose a β glucoside with 1,4 linkage derived from partial hydrolysis of cellulose.
- Gentiobiose, a β glucoside with 1,6 linkage derived from roots of Gentiana lutea.
- Trehalose, α glucoside with 1,1 linkage obtained from yeast and mushrooms.
- Isomaltose, α glucoside with 1,6 linkage formed as a side product of hydrolysis of starch by amylase enzyme.
Lactose (β-D-galactopyranosyl-(1→4) β-D-glucopyranose) (Fig. 1B-2): Lactose give rise to one molecule of glucose and galactose upon enzymatic (lactase) or acid hydrolysis. Lactose is normally present in milk and in the urine of women during later half of pregnancy and during lactation. It is dextrorotatory, shows mutarotation in solution. It reduces metallic ions, forms lactosazone with phenylhydrazine. It is a galactoside since the carbon number 1 of galactose is involved in the β galactoside bond with the carbon number 4 of glucose.
Sucrose (α-D-glucopyranosyl-β-D fructofuranoside): (see Fig. 1B-3).
Hydrolysis of sucrose yields one molecule of glucose and one molecule of fructose. Sucrose is dextrorotatory. After hydrolysis by enzymes or weak acids, it becomes levorotatory. This is because of the formation of fructose upon hydrolysis, which is strongly levorotatory than the glucose. Thus the change of optical rotation of sucrose solution from dextro to levo rotation upon hydrolysis is known as inversion and the mixture of glucose and fructose obtained is called invert sugar.
Sucrose do not reduce metallic ions (do not answer Benedict's and Barfoed's tests) and also do not form osazone with phenylhydrazine.
But prolonged boiling with phenylhydrazine in acid medium will form osazone due to the reaction of products of hydrolysis of sucrose with phenylhydrazine and not due to the reaction of intact sucrose molecules with phenylhydrazine.
REACTIONS OF DISACCHARIDES
1. Molisch Test
Principle: Response of the disaccharides: All the disaccharides that are experimented routinely give the positive reaction – reddish violet ring as this is a general test to detect the presence of carbohydrate.
2. Benedict's Test
Response of the Disaccharides
Based on Benedict's test disaccharides are classified into:
- Reducing disaccharides, e.g. Lactose, Maltose. These disaccharides have a free carbonyl (keto/aldehyde) group which is not involved in glycosidic linkage will reduce cupric ions in the alkaline medium as explained under the monosaccharides, E.g. Lactose, maltose.
- Nonreducing disaccharidesE.g. Sucrose, Trehalose. These are the disaccharides in which the functional groups of constituent monaosaccharides are in linkage.
3. Barfoed's Test
Principle: Response of the disaccharides: Disaccharides will not reduce cupric ions in the weak acid medium within the prescribed keeping time of 2 minutes in the boiling water bath and do not give a positive response to the test.
Application: Useful to differentiate monosaccharides from disaccharides.
Points to Ponder
- If the heating time is prolonged disaccharides will also give a positive response to Barfoed's test.
- If the concentration of disaccharide solution is high, Barfoed's test tends to become positive.
4. Osazone Test
Procedure: Same as given under monosaccharides except for the period for which the reaction tube to be placed in the boiling water bath – it is 45 minutes for disaccharides.
Lactose gives a characteristic yellow puff shaped lactosazone crystals (see Fig. 1B-4).
Maltose: Individual crystals of maltosazone looks like a yellow colored petal and when grouped looks like a sun flower (see Fig. 1B-5).
Inference
Lactose | → | Puff shaped lactosazone crystals |
Maltose | → | Petal shaped or sunflower shaped maltosazone crystals |
Sucrose | → | will not form osazone |
Principle: Reducing disaccharides with a reactive carbonyl group condense with phenyl hydrazine to form respective osazone crystals with characteristic shapes as detailed above.
Application: Useful to differentiate disaccharides.
5. Seliwanoff's Test
Procedure: Same as given under monosaccharides.
Observation: Sucrose gives bright red color (see fig. 1A-8) whereas lactose and maltose do not give red color.
Inference: Sucrose upon acid hydrolysis by the HCl in the Seliwanoff's reagent yields a keto sugar, fructose. Fructose being a keto sugar gives positive response to Seliwanoff's test as described under monosaccharides. Whereas lactose (galactose + glucose) and maltose (glucose + glucose) contain no keto sugar and cannot give positive response to this test upon acid hydrolysis by the HCl present in the Seliwanoff's reagent.
Principle: The disaccharide sucrose contains glucose and fructose. Fructose formed from sucrose upon acid hydrolysis by the HCl of Seliwanoff's reagent, is dehydrated by the acid HCl to form hydroxymethyl furfural which then condenses with the resorcinol of Seliwanoff's reagent to form a red colored complex.
6. Rapid Furfural Test
Procedure: Same as given under monosaccharides.
Observation: Sucrose gives violet color (see Fig. 1A-6) whereas lactose and maltose do not give violet color.
Inference: Sucrose upon acid hydrolysis by the HCl added in the test yields a keto sugar fructose. Fructose being a keto sugar gives positive response to Rapid furfural test as described under monosaccharides. Where as lactose (galactose + glucose) and maltose (glucose + glucose) contain no keto sugar and cannot give positive response to this test.
Principle: The disaccharide sucrose contains glucose and fructose. Fructose formed from sucrose upon acid hydrolysis by the HCl, is dehydrated by the same HCl to form hydroxymethyl furfural which then condenses with the α-naphthol of Molisch reagent to form a violet colored complex.
7. Specific Sucrose Test (Fig. 1B-6)
Procedure: It is done in two steps.
Step 1: Hydrolysis
To 5 ml of sucrose solution add 1 drop of thymol blue indicator and one or two drops of dilute HCl to make the solution acidic as shown by the development of pink color. Divide it into two equal parts. Boil one part for 1 minute and the other part is kept as control. Neutralize both parts 13by adding 2% sodium carbonate drop by drop until a blue color develops.
Step 2: Benedict's Test
Perform Benedict's test with each portions.
Observation: Unboiled sucrose solution will not give a positive response to Benedict's test where as boiled portion gives a positive response.
Inference: Sucrose is hydrolyzed by HCl in the first step to form glucose and fructose and the medium is neutralized by the 2% sodium carbonate.
In the second step, products of acid hydrolysis reduce cupric ions to red cuprous oxide.
Precautions
- Avoid adding excess acid because it will dehydrate sugar to form furfural derivatives and that will interfere the test.
- Always remember to add alkali as per the test procedure since neutralization of acidic pH is needed for getting correct reaction in the second step.
Thymol blue indicator contains two components that work at acid range (pH range 1.2–2.8; color change – red to yellow) and at alkaline range (pH range 8.0–9.6; color change – yellow to blue).
1C. REACTIONS OF POLYSACCHARIDES
INTRODUCTION
The polysaccharides are complex carbohydrates of high molecular weight, which on hydrolysis yields monosaccharides or products related to monosaccharides. The various polysaccharides differ from one another with respect to their constituent monosaccharide composition, molecular weight and other structural features.
In all types the linkage between the monosaccharide units is the glycosidic bond.
This may be α or β which join the respective units through 1 → 2, 1 → 3, 1 → 4 or 1 → 4 linkages in the linear sequence or at branch points in the polymer.
Polysaccharides are classified based on the type of monosaccharide units present in them.
1. Homopolysaccharide
Contains only one type of monosaccharide, e.g. Starch, Glycogen.
2. Heteropolysaccharide
Contains more than one type of monosaccharide units, e.g. Glycosaminoglycans (heparin, hyaluronic acid).
We will discuss the reactions of starch in this chapter in order to understand the chemical properties of polysaccharides in general.
REACTIONS OF STARCH
1. Molisch Test
Procedure:
Observation and Inference: Same as given under monosaccharides.
Principle: The test is answered by all furfural yielding substances and hence all the carbohydrates.
2. Iodine Test
Procedure: To 2–3 ml of starch solution add 2 drops of dilute (0.05 N) iodine solution. Observe the changes on heating and on subsequent cooling.
Observation: Deep blue color appears which then disappears on heating and then reappears on cooling (see fig. 1C-1).
Inference: Starch forms a adsorption complex with iodine to give a blue color. The blue color disappears on heating due to the breaking of the Iodine starch adsorption complex and appears on cooling due to reformation of the adsorption complex.
3. Benedict's Test
Procedure: Same as given with monosaccharides.
Observation: No colored precipitate.
Inference: Starch is a nonreducing carbohydrate.
4. Starch Hydrolysis Test
Procedure: Take 25 ml of starch solution in a beaker. Add 10 drops of concentrated HCl and boil gently. At the end of each minute, transfer a drop (using glass tube) of the solution on to a plate for doing the iodine test and 3 drops to 5 ml of Benedicts solution (Set tubes containing 5 ml of Benedict's reagent in series). Continue until the iodine test becomes negative. Then place the tubes for the Benedict's test in the boiling water bath for 3 minutes.
Observation: See Table 1C-1.
Inference: Starch upon hydrolysis by HCl gives the following products.
Starch → Soluble starch → Amylodextrins → Erythrodextrins → Achrodextrins → Maltose → Glucose. When the hydrolytic stage reaches to the level of formation of maltose and glucose iodine test becomes negative and Benedict's test becomes positive.
1D. IDENTIFICATION OF UNKNOWN CARBOHYDRATES
1E. QUESTIONS
- Name the following:
- General test for detecting carbohydrates
- Reduction test for monosaccharides
- Sugars giving positive response for Rapid furfural test and Seliwanoff's test
- The disaccharide yielding puff shaped osazone crystals
- Tests based on reduction property of sugars
- The test used to detect sugar in urine
- Reducing disaccharides
- Nonreducing disaccharides
- Give the principle of the following tests:
- Molisch test
- Benedict's test
- Barfoed's test
- Osazone test
- Iodine test for starch
- Rapid furfural test
- Seliwanoff's test
- Give the ingredients of following reagents:
- Molisch's reagent
- Benedict's reagent
- Barfoed's reagent
- Seliwanoff's reagent
- Benedict's test is described as a semiquantitive test. Explain.
- Unlike Benedict's test, Barfoed's test is not suitable for testing glucose in urine. Why?
- Give the difference between Benedict's and Barfoed's test.
- Why do glucose, mannose and fructose give similar osazone crystals?
- Sucrose do not form osazone crystals with osazone test. Why?
- Make a scheme for the detection of an unknown carbohydrate solution.
1F. REAGENT PREPARATION
- Molisch's Reagent: Dissolve 5 g of α-naphthol in 100 ml of 95% of alcohol.
- Benedict's Qualitative Reagent: Heat to dissolve 173 g sodium citrate and 100 g sodium carbonate in about 800 ml of water in a conical flask. Transfer to a graduated cylinder through a folded filter paper placed in a funnel or beaker of 1L capacity. Dissolve 17.3 g copper sulfate in about 100 ml of water. Add the copper sulfate solution slowly with constant stirring to the carbonatecitrate solution and make up to 1L.
- Barfoed's Reagent: Dissolve 13.3 g neutral copper acetate crystals in 200 ml water. Pass through a filter paper placed in a funnel to remove the particles if present to another graduated beaker. Then add 1.8 ml glacial acetic acid.
- Seliwanoff's Reagent: Dissolve 0.05 g resorcinol in 100 ml dilute HCl.
- Phenylhydrazine Mixture: Mix 2 parts phenyl-hydrazine hydrochloride and 3 parts sodium acetate by weight thoroughly in a mortar (Mixture with longer shelf life may be prepared by using equal weights of phenylhydrazine hydrochloride and anhydrous sodium acetate).
- 0.1 N iodine Solution: Dissolve 1.27 g iodine and 3 g pure KI (potassium iodide) crystals in 100 ml distilled water. Dilute 1:10 in distilled water before use.
- Glucose, Fructose, Lactose, Maltose, Sucrose, Starch Solutions: 1% solutions-Weigh 1 gm of respective sugars and dissolve in 100 ml of water.