- Cataract Etiology
- Biochemistry of the Lens
- History of Phacoemulsification
- Intraocular Lens Power Calculations
- IOL Power Calculation in Complex Cases
- IOLMaster for Determining the IOL Power at the Time of Surgery
- Corneal Topography in Cataract Surgery
INTRODUCTION
The term cataract is derived from the Latin cataracta and from the Greek katarraktes which denotes a waterfall or a portcullis. Analogously a cataract is a complete or partial opacification of sufficient severity, on or in the human lens or capsule, to impair vision.
Vision is one of the most valued senses. Proper vision is achieved by a series of eye tissues working harmoniously in concert. Most eye debilities involve dysfunction in the lens or retina, and hence this chapter will focus on and elucidate etiological factors which may affect the proper function of the lens as target organ.
The lens is an elegantly simple tissue. It is made up of only two types of cells.
- Epithelial cells, which have not yet completely differentiated and not yet elaborated the major gene products, and
- Fiber cells, in which these processes have been initiated or even completed.
Cataract is one of the major causes of visual impairment leading eventually to blindness. In the USA alone 1.35 million cataract extractions are performed annually. In developing countries, the magnitude of the problem is overwhelming.
Management of this age-old impairment of vision requires one of the three following approaches, or a combination of these approaches.
- Surgical, i.e. extracapsular lens extraction (either manually or by phacoemulsification) and intraocular lens (IOL) implantation;
- Development and application of drug-related strategies to counteract the development of cataract;
- Identification and elimination of risk factors.
It is now well-established that cataract formation is a multifactorial disease. Several of the etiological factors are constitutional and hence difficult to manipulate. Others are environmental in nature and a little easier to control whilst a significant number are behavioral in nature and fall well within the individuals' own ability to control or modify.
- This review will briefly touch on congenital and infantile cataract but will focus on etiological factors in adults (Figure 1.1) and especially those implicated as risk factors in age-related cataract.
CONGENITAL AND INFANTILE CATARACT
Congenital cataract is numerically the most important cause of remediable blindness in children, being far more common than, for example, retinoblastoma or congenital glaucoma.
The prevalence of infantile cataract has been reported to be between 1.2 and 6 cases per 10,000 births. Furthermore, it has been estimated that between 10% and 38.8% of all blindness in children is caused by congenital cataract (Figures 1.2 to 1.4) and that one out of every 250 newborns (0.4%) has some form of congenital cataract.
The etiology of infantile cataract (Table 1.1) can be established in up to one-half of children with bilateral cataract, but in a smaller proportion of infants with unilateral cataract. Infantile cataract most commonly occurs secondary to genetic or metabolic diseases, intrauterine infections or trauma. Less commonly they may occur as a side effect of treatment with certain medications or radiation therapy.
FIGURE 1.4: Congenital cataract with coloboma of the lens(Courtesy: Dr Agarwal's Eye Hospital, India)
GENETIC
Infantile cataract may be inherited as autosomal dominant, autosomal recessive or X-linked recessive traits. Autosomal dominant cataracts are most commonly bilateral nuclear opacities, but marked variability can be present even within the same pedigree. In an extended pedigree of 28 patients with autosomal dominant nuclear cataract, Scott et al reported that 19 of the affected family members had unilateral cataract while 9 had bilateral cataract. Less commonly, anterior polar, posterior polar, and posterior lentiglobus cataract can be autosomal dominantly inherited. In the United States, infantile cataracts are most commonly inherited as autosomal dominant traits, however, in countries where there is a high prevalence of parental consanguinity, infantile cataracts are more commonly inherited as autosomal recessive traits. In Egypt, where one-third of all marriages are consanguineous, Mostafa et al reported autosomal recessive inheritance for six of seven pedigrees with inherited infantile cataract. Linkage analysis has been used to determine the genetic loci of certain autosomal dominant cataract. Coppock-like cataract has been linked to the gamma E-crystalline gene on chromosome 2, Coppock cataract to chromosome 1q21-q25, Marner cataract to 16q22, and cerulean cataract to 17q24. The Cerulean cataract links closely to the galactokinase gene, but galactokinase levels in these patients are normal.
METABOLIC
The most common metabolic disturbance causing cataract during infancy is galactosemia. Galactosemia may be caused by a transferase, galactokinase or epimerase deficiency. Galactose-1-phosphate uridyl transferase (GALT) deficiency occurs in 1:40,000 newborns in the United States and 1:23,000 newborns in Ireland. A homozygous mutation of Q188R on exon 6 of the GALT gene on chromosome 9 is found in two-thirds of children with the transferase deficiency. This results in the accumulation of galactose 4-phosphate in the blood. Galactose is then converted to galactitol in the crystalline lens, resulting in an influx of water into the lens by osmosis. The hydration of the lens then disrupts the normal structure of the lens fibers, resulting in a loss of transparency. Early on, these lens changes have the appearance of an oil-droplet in the center of the lens. These changes are initially reversible with the elimination of galactose from the diet. If left untreated, a lamellar cataract develops which may then progress to a total cataract. In addition to cataract, these children have failure to thrive as infants, which may lead to death if milk and milk products are not eliminated from their diet. Later in childhood, these children may have delayed development, abnormal speech, growth delay, ovarian failure and ataxia. While eliminating galactose from the diet can prevent the life-threatening problems which occur during infancy, dietary compliance does not always correlate closely with the formation of cataract in later childhood or with the associated abnormalities of late childhood. The N314D mutation of the GALT gene causes the milder Duarte form of galactosemia. Combinations of Q188R, N314D and unknown mutations may result in phenotypically different forms of galactosemia.
Galactokinase deficiency may cause cataract with few or no systemic abnormalities. The galactokinase gene is on chromosome 17 and has recently been cloned and found to harbor homozygous mutations in some patients with cataract. Heterozygotes for galactokinase deficiency have half normal values on blood tests. Conflicting results have been reported in the literature as to whether partial loss of enzyme activity leads to presenile cataract. Alpha mannosidosis can also be associated with early onset cataract.
Lamellar cataract may also develop in children with neonatal hypoglycemia or hypocalcemia. Neonatal hypoglycemia is more common in low birth weight infants.
INFECTIOUS
The congenital rubella syndrome was one of the most common causes of congenital cataract in the United States until the widespread employment of the rubella vaccine. During the rubella epidemic in the United States during 1963-64, 16% of all children with the congenital rubella syndrome developed cataract. Infantile cataract also occurs occasionally in children after intrauterine varicella, toxoplasmosis and herpes simplex infections, or after bacterial or fungal endophthalmitis. Cataract may also develop after a varicella infection during early childhood.
PREMATURITY
Transient cataract occurs occasionally in premature infants. They are usually bilateral and begin as clear vacuoles along the apices of the posterior lens suture. They may progress to posterior subcapsular vacuoles. In most cases, they clear completely over the course of several months. All of the premature infants with transient cataract reported by Alden et al were septic and had been treated with Kanamycin, 80% of these infants also had an unexplained metabolic acidosis. These authors suggested that osmotic changes in the lens of these infants might have caused these cataract.
TRAUMA
While trauma is not a common cause of cataract during infancy it should be considered, particularly when a cataract is associated with other ocular signs suggestive of a traumatic injury. The trauma can be either blunt or penetrating. Nonaccidental causes for the trauma must always be considered. Eyes with suspected traumatic cataract should also be examined carefully for both retinal and optic nerve injuries.
LASER PHOTOCOAGULATION
Laser photocoagulation has been used in recent years to ablate the avascular retina of infants with threshold retinopathy of prematurity (ROP). Laser-induced cataracts are transient in some instances, but progress in some cases 6to total opacification of the lens. Drack et al reported cataract in six eyes following argon laser photoablation of the avascular retina in four infants with threshold retinopathy of prematurity.
RADIATION INDUCED
Radiation used to treat ocular and periocular tumors may induce cataract in children. A radiation dose of 15 Gy has been shown to be associated with a 50% risk of cataract formation. Radiation usually causes posterior subcapsular cataract, which typically have their onset 1 to 2 years after the completion of radiation therapy.
MEDICATIONS
Systemic corticosteroids cause cataract in up to 15% of children once a cumulative dose of 1000 mg of prednisone or the equivalent has been reached. This cataract usually begins as central posterior subcapsular opacities, but may progress to involve the entire lens.
IDIOPATHIC
In most series, at least 50% of bilateral infantile cataracts are idiopathic. The percentage of idiopathic unilateral infantile cataract is even higher.
AGE-RELATED CATARACT
PERSONAL FACTORS
Gender
It has often been observed that more females than males have cataract and undergo cataract surgery. This is partly explicable by the longer life span of women and therefore their over-representation in the age groups where cataract is most common. It does appear, however, that there is an additional effect—a true excess risk of cataract in females. In Nepal the prevalence of cataract was greater in females than in males at all ages. The overall risk ratio was 1.4, which would be detectable only in larger studies. In most case-control studies, the two groups were age- and sex-matched so that the effect of sex could not be explored. Hiller et al had to combine the results from three earlier studies in the United States and India to find a significant excess relative risk of 1.13 in females. This follow-up study of data from the National Health and Nutrition Examination Survey (NHANES) also suggested that such an excess risk for women is specific to cortical cataract. In a population-based prevalence survey in Beaver Dam, Wisconsin, women had more cortical opacities compared to men within similar age groups. The Beaver Dam Study reported a protective effect for nuclear opacities with current use of postmenopausal estrogens. Older age at menopause was associated with decreased risk of cortical opacities, suggesting hormonal influences in cataractogenesis. It was also suggested that hormone replacement therapy (HRT) may protect against cortical cataract. The Epidemiology of Cataract in Australia study found that a protective relationship of HRT and cortical cataract exists at the univariate level, but that this relationship was not significant in multivariate analysis. Nuclear cataract cases were more likely to be female in the above study, even after age adjustment. They were, however, unable to support the hypothesis that HRT is protective against nuclear cataract.
Tavani et al studied 287 Italian women who had undergone cataract extraction and 1277 control subjects who were in the hospital for acute, nonneoplastic, nonophthalmologic, nonmetabolic, nongastroenterologic diseases in a case-control study in Northern Italy. The results of this study support the association in women between cataract extraction and diabetes, (OR 4.6 for those younger than 60 years and 1.7 for those age 60 and over) current overweight, (OR 2.2) history of clinically relevant obesity, (OR 1.5) hypertension(OR 1.5) and hyperlipidemia (OR 1.8). They suggest that these factors may have some biologically independent impact on the risk of cataract in women and therefore support the association in women between cataract extraction on the other hand and diabetes, current overweight, history of clinically relevant obesity, hypertension and hyperlipidemia on the other.
Body Mass Index
Body mass index (BMI) is computed as weight in kilograms divided by the square of the height in meters (kg/m²) and is frequently identified as a risk factor for cataract, but the nature of the association is unclear. Several mechanisms may play a role:
- BMI affects glucose levels, which are associated with increased risk of cataract
- Higher BMI also increases uric acid concentrations and the risk of gout, which were associated with cataract in some studies
- BMI is also an important determinant of hypertension which has a controversial relationship with cataract.
Experimental evidence also supports a possible protective effect of restriction of energy intake on the risk of cataract by protection against oxidative stress to the lens.
In developing countries, some studies have associated low BMI with cataract. A recent case control study in India, however, failed to confirm this association.
Hankinson et al in a prospective study examined the association of BMI with cataract extraction in a large cohort of women and found elevated rates of cataract in those with higher BMI. Women with BMI of 23 or above had significantly elevated rates of extraction, between 46% and 65% higher than those with BMI of less than 21. Glynn et al in a prospective cohort study of a total of 17,764 apparently healthy US male physicians aged 40 to 84 years who were free of cataract at baseline were followed for 5 years. In this group higher BMI was especially strongly related to risk of posterior subcapsular and nuclear sclerotic cataract and was also significantly related to risk of cataract 7extraction. Furthermore, BMI below 22 appeared especially protective against posterior subcapsular cataract, with reductions in risk of 50% or more relative to each of the groups with a higher BMI. They concluded that BMI appears to be a strong and independent risk factor for cataract in this well-nourished and socioeconomically homogeneous study population. Even modest elevations in weight were associated with increased risk.
In so far as BMI index is modifiable, cataract caused by overweight is therefore potentially preventable.
Social Economic Status
Less education and lower income are related to increased morbidity and mortality from a number of diseases, even after controlling the known risk factors. These relations have been attributed to underuse of health care resources, high-risk behaviors, exposure to noxious work or adverse home environment, and poor nutrition. In population studies, less education and lower income consistently have been associated with impaired vision and cataract. The relationship of education, income, marital status, employment status to age-related cataract and impaired vision was addressed in the population-based Beaver Dam Eye Study.
A private census of the population of Beaver Dam, Wisconsin, was performed from September 15, 1987 to May 4, 1988. Eligibility requirements for entry into the study included living in the city or township of Beaver Dam and being 43 to 84 years of age at the time of the census. A total of 5924 eligible people were identified. Of these, 4926 (83.1%) participated in the examination.
While controlling for age and sex in this study, less education was significantly (P< 0.05) related to higher frequency of nuclear sclerotic and cortical cataract. Lower reported total household income was significantly associated with higher frequen-cies of cortical and posterior subcapsular cataract. These relations between total household income and cataract were observed in both men and women.
Less education has been associated with higher frequencies of history of heavy drinking, cigarette smoking and less vitamin supplement intake, all of which have been found to be related to specific types of cataract. However, the association of education and income with cataract persisted, despite controlling these exposures in their population. It is possible that poorer nutrition occuring earlier in life, may be related to the development of age-related cataract. A second possible reason explaining the relation of education and income to cataract is that people with less education or lower income are less likely to see an ophthalmologist or have cataract surgery.
Marital status is a measure of social support which is postulated to be an important factor in developing and managing complications associated with disease. While controlling for age and sex, people who were never married had a higher frequency of impaired vision than those currently married. This may be due to the fact that married people may have more social pressure to seek health care and to maintain familial responsibilities, and they may have more transportation assistance than their unmarried/widowed counterparts.
In summary, less education and income are related to cataract and visual impairment, but not to age-related maculopathy. These data suggest that access to medical, surgical, and low vision care may be of benefit to people with low socioeconomic status.
SOCIAL FACTORS
Smoking
Tobacco is the leading preventable cause of disease, disability and premature death.
Tobacco smoking is considered a major risk factor for 6 of the 15 leading causes of death. An individual who smokes has about twice the risk of premature death as a non-smoker, and the heavier the cigarette consumption, the higher the risk.
Of the 4,000 active substances in tobacco smoke, most are hazardous to human health. More than 40 of these chemicals are carcinogens and many others are deleterious to the cardiovascular and the pulmonary systems. They include nicotine, tars, nitrosamines, polycyclic aromatic hydrocarbons, hydrogen cyanide, formaldehyde, and carbon monoxide. Cigarette smoking is also a substantial source of intake of heavy metals and toxic mineral elements, such as cadmium, aluminum, lead, and mercury, all known to be poisonous in high concentrations.
Tobacco smoke also contains numerous compounds with oxidative properties; their existence is linked to the pathogenesis of several of the most common eye disorders, such as cataract and age-related macular degeneration.
Epidemiological data link cigarette smoking to several ophthalmologic disorders like ocular irritation, ocular ischemia, age-related macular degeneration (AMD), cataract, thyroid ophthalmopathy, tobacco-alcohol amblyopia, primary open-angle glaucoma, conjunctival intraepithelial neoplasia, uveal melanoma, Leber hereditary optic neuropathy, type II diabetes, ocular sarcoidosis and strabismus in the offspring of smoking mothers. The effects of smoking on ocular disorders show significant dose dependence; higher levels of smoking increase the risk of developing cataract. It is important to note, however, that the interpretation of results of different studies may be inherently biased, as smokers in these studies use cigarettes of different types, containing different concentrations of toxic substances. Moreover, some of the cigarettes have filters and others do not. Smoking habits may also be associated with other potentially noxious habits, such as excessive alcohol consumption, which may contribute a further bias to the results.
Table 1.2 summarizes five very thought-provoking studies all supporting the view that smoking is associated with the development of cataract.
Several authors have reported a significant link between tobacco smoking and an increased risk of cataract development.8
|
Nuclear sclerosis appears to be the type of cataract most commonly associated with smoking.
In the Beaver Dam Eye Study, the relationship between cigarette smoking and lens opacities was examined in 4926 adult subjects. A significant correlation was found between severe levels of nuclear sclerosis and the number of pack-years smoked. For both sexes, the odds ratio associated with 10 years was 1.09 (confidence interval, 1.04–1.16). The frequency of posterior subcapsular opacities was also increased (odds ratios, 1.05 [confidence interval, 1.00–1.11] for men and 1.06 [confidence interval, 0.98–1.14] for woman). Cortical opacities were not found to be linked to smoking. Leske et al studied, 1380 patients with cataract, aged 40 to 79 years, in an attempt to identify possible risk factors for the development of cataract, current smoking was correlated with the risk of developing nuclear cataract (odds ratio, 1.68; confidence interval, 0.96–1.94), but not other forms of cataract. The City Eye Study reported epidemiological data concerning 1029 volunteers, aged 54 to 65 years, from London, UK. The findings showed a significant relationship between nuclear lens opacities and moderate to heavy cigarette smoking. The relative risk for nuclear-type cataract ranged from 1.0 for past light smokers to 2.6 for past heavy smokers, and 2.9 for current heavy smokers.
Klein et al presented evidence that smoking has a detrimental effect on the development of cataract in the type II diabetic population.
Several prospective studies have investigated the relationship between cataract formation and cigarette smoking. In an 8-year prospective study, Hankinson et al examined the association between cigarette smoking and the risk of cataract extraction in 50,828 female nurses aged 45 to 67 years. The age-adjusted relative risk among female smokers of at least 65 pack-years was 1.63 (confidence interval, 1.18–2.26).
Smoking was also strongly associated with posterior subcapsular opacities for smokers of 65 or more pack-years (relative risk 2.59). In a 5-year prospective study of 22,071 men aged 40 to 84 years, current smokers of at least 20 cigarettes a day showed a significantly increased risk of developing cataract (relative risk 2.16; confidence interval, 1.46–3.20). When calculated for the different types, the relative risk was 2.24 for developing nuclear sclerosis and 3.17 for posterior subcapsular cataract. Past smokers were also at increased risk of developing posterior subcapsular opacities (relative risk 1.44), whereas current light smokers had the same chance of developing any type of cataract as subjects who had never smoked. In a study of 838 watermen from Chesapeake Bay, Maryland, West et al found a significantly increased risk of development of nuclear opacities associated with cigarette smoking (relative risk 2.40; confidence interval, 1.00–6.00).
A 5-year prospective study of this cohort of subjects reported an increase in the incidence and degree of nuclear opacities with increasing age. The risk of progression of nuclear opacities from less than grade 3 at baseline to grade 3 or worse was 2.4-fold higher among current smokers than among ex-smokers or non-smokers.
A significant increase (18%) in the risk of cataract progression was associated with each pack-year that a subject had smoked during the 5-year study period.
Mechanism The way in which smoking induces cataract formation is probably through its effect on the oxidant-antioxidant status of the lens. Oxidative damage plays a major role in cataractogenesis. Animal, laboratory, clinical, and epidemiological data support the relationship between cataract prevention and diets rich in nutritional factors with antioxidant properties, such as riboflavin, vitamins C and E, and the carotenoids.9
Smoking appears to further impair lenticular function by imposing an additional oxidative challenge as well as by contributing to the depletion of endogenous anti-oxidant pools. Tobacco smoke also contains large amounts of heavy metals, such as cadmium, lead and copper, which appear to accumulate in the lens and exert further toxicity.
The above data strongly support an association between tobacco smoking and cataract formation. Given the magnitude and seriousness of the cataract problem, an important preventive measure in fighting this disorder is to quit smoking. It is important to note that smoking is on the increase in the developing world, where cataract surgery is not always readily available.
Alcohol
Excessive alcohol use is associated with numerous chronic health problems, such as liver disease, varicosities, blood dyscrasias, and elevated blood pressure. Some studies have reported a relationship between alcohol consumption and cataract, while other studies have found no relationship. One study reported that both abstainers and heavy drinkers were more likely to have cataract than moderate users, while another found that total abstainers were more likely to have cataract than alcohol users.
As far back as 1973 Sabiston clinically observed in 40 patients over a 5-year period a definite correlation between alcohol intake, Dupuytren's contracture, and cataract. He stated that the mechanism of cataract formation was uncertain, but that an element of chronic dehydration was possible. In New Zealand, where he did his observations, heavy drinking often commenced with the ingestion of large quantities of beer. The national average consumption of beer there is 100 liters per head annually, with manual laborers ingesting a daily total of 4 liters of beer per person per day on average. These persons were almost invariably heavy cigarette smokers as well. He further noted that the cataract commenced in a posterior subcapsular position, and could progress to almost full maturity in six months. There was almost universally a history of heavy cigarette smoking as well. Malnutrition was only sometimes seen.
Drews in 1970 also drew attention to the association of ethanol and cataract. Two decades later he writes: “A patient in his or her 40s or 50s who appears with a posterior subcapsular cataract should be investigated for alcoholism… In the author's practice, about 25% of patients younger than age 65 years who present with cataract are found to be alcoholic on careful investigation. It has been his experience that if the opacities are incipient and if the consumption of alcohol is stopped completely, the posterior subcapsular changes may reverse and even disappear.”
Two decades later attention is once again drawn to the possible link between alcohol and cataract in the Archives of Ophthalmology by two different sets of authors. Munoz et al from the Wilmer Eye Institute, Baltimore, MD, USA conducted a follow-up study of surgical cases of posterior subcapsular cataract (PSC) and their controls to evaluate the possible association of alcohol intake and posterior subcapsular opacities. Two hundred thirty-eight cases and controls were interviewed. Current alcohol intake and usual and maximum weekly consumption ever were assessed. In this population, 57% of the cases and 56% of the controls were nondrinkers, 22% of the cases and 36% of the controls had an average of seven or fewer drinks per week, and 17% of the cases and 8% of the controls had more than seven drinks per week. Heavy drinkers were more likely to be cases than were nondrinkers (odds ratio, 4.6;P<0.05), and light drinkers were not at an increased risk. Light drinkers, defined as those who drink less than 91 g/wk (i.e. one drink or less per day), were at a lower risk than were nondrinkers, although this difference did not reach statistical significance. Moderate to heavy drinkers, that is, those drinking an average of more than one drink per day (more than 91 g/wk) were 2.7 times more likely to have PSC. This U-shaped relationship between alcohol and the risk of PSC was more pronounced in the logistic regression model when controlling for all the factors found to be related to PSC. Some studies have suggested that heavy drinking patterns are associated with lower socioeconomic status. In this study after adjustment for education level, the risk of PSC was still higher among drinkers. Smoking was also not related to PSC. Heavy alcohol use has been linked to poor nutritional status, so the presence of PSC may be related to poor nutrition rather than alcohol consumption per se. Dietary assessment however, was not performed in this study. In summary, this study concluded that moderate to heavy alcohol consumption is associated with a four-fold increase of PSC cataract whereas light drinkers, those consuming one drink per day or less, were not at an increased risk.
The second study reported in the same journal was on alcohol use and lens opacities in the Beaver Dam Eye Study group of patients. The relationship between alcohol use and lens opacities was examined in a large (N = 4926) population-based study of adults. Alcohol history was determined by a standardized questionnaire and the cataract severity was determined by masked grading of photographs obtained using a slit lamp camera and retroillumination. Several significant findings were made and conclusions drawn:
- In both sexes and every age group, a higher percentage of current heavy drinkers had late nuclear sclerotic changes. Similar results were seen for cortical and PSC changes.
- Past heavy drinkers were found to have increased odds of nuclear sclerosis (OR, 1.34; 95% confidence interval [CI], 1.12 to 1.59). There was an additional significant effect of past heavy drinking on the severity of cortical opacity (OR, 1.36; 95% CI, 1.04 to 1.77). The presence of posterior subcapsular opacity was also significantly associated with past heavy drinking (OR, 1.57; 95% CI, 1.10 to 2.25).
- Wine was associated with less severe nuclear sclerosis 10(OR, 0.84; 95% CI, 0.74 to 0.94) in general. Participants who drank liquor were less likely to have severe nuclear sclerosis than those who did not (OR, 0.81; 95% CI, 0.72 to 0.95). Liquor use was also associated with lower frequencies of any cataract (OR, 0.83; 95% CI, 0.72 to 0.94) and fewer past cataract surgeries (OR, 0.75; 95% CI, 0.57 to 0.98).
- A significant relationship was found between beer consumption and cortical cataract. Those who drank larger amounts of beer were more likely to have cortical cataract than those who drank smaller quantity of beer. An increased risk of cortical cataract was associated with increased beer consumption. An increased risk of cortical cataract was not associated with consumption of wine, hard liquor, or a combination of alcohol types when considered as continuous variables. These different relationships for the different types of alcohol (wine and hard-liquor consumption was generally associated with OR's or less than 1, while beer consumption was associated with OR's of more than 1) raises the possibility that other components of wine or hard liquor confer protective effects on cataract development. However, no such theoretical links have yet been established.
Alcohol has many metabolic effects, and modifies the absorption of drugs and dietary components. These effects may be important in the alcohol-cataract relationship. However, one cannot exclude the possibility that alcohol itself, especially when consumed in high volume, may be a direct toxin to the human phacos.
METABOLIC FACTORS
Diabetes Mellitus
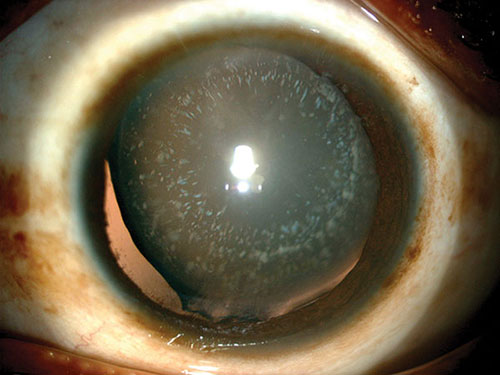
Juvenile diabetic cataract classically known as the “snow flake cataract” is now uncommon with the advent of effective hypoglycemic therapy. It occurs in insulin-dependent diabetics whose onset was before the age of 30. The limited period over which snow flake cataract may occur (chiefly in the first two decades of life) contrasts with the extended period over which lenticular change occurs (from youth into the eighth decade). It is of interest that snow flake cataract occurs at a period of life when the lens is undergoing a major physiological shape change, with negligible sagittal and major equatorial expansion. It may very well be that the mechanisms for refractive change and cataract are the same but age-related factors such as the decreasing ability of the lens to swell may protect the older lens from this type of cataract formation. Other typical features of this type of cataract are subcapsular and cortical “snow flakes”, and polychromatic opacities and vacuoles (Figure 1.5). These may proceed to mature cataract within weeks or months and rarely, may be reversible after normalization of blood glucose over some weeks or even as rapidly as 24 hours.
The rat sugar cataract model is an attractive model for juvenile diabetic cataract in terms of its acute development and other features. It is also relevant to human galactosemic cataract, in which the lens is exposed to high levels of aqueous galactose. The first visible indication of galactosemic cataract is the “oil-droplet” change on retroillumination, due to a change in refractive index between the inner and outer parts of the lens.
It has been noted that there are difficulties in accepting a role of aldose reductase in human cataract. Even though sorbitol is found in increased amount in the human diabetic lens, the amounts detected have been quite low, and insufficient on a lens mass basis to account for osmotic damage.
Data on a cell-to-cell basis, which would be appropriate, are not available.
Although Vadot and Guibal considered that there was sufficient sorbitol in young diabetic lenses to induce cataract, Lerman and Moran could not demonstrate the accumulation of significant amounts in sugar-incubated lenses over the age of 20 years. There is no information about levels in juvenile diabetic cataract itself. Jedziniak et al found a higher aldose reductase activity in the young lens than in the adult lens and calculated that it was sufficient to generate a significant osmotic stress. However, these calculations referred to the lens epithelium and assumed that sorbitol was not removed. Since polyol dehydrogenase is more active than aldose reductase in the human lens, the calculated levels would be expected to be lower. Lin et al demonstrated accumulation of dulcitol and loss of myoinositol in 72- hour cultures of infantile human lens epithelium in a 30 mlQI galactose medium, associated with vacuolar changes at ultrastructural level. Sorbinil and AL1576 reversed these changes. Similar changes have been produced in dog epithelial culture within 6 hours. Lin et al suggest that damage in the human lens may reflect compartmentalization of aldose reductase activity, for instance in the epithelium. If sorbitol accumulation in the epithelium (and not the fibers) were the basis of juvenile cataract, then a failure of epithelial permeability or pumping functions would be a more likely cause of lens swelling 11and cataract than an osmotic mechanism. There is no information available as to whether an oxidative mechanism, dependent on the polyol pathway or not, is operative in juvenile diabetic cataract.
Cataract in diabetic adults Cataract has a greater prevalence in diabetics with a greater risk for women, and is dependent on the duration of diabetics. The morphology is no different from that of age-related cataract, although the frequency of some subtypes is increased.
Klein et al in a population study found cataract to be more prevalent in early and late onset diabetes with significant association with age, severity of retinopathy and diuretic usage. Diabetes duration and the level of glycosylated hemoglobin were also associations in early onset diabetics. In a second report, cataract was found to be the second most common cause of severe visual loss in adult onset diabetics. Various other reports have shown an association between cataract, and diabetes duration or retinopathy.
The frequency of cataract extraction is greater in diabetics than non-diabetics. The Framingham study showed a significant excess risk in the 50 to 64 years age group (relative risk 4.02), while the HANES study showed a relative risk of 2.97 in this age group and 1.63 in the 65 to 74 years age group. Both studies reported an excess prevalence of cataract in diabetics in 50 to 64 years age groups, which disappeared at an older age. This has been attributed to the higher mortality in diabetics with cataract. However, a case-control study in Oxford found an increased risk for cataract extraction in diabetics in the age group of 50 to 79 years, and a small increase in risk for women relative to men.
As has been noted, the morphology of cataract in the adult diabetic resembles that seen in age-related cataract in the absence of diabetes. Thus the major features are nuclear cataract (increased nuclear scattering and brunescence) and cortical spoke and posterior subcapsular cataract. In the Lens Opacity Case Control study, diabetes increased the risk of posterior subcapsular, cortical and mixed forms of cataract. Individual features may not have an identical etiology, but it is likely that those metabolic changes identified in experimental cataract are relevant for the human are in varying degrees. There is no relation between cataract type, and the level of either sorbitol or myoinositol in lens epithelium from patients with cataract and diabetes.
It has been suggested that the increased nuclear scattering and brunescence in diabetic lenses is likely to be the result of increased glycation and the formation of advanced glycation end products.
There is evidence for a fall in free lysine amino groups in the human diabetic lens. It is also possible to induce a change in tertiary structure in alpha-crystalline (bovine) incubated with glucose and glucose-6-phosphate.
A three-fold increase in glycation was measured in diabetics and controls by Vidal et al but there was no correlation with the degree of browning of the lenses measured spectrophotometrically, and they concluded that other chromophores were responsible for the browning at the relevant wavelengths.
Certainly a number of other factors have been proposed to contribute to nuclear brunescence of the non-diabetic lens, but since the diabetic state is not anticipated to increase their concentration, glycation products are still the most likely candidates responsible for diabetic cataract.
Cortical cataract can be caused experimentally by agents which interfere with membrane permeability, ion and water control. The non-diabetic, aging human lens, free of cataract, has an increased membrane permeability which parallels the increase in optical density which occurs from about the fifth decade. There is evidence of degradation of the lens protein MIP26 with age in non-diabetics, which could be responsible for a functional abnormality. This channel protein has until recently been regarded as the gap junctional protein, but may, in fact, serve as a volume regulating channel. Disturbance of either function could increase the risk of cataract. It would be of interest to examine these events in the diabetic lens. The greater thickness of the diabetic compared to the non-diabetic lens could be relevant to this point. The disturbance in Na’ K’-ATPase kinetics reported by Garner and Spector during exposure to glucose-6-phosphate is similar to the change noted in diabetic human lenses. Hydrogen peroxide is present in normal human aqueous, and present at raised levels in the aqueous of patients with cataract. Higher levels are found in the aqueous of diabetic patients with cataract. Simonelli et al have also shown an increase in malondialdehyde in cataractous compared with non-cataractous lenses which is greater in the cataract of diabetic patients. Malondialdehyde is a product of lipid peroxidation of cell membranes, and is regarded as an indicator of oxidative membrane damage. These are important findings, although the methods of measurement are not entirely specific.
The potential role of the sorbitol (polyol) pathway in juvenile cataract was discussed earlier. Recent studies of cultured lens epithelium from cataract patients have shown negligible or absent levels of sorbitol in the epithelium of non-diabetics. In diabetic epithelium sorbitol levels are higher than blood glucose levels, while there is an inverse relationship between blood glucose and myoinositol.
It has been noted that oxidative stress may cause lens membrane damage experimentally. It may also cause damage to DNA. Subcapsular cataract may be regarded as due to an aberration of lens mitosis and lens fiber differentiation, and could be the result of oxidative damage. There are no data, which link this to human subcapsular cataract.
Other cataract-related events A higher rate of capsular rupture reported in diabetics undergoing intracapsular or extracapsular extraction could be related to structural and chemical changes which are known to occur in the capsule. There is an increased risk for death in patients with cataract and diabetes. Cohen et al found lens opacities to be a powerful predictor of death, independent of other factors 12and with an odd ratio of 2.4 (95% confidence interval 1.5-3.9).
Dyslipidemia
Lens opacification and cardiovascular disease are two of the main causes of morbidity worldwide. Lens opacity, manifesting as cataract, is responsible for an estimated 40% of the 42 million cases of blindness in the world. On the other hand, heart disease is the single greatest cause of death in developed countries. The relationship between cholesterol and cardiovascular heart disease is well documented. The relationship between cholesterol and lens opacity is, however, far less well-appreciated.
Issues relating to drug safety and inherited defects in enzymes mediating cholesterol metabolism have brought renewed attention to a possible inter-relationship between lipid metabolism and cataract induction in humans. The lens is unique in that it contains a relative abundance of cholesterol in the fiber cell plasma membrane, (the highest of any cell group in the body) and furnishes its needs for cholesterol by onsite biosynthesis. It has been shown that inhibition of cholesterol synthesis in the lens leads to cataract formation in man.
Smith-Limli-Opitz syndrome, mevalonic aciduria and cerebrotendinous xanthomatosis are inherited disorders of cholesterol metabolism and affected patients may present with lens opacities. Triparanol, a hypolipidemic agent that inhibits cholesterol biosynthesis was withdrawn from clinical use because of its propensity to induce cataract formation in humans. The very widely used vastatin class of hypolipidemic medicines is potent inhibitors of cholesterol biosynthesis and is able to lower serum lipid concentration effectively. Although high ocular safety in older patients over periods of up to 5 years, has been reported, it is still not clear whether these agents have the potential to be cataractogenic, particularly in younger patients and over longer periods.
In order to assess the prevalence of lenticular opacities in patients with dyslipidemia (raised serum cholesterol and triglycerides) a group of 80 dyslipidemic patients were subjected to a general physical examination and an ophthalmic examination of the fully dilated eye at the Tygerberg Academic Hospital, University of Stellenbosch, South Africa (unpublished data).
Patients (n = 80) of both genders and irrespective of age were enrolled in the trial if they met the inclusion criteria for dyslipidemia. Patients were included if their fasting serum cholesterol and triglyceride concentrations were > 5.2 mmol/l and > 2.3 mmol/l, respectively when measured on three separate occasions over a one-month period (Figure 1.6). Patients were excluded if they suffered from any condition known to cause or predispose them to elevated lipid levels or lenticular opacification.
Results The study group was predominantly male Caucasian and smokers. Most patients—68.8% admitted regular alcohol consumption. The mean systolic and diastolic blood pressure data, 134 ± 18 and 84 ± 9 mm Hg, respectively, fell within the normal range for age. The BMI of the group was significantly greater than the norm (i.e. 28.89 ± 4.82 kg/m2).
The prevalence of lenticular opacities divided the study group into two cohorts, i.e. those with normal lenses (62%) and those with opacities (39%) (Figure 1.7).
The prevalence of lenticular opacity in dyslipidemic patients in the age group of 30 to 40 years was 33%. This age group was not studied in the Barbados Eye Study (BES) or in The Beaver Dam Eye Study (BDES) and consequently data for comparison are not available (Table 1.3). In the 40 to 50 year age group, the prevalence of lenticular opacity in our patients was 50% compared to 4.7% in the BES and 8.3% in BDES. Differences in the older age groups were not prominent (Figure 1.8).
Modern medicine today aspires to early detection of disease processes with the aim of early intervention in an attempt either to halt the progression or to reverse the process.
Although the classic systemic signs of dyslipidemia are well-appreciated, i.e. xanthomata, xanthelasma, thickening of the Achilles tendon and corneal arcus, in our study the prevalence of one or more of the ocular signs was far greater than that of the systemic signs, 23.8% for the former as opposed to 47.3% for the latter.
FIGURE 1.8: Prevalence of lenticular opacities in two population-based studies compared to the dyslipidemic study group
| |||||||||||||||||||||||||||||||||||||
The distribution of dyslipidemia-related signs in this study was:
- Xanthelasma—7.5%
- Corneal arcus—8.8%
- Achilles tendon involvement—16.3%
- Cortical lenticular opacity—31.0%.
It is noteworthy that the most frequent ocular sign—cortical lenticular opacity—occurred twice as frequently as the most frequent systemic sign—Achilles tendon thickening (Figure 1.9).
This work leads the investigators to conclude that:
- Dyslipidemic patients are more likely to develop cortical opacification than the normal population.
- Cortical lens opacification in dyslipidemics manifests at a younger age than does nuclear opacification.
- Cortical lens opacification in the patient younger than 50 years of age should alert the ophthalmologist to arrange for diagnostic serum lipid assessment.
- Cortical lenticular opacification should be regarded as one of the most common, and hence reliable, clinical signs of dyslipidemia.
Jahn et al attempted to determine the role of glucose and lipid metabolism in the formation of cataract in elderly people undergoing cataract extraction. They found that patients with posterior subcapsular cataract had higher concentrations of fasting serum triglycerides and were significantly younger than patients with nuclear or cortical cataract. Their results furthermore suggest that the association of hypertriglyceridemia, hyperglycemia and obesity favors the formation of a specific morphologic type of lens opacity, posterior subcapsular cataract, occuring at an early age. Because these factors are potentially modifiable by lifestyle changes, these observations may prove important as the modification of these parameters could constitute an effective mode of prevention or retardation in a subgroup of patients developing cataract at an early age.
Acetylator Status
The human acetylation polymorphism has been known for more than three decades since its discovery during the metabolic investigation of the antituberculous hydrazine drug, isoniazid.14
The trait was originally known as the “isoniazid acetylation polymorphism” but is now usually abbreviated as “acetylation polymorphism” because acetylation of numerous hydrazine and arylamine drugs and other chemicals are subject to this trait. Individuals phenotype as “slow” acetylators when homozygous for the slow acetylator gene, “rapid” when homozygous for the rapid acetylator gene or “intermediate” when heterozygous. The acetylator phenotype is a life-long, relatively stable characteristic of the individual that can phenotypically be determined by procedures using any of several test agents (e.g. caffeine, isoniazid, sulfamethazine, sulfapyridine). Certain disease states such as AIDS can change the phenotype expression in an individual. On the other hand, acetylator genotype can be determined by specialized polymerase chain reaction (PCR) methods.
Several diseases have been linked to acetylator pheno- and/or genotype. The best documented are bladder cancer (slow), colorectal adenomas (rapid), Gilbert's syndrome (slow), allergic diseases Type I diabetes mellitus (fast), Type II diabetes mellitus (slow) and familial Parkinson's disease (slow).
Recent work (PhD level, unpublished) at the departments of Ophthalmology and Pharmacology at the University of Stellenbosch, South Africa, have also established an association between age-related cataract and acetylation status as determined both phenotypically and genotypically. Sixty adult patients of both sexes with classic age-related lens opacities presenting for cataract surgery were enrolled in a prospective controlled study. Patients were included in the trial if they perceived themselves to be colored and if this was verified by at least one independent observer. The South African population of mixed ancestry (including Malay, Khoisan, Negroid and Caucasoid stock) is referred to as “colored” and all patients were selected from this well studied subgroup of the population. Care was taken to exclude all patients with well-known etiological factors for cataract formation such as diabetes mellitus, previous ocular trauma, other metabolic and/or inherited diseases.
One hundred and twenty patients of the same race group served as controls.
Figure 1.10 demonstrates that in the control group (representing the population at large) the distribution of the phenotypic acetylation status was 20% “rapid”, 50% “intermediate” and 30% “slow”. In the cataract group the distribution was 5% “rapid”, 42% “intermediate” and 53% “slow”. This clearly seems to suggest that cataract possibly occurs more frequently in slow acetylators than in the rest of the population. Could this finding perhaps suggest a possible etiologic role for chemical substances possessing a primary aromatic amine or hydrazine group in human lenticular opacification?
Lipid Peroxidation, Free Radicals and Nutritional Influences on Cataract Formation
Oxygen and oxygen-derived free radicals and a failure of intracellular calcium homeostatic mechanisms are recurring themes in a wide variety of cell injuries.
The addition of electrons to molecular oxygen leads to the formation of toxic free oxygen radicals or reactive oxygen species (ROS), e.g.
Iron is very important in this process according to the Haber-Weiss reaction:
These free radical species cause lipid peroxidation and other deleterious effects on cell structure. Recent studies have shown that lipid peroxidation, an event caused by imbalance between free radical production and antioxidant defense, may play a role in the genesis of cataract. Higher levels of malondialdehyde (MDA), a final product of the lipid peroxidation process, have been observed in diabetic and myopic cataract compared with senile cataract. Protection of the cell against damage by these free radicals takes place indirectly (enzymatically) by antioxidant enzymes—superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT). Direct protection is offered by mainly dietary antioxidants—ascorbate (Vit C), tocopherol (Vit E), carotenoids(Vit A) and glutathione (GSH).
Light and oxygen as risk factors for cataract Various epidemiological studies demonstrate associations between elevated risk of various forms of cataract and exposure to higher intensities of incident and/or reflected ultraviolet light (Table 1.4).
Elevated levels of oxygen exposure perhaps show the clearest causal relationship between oxidative stress and cataract. Nuclear cataract was observed in patients treated with hyperbaric oxygen therapy, and markedly elevated levels of mature cataract were observed in mice that survived exposure to 100% oxygen twice weekly for 3 hours. A higher incidence of cataract was noted in lenses exposed to hyperbaric oxygen in vitro. Very early stages of cataract in guinea pigs exposed to hyperbaric oxygen was noted by Giblin.
Role of cellular antioxidants against lens damage Protection of the organism against photooxidative insult can be viewed as two interrelated processes. Primary defenses offer protection of proteins and other lens constituents by lens antioxidants and antioxidant enzymes whereas secondary defenses include proteolytic and repair processes. The primary defenses shall form the focus of our attention.
The major aqueous antioxidants in the lens are ascorbate and GSH.
Ascorbate is probably the most effective, least toxic antioxidant identified in mammalian systems. The following has been observed:
- The lens and aqueous concentrate ascorbate >10 times the level found in human plasma.
TABLE 1.4 Extent of light expoxure and the risk of cataract StudyExposurePR95% CIUSA: NHANESDaily hours of sunlight< 6.6 h1.0surveyin area; ages 65–747.1–7.7 h1.71.2–2.7>8.2 h2.71.6–4.6AustraliaDaily hours of sunlight<8 h1.0in area8.5–9 h2.90.6–13.2>9.5 h4.20.9–18.9Average mean erythemal20001.0dose of area25001.30.8–2.330001.81.0–3.4NepalAverage hours of sunlight7–9 h1.010–11 h1.20.9–1.4>12 h2.52.1–3.0PR = prevalence ratio CI = confidence interval - The concentration of ascorbate in the lens nucleus is only 25% that of the surrounding cortex.
- Ascorbate levels in normal lenses are higher than in cataractous lenses.
- Ascorbate levels are higher in the older guinea pig lens than in younger animals despite the same dietary intake of ascorbate.
- Increasing lens ascorbate concentrations by two-fold is associated with protection against cataract-like damage.
With this basic science knowledge, several epidemiological, clinical and even interventional studies have been undertaken. Vitamin C was considered in approximately 9 published studies and observed to be inversely associated with at least one type of cataract in eight of these studies.
In the Nutrition and Vision Project, age-adjusted analyses bases on 165 women with high vitamin C intake (mean = 294 mg/day) and 136 women with low vitamin C intake (mean = 77 mg/day) indicated that the women who took vitamin C supplements for > 10 years had > 70% lower prevalence of early opacities (RR: 0.23; CI: 0.09-0.60) and > 80% lower risk of moderate opacities (RR: 0.17; CI: 0.03-0.87) at any site compared with women who did not use vitamin C supplements.
In comparison to the above data, Mares-Perlman et al report that past use of supplements containing vitamin C was associated with a reduced prevalence of nuclear cataract, but an increased prevalence of cortical cataract after controlling for age, sex, smoking, and history of heavy alcohol consumption.
Glutathione (GSH) levels in the lens are several fold the levels found in whole blood and plasma. GSH levels also diminish in the older and cataractous lenses. Pharmacological opportunities could be suggested by observations that incorporating the industrial 0.4% butylated hydroxytoluene in diets of galactose-fed (50% of diet) rats diminished prevalence of cataract. Clinical studies, however, have not yet been forthcoming.
Vitamin E, a natural lipid-soluble antioxidant, can inhibit lipid peroxidation and appears to stabilize lens cell membranes. Consumption of Vit E supplements was inversely correlated with cataract risk in two studies. Robertson et al found among age- and sex-matched cases and controls that the prevalence of advanced cataract was 56% lower (RR: 0.44; CI: 0.24–0.77) in persons who consumed vitamin E supplements (>400 IU/day) than in persons not consuming supplements. Jacques and Chylack (unpublished) observed a 67% (RR: 0.33; CI:0.12–0.96) reduction in prevalence of cataract for vitamin E supplement users after adjusting for age, sex, race and diabetes.
Two prospective studies demonstrated a reduced cataract progress among individuals with higher plasma vitamin E. Rouhianen et al found a 73% reduction in risk for cortical cataract progression (RR:027; CI: 0.08–0.83), whereas Leske et al reported a 42% reduction in risk for nuclear cataract progression (RR: 0.58; CI: 0.36–0.94). Vitamin E supplementation was related to a lower risk for progress of nuclear opacity (RR:0.43; CI: 0.19–0.99).
The carotenoids, like vitamine E, are also natural lipid-soluble antioxidants. Beta-carotene is the best known carotenoid because of its importance as a vitamin A precursor. However, it is only one of the 400 naturally occuring carotenoids, and other carotenoids may have similar or greater antioxidant potential. In addition to β-carotene, α-carotene, lutein and lycopene are important carotenoid components of the human diet.
Jacques and Chylack were the first to observe that persons with carotene intakes above 18,700 IU/day had the same prevalence of cataract as those with intakes below 5,677 IU/day (RR:0.91; CI:0.23–3.78). Hankinson et al followed this report with a study that reported that the multivariate-adjusted rate of cataract surgery was about 30% lower (RR: 0.73; CI: 0.55–0.97) for women with high carotene intakes (median = 14,558 IU/day) compared with women with low intakes of this nutrient (median = 2,935 IU/day). However, while cataract surgery was inversely associated with total carotene intake, it was not strongly associated with consumption of carotene-rich foods, such as carrots. Rather, cataract surgery was associated with lower intakes of foods such as spinach that are rich in lutein and xanthin carotenoids, rather than β-carotene. This would appear to be consistent with the observation that the human lens contains lutein and zeaxanthin but no β-carotene.
This observation would appear to be consistent with the observation that lutein and zeaxanthin are the most prevalent carotenoids in lens. However, Mares-Perlman did not detect a significantly altered risk for cataract among consumers of these nutrients.
Intervention studies To date only one intervention trial designed to assess the effect of vitamin supplements on cataract risk has been completed. Sperduto et al took advantage of two ongoing, randomized, double-blinded vitamin and cancer trials to assess the impact of vitamin supplements on cataract prevalence. The trials were conducted among almost 4,000 participants aged 45 to 74 years from rural communities in Linxian, China. Participants in one trial received either a multisupplement or placebo. In the second trial, a more complex factorial design was used to evaluate the effects of four different vitamin/mineral combinations:
- Retinol (5000 IU) and zinc (22 mg)
- Riboflavin (3 mg) and niacin (40 mg)
- Vitamin C (120 mg) and molybdenum (30 mg)
- Vitamin E (30 mg), β-carotene (15 mg), and selenium (50 mg).
At the end of the five to six years follow-up, the investigators conducted eye examinations to determine the prevalence of cataract. In the first trial there was a significant 43% reduction in the prevalence of nuclear cataract for persons aged 65 to 74 years receiving the multisupplement (RR: 0.57; CI: 0.36–0.90). The second trial demonstrated 17a significantly reduced prevalence of nuclear cataract in persons receiving the riboflavin/niacin supplement relative to those persons not receiving the supplement (RR: 0.59; CI: 0.45–0.79). The effect was strongest in those aged 65 to 74 years (RR: 0.45; CI: 0.31–0.64). However, the riboflavin/niacin supplement appeared to increase the risk of posterior subcapsular cataract (RR:2.64; CI: 1.31–5.35). The results further suggested a protective effect of the retinol/zinc supplement (RR: 0.77; CI: 0.58–1.02) and the vitamin C/molybdenum supplement (RR:0.78; CI: 0.50–1.04) on prevalence of nuclear cataract.
Conclusion Although light and oxygen are necessary for physiological function, when present in excess they seem to be causally related to cataractogenesis. Aging might diminish the bodies primary antioxidant reserves, antioxidant enzyme abilities, and diminished secondary defenses such as proteases.
The literature creates the strong impression that antioxidant intake might diminish the risk for cataract formation.
Longitudinal studies and more intervention studies are essential in order to establish the value of dietary antioxidants and to determine the extent to which cataract progress is affected by nutritional supplements. This fact becomes significant when one appreciates that poor education and lower socioeconomic status are directly related to poor nutrition. It is, therefore, not irrational to contemplate the value of intervention for populations at risk. The work available, albeit preliminary, indicates that nutrition may provide the least costly and most practicable means to attempt the objectives of delaying cataract.
OCULAR DISEASE
Many ocular diseases have been associated with cataract formation either as direct cause and effect relationships or as common associations.
Myopia
Weale suggested that lenses of myopes are subject to excessive mechanical stress which could lead to cataract. This hypothesis was tested by several investigators and Harding et al during their Oxford case-control studies found that the risk of cataract after the age of 50 was doubled in myopes. Weale (1980) also suggested that there is a graded risk for increasing degrees of myopia. This was eloquently confirmed two decades later by Lim et al in the Blue Mountains Eye Study. Eyes with onset of myopia before age 20 had the greatest posterior subcapsular (PSC) cataract risk (odds ratio [OR] 3.9; confidence interval [CI] 2.0–7.9) Refraction-related increasing odds were found between PSC cataract and myopia: low myopia (OR 2.1; CI: 1.4–3.5), moderate myopia (OR 3.1; CI: 1.6–5.7), and high myopia (OR 5.5; CI: 2.8–10.9). High myopia was associated with PSC, cortical, and late nuclear cataract. Conversely PSC cataract was inversely associated with hyperopia (OR 0.6; CI: 0.4–0.9). They finally concluded that early-onset myopia (before 20 years of age) may be a strong and independent risk factor for PSC cataract, that nuclear cataract was associated with presumed acquired myopia, whereas high myopia was associated with all three types of cataract.
Wensor et al demonstrated that a myopic shift is associated with nuclear cataract. In the population based study of 3,271 Australians an association between myopia of 1 Diopter or more and both nuclear and cortical cataract was observed. Between posterior subcapsular cataract and myopia such a relationship did not exist. It is not sure that a causal relationship exists between cortical cataract and myopia or rather that a myopic shift occurs after people develop cortical cataract. The temporality of this relationship should still be explored in future prospective analyses.
Glaucoma
Glaucoma has been shown to be strongly associated with the pathogenesis of cataract (Figure 1.11) in many studies undertaken in many countries. The relative risk (odds ratio [OR]) of cataract developing in a glaucoma patient can be as high as six times normal. This risk more than doubles to an OR of 14.3 after glaucoma filtration surgery. This rise in risk is most probably due to the trauma of surgery for glaucoma. Vesti in Helsinki, Finland investigated cataract progression after trabeculectomy in a study of 47 eyes with exfoliative glaucoma (EXG) and in 20 eyes with primary open-angle glaucoma (POAG). EXG, age, hypotony (IOP < or = 5 mm Hg) lasting > or = 5 days and early postoperative IOP rise > 30 mm Hg were observed to be risk factors for cataract progression.
Besides formal filtering procedures like full thickness procedures, laser procedures for the management of different types of glaucomas are frequently performed such as argon laser trabeculoplasty, argon laser iridoplasty and Nd-Yag peripheral iridotomy. Each of these procedures carries the risk of inducing a cataract especially of the focal type. Zadok et al has described a previously unreported complication of a posterior chamber intraocular lens (IOL) implanted in a phakic eye.
The left eye of a 25-year old patient with high myopia was treated prophylactically with Nd: YAG laser iridotomy prior to phakic IOL implantation. Slit lamp examination of the same eye disclosed an opacity of the anterior capsule of the crystalline lens under the iridotomy site.
Miotics, particularly long-acting cholinesterase inhibitors, if used for long term, may cause tiny anterior subcapsular vacuoles and, occasionally, more advanced opacities. Cessation of medication may stop, retard or occasionally reverse their progression.
Acute congestive angle-closure glaucoma is associated with the subsequent formation of glaukomflecken consisting of small, gray-white, anterior, subcapsular or capsular opacities in the pupillary zone.
Ophthalmic Surgical Procedures
Many different ophthalmic procedures carry the risk of inducing cataract. Among others are surgical iridectomy, filtration surgery, corneal transplants, retinal detachment surgery with and without intraocular silicone oil as well as pars plana vitrectomy especially in diabetics. Assessing the surgical outcome in a series of 63 consecutive patients treated for rhegmatogenous retinal detachment by primary vitrectomy Oshima reported the reattachment rate by final examination as 100%, but there was a high incidence (53.8%) of cataract progression in phakic eyes.
More recently with the advent of minus power phakic, IOL implantation surgery, several reports have appeared of cataract induction secondary to the implantation of these lenses into the ciliary sulcus. These cataracts have occurred both with silicone and collamer materials. Some have taken as short a time as 6 months, whilst others took 7 years to form. In another series of 38 consecutive eyes with high myopia implanted with a silicone posterior chamber plate-style intraocular lens (Chiron, Adatomed) over a period of 21 months and followed for between 3 and 24 months not a single cataract occurred. The lens style and design may play a significant role in the cataract pathogenesis, because in a recent study Brauweiler et al attempted to assess the effectiveness and safety of implantation of a silicone, posterior chamber IOL in the ciliary sulcus of phakic, highly myopic eyes in a noncomparative consecutive interventional series. Eighteen eyes of 10 patients underwent implantation of a Fyodorov 094M-1 IOL by the same surgeon and were evaluated for a 2-year postoperative period. Cataract formation of the anterior subcapsular (8 eyes) or nuclear (only 1 eye) type was observed in overall 9 (52.9%) of 17 eyes. When considering only the patients with a follow-up of 2 years, the incidence of cataract formation was 81.9% (9 of 11 eyes). Obviously this very high incidence of cataract formation should discourage the implantation of the type of IOL used in this study.
Ocular Trauma
The development of cataract (Figure 1.12) is a known complication following blunt or penetrating ocular trauma. However traumatic cataract and zonular dehiscence is only one complication of the injured ocular tissues. Other complications include glaucoma, retinal detachment, optic nerve damage, extraocular muscle imbalance and injury to the bony orbit.
Ocular trauma is a major cause of monocular blindness in both the developed and developing world, but this is not seen as a significant cause of bilateral blindness. Trauma can, therefore, be considered as a major cause of blind eyes but not of blind people.
Crystalline lens subluxation, total dislocation, or localized cortical or diffuse opacities are often observed secondary to blunt ocular trauma. An unusual complication of blunt trauma is rupture of the posterior capsule with subsequent lens fiber hydration leading to rapidly progressive lens opacification. Posterior capsular breaks have been reported to develop thick, fibrous, opaque margins approximately 6 weeks after blunt trauma.
Secondary Cataract
Uveitis A secondary cataract develops as a result of some other primary ocular disease. The most common cause of secondary cataract is chronic anterior uveitis. The earliest finding is a polychromatic luster at the posterior pole of the lens. If the uveitis is controlled, the progression of cataract may be arrested. If the inflammation cannot be controlled, anterior and posterior subcapsular opacities develop and the lens may become completely opaque. The lens opacification seems to progress more rapidly in the presence of posterior synechiae.
Hereditary posterior segment disease Hereditary fundus dystrophies such as retinitis pigmentosa, Leber's congenital amaurosis, gyrate atrophy, Wagner's and Stickler's syndromes may be associated with posterior subcapsular lens opacities. In a study of 384 eyes of 192 patients with a mean age of 39.1 years who presented with typical retinitis pigmentosa, cataract was found in 46.4% of the eyes.19
Among these, 93.6% showed posterior subcapsular opacification. The incidence of cataract increased with age.
Wagner's vitreoretinal degeneration is characteristically associated with high myopia, glaucoma, choroidal atrophy, retinal detachment and presenile cataract.
Persistent hyperplastic primary vitreous (PHPV) is a congenital disorder that manifests a range of ocular anomalies including leukoria, microphthalmia, a retrolental fibrovascular membrane and cataract. In general the prognosis for visual acuity with PHPV is poor.
Iris color McCarty et al in their Australian population study of 3,271 adults aged 40 years and older found an association between cortical cataract and brown or dark brown irides for all ages that was not explained by country of birth or language spoken. In all age categories, brown iris color was also associated with nuclear cataract. No such association was found for posterior subcapsular cataract.
In the Italian-American Cataract Study, there was an increased, although not significant, risk of cortical cataract in people with brown irides. Dark iris color was not associated with cortical cataract in the Lens Opacities Case-Control Study. In the National Health and Nutrition Examination Survey, blacks, who have dark brown irides, were found to have significantly increased risk of cortical cataract. In both the above mentioned studies, dark iris color was also found to be a significant risk factor for nuclear cataract.
The relationship of nuclear cataract and iris color could result from genetic susceptibility associated with iris color or other factors not yet determined. This finding may partially explain the variation in the prevalence of nuclear cataract observed in different countries with different racial groups.
SYSTEMIC DISEASES
Hypertension
The association between hypertension and cataract was first noted in the Framingham study where earlier detection of elevated blood pressure was more common in those later found to have cataract. It was also noted in the same study that consumption of diuretics which restores normal blood pressure in many patients does not protect against this risk. There may, however, be a variety of interactions in these patients in that hypertension may be associated with high blood glucose, diabetes and other conditions as well as with use of diuretics. Diuretics have different effects on plasma urea levels, with frusemide and acetazolamide associated with the highest levels, and parallel effects on cataract. Overall diuretic use was associated with an odds ratio of 1:6 but cyclopenthiazide (Navidrex), which had least effect on plasma urea, was reported by a greater proportion of controls than cases. Loop diuretics were reported by more than twice the proportion of cases than of controls. Hypertension and diuretic consumption did not appear as risk factors in Oxford but the graded properties of different diuretics did emerge and with a similar sequence to that found in Edinburgh. The only significant association of individual diuretics was an apparent protective effect by cyclopenthiazide and a risk associated with spironolactone which itself is a steroid. There was no significant association of particular sites of opacity with diuretic use.
Dehydrational Crisis
Harding has proposed that frequent episodes of diarrhea may be related to cataractogenesis and may account for the excess prevalence in some developing countries. Four intermediate events have been suggested to explain the role of diarrhea in the development of cataract
- Malnutrition secondary to malabsorption of nutrients
- Relative alkalosis from administration of rehydrating fluids with bicarbonate
- Dehydration induced osmotic disturbance between the lens and the aqueous humor, and
- Increased levels of urea and ammonium cyanate which may denature lens proteins by the process of carbamylation.
Six case-control studies have examined the relationship of severe diarrhea and increased risk of cataract, with discordant results. Two case-control, clinic-based studies done in Madhya Pradesh and Orissa, India have suggested a three- to four-fold increase in the risk for cataract for those with remembered episodes of life-threatening dehydration crises, severe enough to render the patient bedridden for at least three days. However, these findings were not replicated in two other epidemiologic investigations done in India. Using a less stringent definition of diarrhea (confinement to bed for one day), the India-US Case-Control Study found no associations with cataract. Also, a village-based case-control study in Southern India showed no association between severe diarrhea and risk of cataract. Furthermore, an observational study done in Matlab, Bangladesh, revealed that diarrhea from all causes was not significantly associated with cataract, although it was difficult to determine how cataract was defined in the study. The case-control study in Oxford found a marginally significant excess risk of cataract with reported severe diarrhea, but a significant risk in the subgroup aged 70 and older. Adjustments for the other possible confounding factors also found in the study were not done. Considering the potential public health importance of diarrhea as a risk factor, as well as the biologically plausible role of dehydration in cataractogenesis, further research to clarify this association is needed. Prospective studies involving closer follow-up of groups of patients who suffered from acute life-threatening diarrhea may provide more convincing evidence. Moreover, studies that examine the cumulative effect of milder, chronic dehydration episodes in cataractogenesis may also add to the current understanding of this issue.
Renal Failure
Cataract has been reported in many cases of renal failure. Sometimes cataract, often transient, was associated with 20hemodialysis and thought to be caused by the osmotic shock that dialysis causes, but Laqua (1972) noted lens opacities before dialysis and suggested they were caused by uremia. Increased blood urea could lead to cataract in a similar way to that postulated in severe diarrhea. After renal transplantation patients are treated with immunosuppressants usually including corticosteroids that may cause cataract. Posterior subcapsular lens opacities were observed in 19 out of 22 renal transplant recipients, aged 21 to 54 years in Hiroshima. Half of the patients suffered visual loss, attributed to steroid-induced cataract. In a study of diabetic patients receiving renal transplants in the USA, only one patient developed a visually-impairing cataract but lesser degrees of lens opacification were seen in 26% of eyes. Fourteen of 55 non-diabetic renal transplant patients were found to have cataract. The case-control study in Edinburgh did not report on renal failure as such but did find that the mean urea level was significantly higher in the plasma of cataract patients compared with controls. The level was not high enough to indicate renal failure. The raised urea levels are still present when subjects are subdivided by age and sex. Diuretics may raise urea levels and thus contribute to these differences but when all diabetics and individuals receiving diuretics were excluded, a relationship between high plasma urea and cataract remained.
ENVIRONMENTAL FACTORS: ULTRAVIOLET RADIATION
There is considerable international interest in the association (Figures 1.13 and 1.14) between solar ultraviolet B (UVB) radiation and cataract. Much of this interest has resulted from concern about the health effects of the increasing levels of UVB reaching the earth's surface as a consequence of depletion of the stratospheric ozone layer.
Young suggests that sunlight is the primary causal factor in cataractogenesis, and strongly advocates the widespread distribution of sunglasses to prevent cataract. Harding on the other hand suggests that sunlight is not a major etiological factor in human cataract formation.
The lens is known to absorb UVB and UVA and change in lens clarity has been linked in animal experiments with short-term, high intensity exposure and chronic exposure to UVB.
Epidemiologic studies have demonstrated cataract to be more prevalent in sunny countries, such as Israel, than in cloudy countries, such as England. Moreover, in Romania and the United States, cataracts are more prevalent in dry hot areas with more sun exposure within each country than in areas with prolonged cloud cover. The Beaver Dam Eye Study found an association between ultraviolet B radiation exposure and cortical cataract in men only.
The Lens Opacity Case-Control Study did not find an association between sun exposure and any type of cataract development. However, this study investigated only urban populations, and this may explain why no association was found. In both the Italian-American Cataract Study and India-US Case-Control Study, sunlight exposure was associated with cataract formation. Taylor studied 797 watermen and went to great lengths to calculate an ultraviolet radiation exposure index on the basis of field variables such as outdoor hours worked, work location, and attenuation due to spectacle use and hat cover. He found a significant association between ultraviolet B radiation index and cortical cataract but found no association with other morphological cataract types.
Bochow et al studied the relationship between ultraviolet radiation exposure and posterior subcapsular cataract. He not only discovered a significant association but also a dose-response relationship.
Two unique studies, one prospective and one case-control, provide indirect evidence that ultraviolet light plays a role in cataract formation. Schein et al studied the distribution of cortical opacities by lens quadrant in a prospective study of Chesapeake Bay watermen. The prevalence of cortical lens opacities increased with age, with a high degree of concordance between eyes. The inferonasal 21lens quadrant was the most common location involved both for new cataract development and for progression of preexisting cataract. Cataract formation in this quadrant was presumed to be the most consistent with ultraviolet radiation damage on the basis of greater exposure in this area of the lens. Resnikoff et al studied the association of lens opacities with two other presumed ultraviolet radiation-associated ocular diseases, climatic droplet keratopathy and exfoliation syndrome. There was a strong correlation between the diseases in this case-control study.
Based on the available epidemiological evidence, the following conclusions can be drawn:
- There is sufficient experimental evidence that exposure to artificial sources of UVB can cause lens opacities in laboratory animals.
- There is limited evidence suggesting that exposure to solar UVB causes cortical opacities in humans.
- There is also limited evidence suggesting that exposure to solar UVB causes posterior subcapsular cataract in humans.
- The epidemiological evidence is consistent in suggesting that nuclear cataracts are not causally associated with exposure to solar UVB.
DRUG-RELATED FACTORS
A number of well-known and widely used drugs have been implicated in cataract etiology with oral corticosteroids probably the widest recognized of all.
Corticosteroids
In 1930, Hench postulated that a naturally occuring substance might be responsible for the clinical improvement seen in women with rheumatoid arthritis when they became pregnant. He called this substance “compound E”, but it was not until 1948 that this substance (soon to be called cortisone) was synthesized and became available for clinical use. In the 50 years since then, corticosteroids have had an enormous impact in medicine, however, it soon became clear that hydrocortisone has significant mineral corticoid as well as anti-inflammatory activity and that this could produce dose-related toxicity. It is now known that the principal naturally occurring corticosteroids secreted by the adrenal cortex are hydrocortisone (cortisol), a glucocorticoid involved in the regulation of carbohydrate, protein and lipid metabolism and aldosterone, a mineralocorticoid affecting fluid and electrolyte balance. Because hydrocortisone also exerts some mineralocorticoid (salt-retaining) effects, several structurally modified glucocorticoids with relatively greater anti-inflammatory and lower salt-retaining properties were synthesized once the therapeutic potential of their anti-inflammatory and immunosuppressive properties became apparent. Anti-inflammatory and immunosuppressive effects occur at doses above the normal physiological levels of daily glucocorticoid production, i.e. at pharmacological doses. However, since many physiological and pharmacological actions are mediated by the same receptor, it is not surprising that prolonged use of pharmacological doses can lead to adverse physiological effects.
It is estimated that between 10 to 60% of patients using systemic corticosteroids develop cataract, especially of the posterior subcapsular (PSC) type. Glucocorticosteroids are lipophilic and therefore diffuse easily across the cell membrane after which they bind and activate a cytoplasmic glucocorticoid receptor. The resulting receptor steroid complex enters the cell nucleus, binds to the glucocorticoid response elements on the DNA and up- or downregulates the expression of corticosteroid-responsive genes with resultant effects on protein synthesis in target tissues.
Several ways have been identified in which corticosteroids may induce cataract formation including:
- Elevation of glucose level
- Inhibition of Na, K-ATPase
- Increased cation permeability
- Inhibition of glucose-6-dehydrogenase
- Inhibition of RNA-synthesis
- Loss of ATP
- Covalent bonding of steroids to lens proteins.
Posterior subcapsular cataract is the hallmark of steroid cataract. It starts as fine granular and vacuolated opacities at the posterior aspect of the lens. PSC opacities occur frequently with high doses (more than 15 mg prednisone or equivalent per day) and prolonged use (more than one year) of corticosteroids. Clinical trials have shown that PSC opacities secondary to oral corticosteroids may develop within as short a time as 4 months.
Recent studies have suggested that the use of inhaled corticosteroids may be a significant risk factor for the development of cataract, may be even more so than the use of oral corticosteroids. These studies have again pointed out the importance of the “first-order effect”. A drug absorbed through the nasal mucosa or conjunctiva “drains” to the right atrium and ventricle. The drug is then pumped in part, to the head (i.e. the eye as a target organ) before returning to the left atrium and ventricle. The second passage is then to the liver and kidneys, where the drug is metabolized and detoxified. With oral medication—the first pass includes absorption from the gut via the liver where, depending on the drug, more than 90% of the drug is detoxified before going to the right atrium. Therefore, oral medications are metabolized even before the first pass, while ocular or nasally administered drugs are not metabolized until the second pass. This, in part, may be a reason why more potent steroid inhalants have greater ocular exposure and some ocular medications cause significant systemic adverse effects.
Considering the widespread use of corticosteroids and their association with PSC cataract, clinicians should be aware of a patient's medication history and recognize the distinguishing features of PSC cataract.22
Allopurinol
Allopurinol is an antihyperuricemic drug widely used for the treatment of hyperuricemia and chronic gout. It inhibits the terminal step in uric acid synthesis, which results in a reduction of uric acid concentrations in both serum and urine. In about 85% of patients with gout, serum urate concentrations can be normalized by an allopurinol dose of 300 mg/d, and in some patients a dose of 100 to 200 mg/d is sufficient. Treatment with allopurinol is usually well tolerated, with hypersensitivity reactions constituting the most common adverse effects.
In 1982, Fraunfelder et al reported 30 cases of cortical and subcapsular cataract associated with long-term use of allopurinol reported to the National Registry of Drug-Induced Ocular Side Effects (Oregon Health Sciences University, Portland). The observed lens changes appeared to have the characteristics of early age-related cataract. At about the same time, Lerman et al used phosphorescence spectroscopy to demonstrate in vitro the probable presence of allopurinol in cataractous lenses that had been extracted from patients treated with allopurinol. The phosphorescence peaks characteristic of allopurinol could not be demonstrated in lenses from patients who had not ingested allopurinol. Evidence from epidemiologic studies on the possible cataractogenic effects of allopurinol is, however, inconclusive. Two separate epidemiologic studies did not show an increased risk. Another study reported an unusual morphologic thinning of the anterior clear zone of the lens in patients receiving long-term treatment with allopurinol. In the Lens Opacities Case Control Study, wherein gout medications were found to be associated with a 2.5-fold increased risk of mixed cataract, no distinction was made between allopurinol and other medications for gout. In a case control study conducted by Garbe et al using data from the Quebec universal health program for all elderly patients they established that a clear relationship exists between the long-term administration of allopurinol and an increased risk for cataract extraction.
Phenothiazines
In 1965, the occurrence of ocular pigmentation and lens opacity in patients on high dose phenothiazine drugs, particularly chlorpromazine, was reported in several papers. Phenothiazine has been thought to cause pigmentation by virtue of its ability to combine with melanin and form a photosensitive product. It is also postulated that this process might accelerate any predisposition to lens opacification from environmental insults such as solar radiation. A study involving schizophrenic patients showed an association between severity or grade of lenticular pigmentation and equivalent dose of phenothiazine intake. Epidemiologic research on the role of phenothiazines in cataractogenesis is limited. A case-control study done in North Carolina found a two-fold increased risk of cataract in those with history of tranquilizer use, although the types of tranquilizers and cataract were not characterized. A health maintenance organization based, non-concurrent prospective study that controlled for steroid use and diabetes documented at least three-fold increased risk for cataract extraction among current and past (two to five years prior to extraction) users of two groups of tranquilizers: “antipsychotic phenothiazine drugs” (chlorpromazine, thioridazine, trifluoperazine, perphenazine, fluphenazine) and “other phenothiazine drugs” (chlorperazine, prochlorperazine, promethazine, trimeprazine).
Given the paucity and limitations of available epidemiologic data, more studies, such as those characterizing the specific types of senile cataract and phenothiazines, are needed to verify any association.
Diuretics and Antihypertensives
Harding and van Heyningen reported that thiazide diuretics were used less frequently by patients who underwent cataract surgery than control subjects. More recently, the Beaver Dam Eye Study found that use of thiazides was associated with lower prevalence of nuclear cataract and increased prevalence of posterior subcapsular cataract. Several other studies have found that use of diuretics was associated with increased risk of cataract. The Beaver Dam Eye Study also found a raised overall risk (OR, 1.3) for potassium-sparing diuretics, but this was not statistically significant. A cataractogenic effect of potassium-sparing diuretics is biologically plausible, as these diuretics disturb sodium transport across the lens fiber membrane.
The calcium channel blocker nifedipine has been associated with increased risk of cataract extraction and angiotensin-converting enzyme inhibitors with decreased risk of nuclear cataract. Hypertension and other cardiovascular conditions is a potential confounding problem in studies of cataract and antihypertensive medications, including diuretics.
Antimalarial Drugs
Most drugs used in the treatment of malaria produce phototoxic side effects in both the skin and the eye. Cutaneous and ocular effects that may be caused by light include: cataract formation, changes in skin pigmentation, corneal opacity and other visual disturbances including irreversible retinal damage (retinopathy) leading to blindness. The mechanism for these reactions in humans is unknown. A number of studies have been published that suggest a strong relationship between chloroquine use and cataract formation. The basis of the relationship seems to lie in the phototoxicity of chloroquine and related drugs.
Because malaria is a disease most prevalent in regions of high light intensity, protective measures (clothing, sunblock, sunglasses or eye wraps) should be recommended whilst taking antimalarial drugs.
Amiodarone
Amiodarone hydrochloride is a benzofurane derivative used for cardiac abnormalities. Its use is commonly associated with an asymptomatic keratopathy. The antiarrhythmic drug 23also produces anterior subcapsular lens opacities that are usually asymptomatic. Anterior subcapsular lens opacities were observed in 7 of 14 patients treated with moderate to high doses of amiodarone at the Veterans Administration Medical Center in San Francisco in 1982. In 1993, a report was published that summarized the status of these same 14 patients 10 years later. Anterior subcapsular lens opacities developed or progressed in all patients continuing treatment with this antiarrhythmic agent during the ensuing 10-year interval. Although Snellen visual acuities were not decreased, subtle visual impairment was present as measured by contrast sensitivity measurements with and without glare. The authors of the report concluded that decrease in visual acuity should not be a contraindication for therapy with this potentially life-saving drug.
Hypocholesterolemic Drugs
Cataract in animals and men are in some instances associated with genetic defects in enzymes that regulate cholesterol metabolism and the use of drugs which inhibit lens cholesterol biosynthesis. The basis of this relationship apparently lies in the need of the lens to satisfy its sustained requirement for cholesterol by on-site synthesis, and impairing this synthesis can lead to alteration of lens membrane structure. The lens membrane contains the highest cholesterol content of any known membrane. The genetic defects Smith-Lemli-Opitz syndrome, mevalonic aciduria, and cerebrotendinous xanthomatosis all involve mutations in enzymes of cholesterol metabolism, and affected patients can develop cataract. Questions about the ocular safety of drugs, which can inhibit lens cholesterol biosynthesis, persist. Concern over potential damage to the lens from the use of hypocholesterolemic drugs stems from the reports in 1962 by Kirby et al and Laughlin et al that treatment of patients with Triparanol (Mer 29, Wm S Merrel Co.) to lower blood cholesterol was associated with development of cataract. Drugs used to lower blood cholesterol are among the most widely prescribed medicines. One drug in the group, lovostatin (Mevacor, Merck), is alone the third most prescribed drug in the United States. This drug can inhibit cholesterol synthesis in lens and produce cataract in dogs. Whether these drugs inhibit cholesterol biosynthesis in human lenses at therapeutic doses is unknown.
The clinical safety trials indicate that treatment with lovastatin for up to five years does not significantly increase the development of cataract or grossly alter visual function. The ocular safety in an older patient population (>50 years) appears high. This seems also to apply to simvastin, except that one clinical trial showed a significant increase in cortical opacities with the use of this drug (Figure 1.15).
An unpublished study conducted at the University of Stellenbosch Medical School found that the rate at which opacification occurs in dyslipidemic patients on Cerivastatin was 4.5% per year (Figure 1.15). Although this rate of opacification is not statistically noteworthy it would seem that if these data are projected over a period of 20 years and compared the normal rate of opacification reported by Boccuzzi and Leino et al, an alarming amount of opacities would be present in the group of patients on cerivistatin.
Hypolipidemic drugs are intended for life-long use and patients as young as 18 years can receive these drugs. Although the human lens grows throughout life, the rate of growth is slow after 10 years of age. About 40 years are required for the lens cortex to double in width. The size of the nucleus remains essentially constant after 10 years of age. Thus, the consequences of inhibiting lens growth due to block of cholesterol biosynthesis may be difficult to assess in only a 1 to 5 years period. A considerable body of evidence indicates that sustained alteration of lens sterol content and composition due to genetic mutations or exposure to drugs can lead to altered lens clarity. Long-term ocular safety of the vastatin drugs should perhaps be viewed in units of 10 to 20 years. Certainly a 20-year-old person required to have cataract surgery at age 40 because of some chronic treatment would constitute a medical crisis for this individual, particularly if a less toxic treatment had been available.
The question of whether the vastatin drugs inhibit lens cholesterol biosynthesis in humans treated with standard therapeutic doses is unanswered. Since very low concentrations of lovastatin and simvastatin are required to inhibit cholesterol synthesis in animal lenses (3–22 nM), and only five times the therapeutic dose of lovostatin decreased cholesterol accumulation by the rat lens, it at least appears possible that therapeutic doses could inhibit lens cholesterol biosynthesis in humans.
CONCLUSION
Human lenticular opacification leading to the clinical challenge of cataract formation is etiologically multifactorial. It does seem, however, that evidence is slowly mounting to encourage clinicians to consider cataract as belonging to the growing list of preventable ocular diseases.
FURTHER READING
- Adler NE, Boyce T, Chesney MA, et al. Socioeconomic inequalities in health. No easy solution. JAMA 1993; 269: 3140–5.
- Alden ER, Ralina RE, Hodson WA. Transient cataract in low-birth-weight infants. J Pediatr 1973; 82: 318–31.
- Amaya LG, Speedwell L, Taylor D. Contact lenses for infant aphakia. Br J Ophthalmol 1990; 74: 154–6.
- Ames GM, Janes CR. Heavy and problem drinking in an American blue-collar population: implications for prevention. Soc Sci Med 1987; 8: 949–60.
- Ansari NH, Awasthi YG, Srivastava SK. Role of glycosy-lation in protein disulphide formation and cataracto-genesis. Exp Eye Res 1980; 31: 9–19.
- Armitage MM, Kivun JD, Farrell RE. A progressive early onset cataract gene maps to human chromosome 17q24. Nature Facet 1995; 937–40.
- Assmann, et al. Lipid Metabolism Disorders and Coronary Heart Disease. MMV-Medizin-Verl (2nd ed), 1993.
- Baghdassarian SA, Tabbara KF. Childhood blindness in Lebanon. Am J Ophthalmol 1975; 79: 827–30.
- Bandmann O, Vaughan J, Holmans P, et al. Association of slow acetylator genotype for N-Acetyltransferase 2 with familial Parkinson's disease. Lancet 1997; 350: 1136–9.
- Barnes PJ. Anti-inflammatory mechanisms of glucocorticoids. Biochem Soc Trans 1995; 23: 940–5.
- Behrens-Baumann W, Thiery J, Wieland E, et al. 3-Hydroxy-3-methylglutaryl coenzyme—a reductase inhibitor simvastatin and the human lens: clinical results of 3-year follow-up. Arzneim-Forsch 1992; 42 (11): 1023–4.
- Beigi B, O'Keefe M, Bowell R, et al. Ophthalmic findings in classical galactosaemia—prospective study. Br J Ophthalmol 1993; 77: 1624–64.
- Belpoliti M, Maraini G. Sugar alcohols in the lens epithelium of age-related cataract. Exp Eye Res 1993; 56: 3–6.
- Benos DJ. Amiloride: a molecular probe of sodium trans-port in tissues and cells. Am J Physiol 1982; 242: C131–45.
- Benson WH, Farber ME, Caplan RJ. Increased mortality rates after cataract surgery: a statistical analysis. Ophthalmology 1988; 95: 1288–92.
- Berger J, Shepard D, Morrow F, et al. Relationship between dietary intake and tissue levels of reduced and total vitamin C in the guinea pig. J Nutr 1989; 119: 1–7.
- Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol 1967; 12 (5): 415–47.
- Bhatnagar R, West KP (Jr), Vitale S, et al. Risk of cataract and history of severe diarrheal disease in Southern India. Arch Ophthalmol 1991; 109: 696–99.
- Bhuyan KC, Bhuyan DK, Podos SM. Free radical enhancer xenobiotic is an inducer of cataract in rabbit. Free Radical Res Comm 1991; 12-13: 609–20.
- Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci 1986; 38: 1463–71.
- Bialas MC, Routledge PA. Adverse effects of cortico-steroids. Adverse Drug React Toxicol Rev 1998; 17 (4): 227–35.
- Björkhem, I, Boberg KM. Inborn errors in bile biosynthesis and storage of sterols other than cholesterol. Metabolic Basis of Inherited Disease; McGraw-Hill New York, 1995; 7: 2073–99.
- Blondin J, Baragi VJ, Schwartz E, et al. Delay of UV-induced eye lens protein damage in guinea pigs by dietary ascorbate. Free Radic Biol Med 1986; 2: 275–81.
- Boccuzzi SJ, Bocanegra TS, Walker JF, et al. Long-term safety and efficiency profile of simvastatin. Am J Cardiol 1991; 86: 1127–31.
- Bochow TW, West SK, Axar A, et al. Ultraviolet light exposure and risk of posterior subcapsular cataract. Arch Ophthalmol 1989; 107: 369–72.
- Bonting SJ. Na'K’ activated adenosine triphosphatase and active cation transport in the lens. Invest Ophthalmol 1965; 4: 723.
- Brauweiler PH, Wehler T, Busin M. High incidence of cataract formation after implantation of a silicone posterior chamber lens in phakic, highly myopic eyes. Ophthalmology 1999; 106 (9): 1651–5.
- Brilliant LB, Grasset NC, Pokrel RP, et al. Associations among cataract prevalence, sunlight hours and altitude in the Himalayas. Am J Epidemiol 1983; 118: 250–64.
- Brown CA, Burman D. Transient cataract in a diabetic child with hyperosmolar coma. Br J Ophthalmol 1973; 57: 429–33.
- Burke JP, O'Keefe M, Bowell R, et al. Ophthalmic findings in classical galactosemia: a screened population. Pediatr Ophthal Strabismus 1989; 26: 165–8.
- Caird Fl, Pirie A, Ramsell TG. Diabetes and the Eye. Blackwell Scientific: Oxford 1969.
- Caird Rl, Hutchinson M, Pirie A. Cataract and diabetes. BMJ 1964; 2: 665–8.
- Cenedella RJ. Cholesterol and cataract. Surv Ophthalmol 1996; 40: 320–37.
- Chatterjee A, Milton RC, Thyle S. Prevalence and aetiology of cataract in Punjab. Br J Ophthalmol 1982; 66: 35–42.
- Chiba M, Masironi R. Toxic and trace elements in tobacco and tobacco smoke. Bull World Health Organ 1992; 70: 270–6.
- Christen WG, Manson JE, Seddon JM, et al. A prospective study of cigarette smoking and risk of cataract in men. JAMA 1992; 268: 989–93.
- Chylack LT Jr, Henriques H, Tung W. Inhibition of sorbitol production in human lenses by an aldose reductase inhibitor. Invest Ophthalmol Vis Sci 17: ARVO (Suppl): 1978; 300.
- Cigala O, Pancallo MT, Della Valle M, et al. La simvastatina nel trattamento delle piercolesterolemie. La Clinica Terapeu 1991; 137: 333–7.
- Clair WK, Chylack LTJ, Cook EF, et al. Allopurinol use and the risk of cataract formation. Br J Ophthalmol 1989; 73: 173–6.
- Clayton RM, Cuthbert J, Duffy J, et al. Some risk factors associated with cataract in SE Scotland: a pilot study. Trans Ophthalmol Soc UK 1982; 102: 331–6.
- Clayton RM, Cuthbert J, Philips CJ, et al. Epidemiological and other studies in the assessment of factors contributing to cataractogenesis. Ciba Fdn Symp 1984; 106: 25–47.
- Clayton RM, Cuthbert J, Philips CJ, et al. Some risk factors associated with cataract in SE Scotland: A pilot study. Trans Ophthalmol Soc UK, 1982; 102: 331–6.
- Clayton RM, Cuthbert J, Phillios CI, et al. Analysis of individual cataract patients and their lenses: a progress report. Exp Eye Res 1980; 31: 553–6.
- Cohen DL, Neil HA, Sparrow J, et al. Lens opacity and mortality in diabetes. Diabetic Med 1990; 7: 615–7.
- Collman GW, Shore DL, Shy CH, et al. Sunlight and other risk factors for cataract: an epidemiological study. Am J Public Health 1988; 78: 1459–62.
- Cooperative Study of Lipoproteins and Atherosclerosis. Evaluation of serum lipoprotein and cholesterol measurements as predictors of clinical complications of atherosclerosis. Circulation 1956; 14. 2: 691–741.
- Cotlier E, Kwan B, Beatty C. The lens as an osmometer. Bioctfm Biophys Acta 1968; 150: 705.
- Cotlier E, Rice P. Cataract in the Smith-Lemli-Opitz syndrome. Am J Ophthalmol 1971; 72: 955–9.
- Cotlier E. Congenital rubella cataract. In Cotlier E, Lam-bert SR, Taylor D (Eds): Congenital Cataract. RG Landes/CRC: Boca Raton, 1994; 65–76.
- Cotlier E. Congenital varicella cataract. Am Ophihalmol 1978; 86: 627–9.
- Cruickshanks KI, Klein BEK, Klein R. Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health 1992; 82: 1658–62.
- Cuthbert J, Clayton RM, Philips. Cuneiform cataract: a special case? Colloq D'INSERM, 147: 387–96.
- Dawber TR. The Framingham Study: The Eipidemiology of Atherosclerotic Disease. Cambridge Harvard University Press: London 1980.
- de Vries ACJ, Cohen LH. Different effects of the hypo-lipidemic drugs pravastatin and lovastatin on the cholesterol biosynthesis of the human ocular lens in organ culture and on the cholesterol content of the rat lens in viva. Biochim Biophys Acta 1993; 1167: 63–69.
- Dohi K, Fukuda K, et al. Cataract in kidney transplant patients. Horishima J Med Sci 1984; 33: 275–8.
- Dolan BJ, Flach AJ, Peterson JS. Amiodarone keratopathy and lens opacities. J Am Optom Assoc 1985; 56 (6): 468–70.
- Donahue RP, Bias WB, Renwickj H, et al. Probable assignment of the Duffy blood group locus to chromosome I in man. Proc Natl Acad Sci USA 1968; 61: 949–55.
- Dorland's Illustrated Medical Dictionary (28th edn) WB Saunders: Philadelphia 276.
- Drack AV, Burke JP, Pulido JS, et al. Transient punctate lenti-cular opacities as a complication of argon laser photo-ablation in an infant with retinopathy of prematurity. Am J Ophthalmol 1992; 113: 583–4.
- Drews RC. Alcohol and cataract. Arch Ophthalmol 1993; 111: 1312.
- Drews RC. Ethanol cataract. In Solanes M (Ed): XXI Concilium Ophthalmologicum Mexico 1970. the Netherlands Exerpta Medica Amsterdam: 1970; 753–8.
- Duncan G, Hightower KR, Gandolfi SA, Tomlinson J, Maraini G. Human lens membrane cation permeability increases with age. Invest Ophthalmol Vis Sci 30: 1989; 1855–9.
- Dunn JP, Jabs DA, Wingard JC, et al. Bone marrow trans-plantation and cataract development. Arch Ophthalmol 1993; 11: 1367–73.
- Duthie GG, Arthur JR, James WP. Effects of smoking and vitamin E on blood antioxidant status. Am J Clin Nutr 1991; 53 (Suppl): 1061S–64S.
- Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol 1981; 91: 381–95.
- Eiberg H, Marner E, Rosenberg T, et al. Marner's cataract (AM) assigned to chromosome 16: linkage to haptoglobin. Clin Cenet 1988; 34: 272–5.
- Elsas U II, Fridovich-Keil JL, Leslie ND. Galactosemia: a mole-cular approach to the enigma. Pediatr 1993; 8: 101–09.
- Elsas U, Dembure PP, Langley S, et al. A common mutation asso-ciated with the Duarte galactosemia allele. Am J Hum Cenet 1994; 54: 1030–36.
- El-Yazigi A, Johansen K, Raines DA, et al. N-Acetylation Poly-morphism and diabetes mellitus among Saudi-Arabians. J Clin Pharmacol 1992; 32 (10): 905–10.
- Emmelot P. The organization of the plasma membrane of mam-malian cells: structure in relation to function. In Jamieson GA, Robinson DM (Eds): Mammalian Cell Membranes, Butterworths: Boston, 1977; 2: 1–54.
- Emmerson BT. The management of gout. N Engl J Med 1996; 446: 445–51.
- Epstein FH. Cardiovascular Disease Epidemiology; A Journey from the Past Into the Future. Circulation 1996; 93: 1755–64.
- Erdman J. The physiologic chemistry of carotenes in man. Am J Clin Nutr 1988; 7: 101–6.
- Fechner PU. Cataract formation with a phakic IOL. J Cataract Refract Surg 1999; 25 (4): 461–2.
- Fink AM, Gore C, Rosen E. Cataract development after implantation of the Staar Collamer posterior chamber phakic lens. J Cataract Refract Surg 1999; 25 (2): 278–82.
- Finley SC, Finley WH, Monsky DM. Cataract in girl with features of Smith-Lemli-Opitz syndrome. J Pediatr 1969; 75: 706–07.
- Flach AJ, Dolan BJ, Sudduth B, et al. Amiodarone-induced lens opacities. Arch-Ophthalmol 1983; 101 (10): 1554–6.
- Flach AJ, Dolan BJ. Progression of amiodarone induced cataract. Doc Ophthalmol 1993; 83 (4): 323–9.
- Flaye DE, Sullivan KN, Cullinan TR, et al. Cataract and cigarette smoking: the City Eye Study. Eye 1989; 3: 379–84.
- Francois J. Congenital Cataract, Charles C Thomas (Ed): Springfield, 1963.
- Francois J. Late results of congenital cataract surgery. Ophthalmol 1979; 86: 1586–98.
- Franfelder FT. Do inhaled corticosteroids significantly increase cataract surgery in elderly patients? Arch Ophthalmol 1998; 116: 1369.
- Fraunfelder FT, Hanna C, Dreis MW, et al. Cataract associated with allopurinol therapy. Am J Ophthalmol 1982; 94: 137–40.
- Friend J, Chylack LT, Khu P, et al. The MSDRL Study Group: Lack of human cataractogenic potential of lovastatin: results of three-year study. Invest Ophthalmol Vis Sci 1992; 33: 1301.
- Garbe E, Suissa S, Le Lorier J. Exposure to allopurinol and the risk of cataract extraction in elderly patients. Arch Ophthalmol 1998; 116: 1652–6.
- Garner MH, Spector A. ATP hydrolysis kinetics by Na, K- ATPase in cataract. Exp Eye Res 1986 42: 339–48.
- Gibbs ML, Jacobs M, Wilkie AOM, et al. Posterior lens. Surv Ophthalmol 1996; 40 (6).
- Giblin FJ, Padgaonkar VA, Leverenz VR, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res 1995; 60: 219–35.
- Giblin FJ, Schrimscher L, Chakrapani B, et al. Exposure of rabbit lens to hyperbaric oxygen in vitro: regional effects on GSH level. Invest Ophthalmol Vis Sci 1988; 29: 1312–9.
- Gofman, et al. The role of lipids and lipoproteins in atherosclerosis. Science 1950; 111: 166–71.
- Gretton C. Like falling off a cliff. Med Ad News 1994; 3–25.
- Grundy SM. HMG-KoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med 1988; 319: 24: 33.
- Gumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataract. N Engl J Med 1997; 337: 8–14.
- Hankinson SE, Seddon JM, Colditz, et al. A prospective study of aspirin use and cataract extraction in women. Arch Ophthalmol 1989; 111: 503–8.
- Hankinson SE, Stampfer MJ, Seddon JM, et al. Intake and cataract extraction in women: a prospective study. Br Med J 1992; 305: 335–9.
- Hankinson SE, Willet WC, Colditz GA, et al. A prospective study of cigaret smoking and risk of cataract surgery in woman. JAMA 1992; 268: 994–8.
- Harding J. Cataract: Biochemistry, Epidemiology and Pharmacology. Chapman and Hall: London, 1991; 122–3.
- Harding JJ, Crabe MJC. The lens: development, proteins, metabolism and cataract. In Davidson H (Ed): The Eye, Academic Press: London, 207–492.
- Harding JJ, Egerton M, Harding RS. Protection against cataract by aspirin, paracetamol and ibuprofen. Acta Ophthalmol 1989; 67: 518–24.
- Harding JJ, Harding RS, Egerton M. Risk factors for cataract in Oxfordshire: diabetes, peripheral neuropathy, myopia, glaucoma and diarrhoea. Acta Ophthalmol 1989; 67: 510–17.
- Harding JJ, van Heyningen R. Beer, cigarets and military work as risk factors for cataract. Dev Ophthalmol 1989; 17: 13–16.
- Harding JJ, van Heyningen R. Drugs including alcohol that act as risk factors for cataract and possible protection against cataract by aspirin like drugs. Br J Ophthalmol 1989; 73: 579–80.
- Harding JJ, van Heyningen R. Drugs including alcohol, that act as risk factors for cataract, and possible protection against cataract by aspirin-like analgesics and cyclopenthiazide. Br J Ophthalmol 1988; 72: 809–14.
- Harding JJ. Cataract Biochemistry, Epidemiology and Pharma-cology. Chapman and Hall 1991: 116–8.
- Harding JJ. Physiology, biochemistry, pathogenesis, and epidemiology of cataract. Curr Opinion Ophthalmol 1992; 3: 3–12.
- Harding JJ. Possible causes of the unfolding of proteins in cata-ract and a new hypothesis to explain the high prevalence of cataract in some countries. In Regnault F, Hockwin O, Courtois Y (Eds): Ageing of the lens. Proceedings of the symposium on the aging of the lens held in Paris, September 1979. Biomedical Press: Amsterdam, 1980; 71–80.
- Havel, et al. Lovastatin (Mevolin) in the treatment of heterozygous familial hypercholesterolemia. Ann Intern Med 1987; 107: 609–15.
- Hayes RB, Bi W, Rothman N, et al. N-Acetylation phenotype and genotype and risk of bladder cancer in benzidine-exposed workers. Carcinogenesis (United States); 1993; 14 (4): 675–8.
- Henkj M, Whitelocke RAF, Warrington AlP, et al. Radiation dose to the lens and cataract formation. Radiat Oncol Biol Phys 1993; 25: 815–20.
- Hesker H. Antioxidative vitamins and cataract in the elderly. Z Ernahrungswiss 1995; 34: 167–76.
- Hiller R, Giacometti L, Yuen K. Sunlight and cataract—an epidemiologic investigation. Am J Epidemiol 1977; 105.
- Hiller R, Sperduto RD, Ederer F. Epidemiologic associations with cataract in the 1971-1972 National Health and Nutrition Examination Survey. Am J Epidemiol 1983; 118: 239–49.
- Hiller R, Sperduto RD, Ederer F. Epidemiologic associations with nuclear, cortical, and posterior subcapsular cataract. Am J Epidemiol 1986; 124: 916–25.
- Hiller R, Yuen K. Sunlight and cataract: an epidemiological investigation. Am J Epidemiol 1977; 105: 450–9.
- Hing S, Speedwell L, Taylor D: Lens surgery in infancy and childhood. Br J Ophthalmol 1990; 74: 73–7.
- Hockwin O, Koch H. Cataract of toxic etiology. In Bellows (Ed): Cataract and Abnormalities of the Lens. Grune and Stratton 1975; 234–45.
- Hoffmann G, Gibson KM, Brandt IK, et al. Mevalonic aciduria: an inborn error of cholesterol and nonsterol isoprene biosynthesis. N Engl J Med 1986; 314: 1610–14.
- Holowich F, Boateng A, Kolck B. Toxic Cataract. In Bellows JG (Ed): Cataract and Abnormalities of the Lens. Grune and Stratton: New York 1975; 230–43.
- Hunninghake, et al. Lovastatin—follow up ophthalmo-logical data. JAMA 1988; 259: 354–5.
- Hyman L. Epidemiology of eye disease in the elderly. Eye 1987; 1: 330–41.
- Jaafar MS, Robb RM. Congenital anterior polar cataract. Ophthalmology 1984; 91: 249–54.
- Jackson RC. Temporary cataract in diabetes mellitus. Br J Ophthalmol 1955; 39: 629–31.
- Jacques PF, Chylack LT (Jr). Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr 1991; 53: 352S–55S.
- Jacques PF, Taylar A, Hankinson SE, et al. Long-term vitamin C supplement use and prevalence of early age-related lens opacities. Am J Clin Nutr 1997; 66: 911–6.
- Jahn CE, Janke M, Winowski H, et al. Identification of metabolic risk factors for posterior subcapsular cataract. Ophthalmic Res 1986; 18: 112–6.
- Jay B, Black RE, Wells RS. Ocular manifestations of ichthyosis. Br J Ophthalmol 1968; 52: 217–26.
- Jedziniak JA, Chylack LT Jr, Cheng HM, et al. The sorbitol path-way in the human lens: aldose reductase and polyol dehydro-genase. Invest Ophthalmol Vis Sci 1981; 20: 314–26.
- Jick H, Brandt DE. Allopurinol and cataract. Am J Ophthalmol 1984; 98: 355–8.
- Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. II—Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiolo 1977; 106: 33–41.
- Kahn MU, Kahn MR, Sheikh AK. Dehydrating diarrhoea and cataract in rural Bungladesh. Ind J Med Res 1987; 85: 311–5.
- Kallner AB, Hartmann D, Horning DH. On the require-ments of ascorbic acid in men: steady-state turnover and blood pool in smokers. Am J Clin Nutr 1981; 34: 1347–55.
- Kanski JJ. Clinical Ophthalmology (3rd edn). Butterworth-Heineman: Oxford. 1994; 289.
- Kasai K, Nakamura T, Kase N et al. Increased glycosylation of proteins from cataractous lenses in diabetes. Dia-betologia 1983; 25: 36–8.
- Kench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocortico-sterone; compound E) and of pituitary and adrenocorticotrophic hormone on rheumatoid arthritis. Mayo Clin Prvc 1949; 4: 181–97.
- Keys A. Atherosclerosis: a problem in newer public health. J Mt Sinai Hosp 1953; 20: 118–39.
- Kirby TJ, Achor RWP, Perry HO, et al. Cataract formation after triparanol therapy. Arch Ophthalmol 1962; 68: 486–9.
- Klein BEK, Klein R, Lee KE. The incidence of age-related cataract, the Beaver Dam Eye Study. Arch Ophthalmol 1998; 116: 219.
- Klein BEK, Klein R, Linton KL, et al. Cigaret smoking and lens opacities: the Beaver Dam Eye Study. Am J Prev Med 1993; 9: 27–30.
- Klein BEK, Klein R, Linton KL, et al. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol 1993; 137: 190–200.
- Klein BEK, Klein R, Moss SE. Prevalence of cataract in a popu-lation-based study of persons with diabetes mellitus. Ophthalmology 1985; 92: 1191–6.
- Klein BEK, Klein R, Ritter LL. Is there evidence of an estrogen effect on age-related lens opacities? The Beaver Dam Eye Study. Arch Ophthalmol 1994; 112: 85–91.
- Klein R, Klein BEK, Jenses SC, et al. The relation of socioeconomic factors to age-related cataract, maculopathy, and impaired vision. Ophthalmology 1969-79; 101 (21).
- Klein R, Klein BEK, Moss SE. Visual impairment in diabetes. Ophthalmology 1984; 91: 1–8.
- Klein R, Moss SE, Klein BE, et al. Relation of ocular and systemic factors to survival in diabetes. Arch Intern Med 1989; 149: 266–72.
- Kleinman NJ, Spector A. The relationship between oxi-dative stress, lens epithelial cell DNA and cataractogenesis. Exp Eye Res 1992; 55 (Suppl): 1 (abstract 807).
- Koga T, Shimada Y, Kuroda M, et al. Tissue-selective inhibition of cholesterol synthesis in viva by pravastatin sodium, a 3-hydroxy-3-methylglutaryl coenzyme: a reductase inhibitor. Biochim Biophys Acta 1990; 1045: 115–20.
- Köhler L, Stigmar G. Vision screening of four-year-old children. Acta Paediatr Scand 1973; 62: 17–27.
- Kreines K, Rowe KW. Cataract and adult diabetes. Ohio Med J 1979; 75: 782–6.
- Kretzer FL, Hittner HM, Mehta RS. Ocular manifestations of the Smith-Lemli-Opitz syndrome. Arch Ophthalmol 1981; 99: 2000–6.
- Kuchle M, Schonherr U, Dieckmann U. Risk factors for capsular rupture and vitreous loss in extracapsular cataract extraction. The Erlangen Ophthalmology Group. Fortschr Ophthalmol 1989; 86: 417–21.
- Kuriyama M, Fujiyama J, Yoshidome H, et al. Cerebro-tendinous xanthomatosis: clinical and biochemical evaluation of eight patients and review of the literature. J Neurol Sci 1991; 102: 225–32.
- Kuzma JW, Kissinger DG. Patterns of alcohol and cigarette use in pregnancy. Neurobehav Toxicol Teratol 1981; 3: 211–21.
- Lambert SR, Taylor D, Kriss A, et al. Ocular manifestations of the congenital varicella syndrome. Arch Ophthalmol 1989; 107: 52–6.
- Laqua H. Kataract bei chronisher Nierensuffinzeinz und Dialysebehanlung. Klin MBl Augenheilk 1972; 160: 346–9.
- Laties AM, Shear CL, Lippa EA, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results II. Assessment of the human lens after 48 weeks of treatment with lovastatin. Am J Cardiol 1991; 67: 447–53.
- Laughlin RC, Carey TF. Cataract in patients treated with triparanol. JAMA 1962; 181: 339–40.
- Laurent M, Kern P, Regnault F. Thickness and collagen metabolism of lens capsule from genetically prediabetic mice. Ophthalmic Res 1981; 13: 93.
- Law MR, Wald NJ. An ecological study of serum cholesterol and ischeamic heart disease between 1950 and 1990. Eur J Clin Nutr 1994; 48: 305–25.
- Leino M, Pyorala K, Lehto S, et al. Lens opacities in patients with hypercholesterolemia and ischemic heart disease. Doc Ophthalmol 1992; 80: 309–15.
- Lerman S, Megaw JM, Fraunfelder FT. Further studies on allopurinol therapy and human cataractogenesis. Am J Ophthalmol 1984; 97: 205–9.
- Lerman S, Megaw JM, Gardner K. Allopurinol therapy and cataractogenesis in humans. Am J Ophthalmol. 1982; 94: 141–6.
- Lerman S, Moran M. Sorbitol generation and its inhibition by Sorbinil in the aging normal human and rabbit lens and human diabetic cataract. Ophthalmic Res 1988; 20: 348–52.
- Leske MC, Chylack LT (Jr), He Q, et al. The LSC Group: Anti-oxidant vitamins and nuclear opacities—The longi-tudial study of cataract. Ophthalmology 1998; 105: 831–6.
- Leske MC, Chylack Lt (Jr), Wu S. The lens opacities case-control study: Risk factors for cataract. Arch Ophthalmol 1991; 109: 244–51.
- Leske MC, Connel AMS, Schadat A. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol 1997; 115: 105.
- Lessel S, Forbes AP. Eye signs in Turner's syndrome. Arch Ophthalmol 1966; 76: 211–3.
- Letson RD, Desnick RJ. Punctate lenticular opacities in Type II mannosidosis. Am J Ophthalmol 1978; 85: 218–24.
- Leveille PJ, Weidruch R, Walford RL, et al. Dietary restriction retards age-related loss of gamma crystalline in the mouse lens. Science 1998; 224: 1247–9.
- Liang J, Chakrabarti B. Sugar-induced change in near ultraviolet circular dichroism of alpha-crystallin. Biochem Biophys Res Commun 1981; 102: 180.
- Libondi T, Menzione M, Auricchio G. In vitro effect of alpha-tocopherol on lysophosatiphatidylcholine-induced lens damage. Exp Aye Res 1985; 40: 661–6.
- Lim R, Mitchell P, Cumming RG. Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci 1999; 40 (12): 3021–6.
- Lin LR, Reddy VN, Giblin FJ, et al. Polyol accumulation in cultured human lens epithelial cells. Exp Eye Res 1991; 52: 93–100.
- Liu CS, Brown NA, Leanard TJ, et al. The prevalence and morpho-logy of cataract in patients on allopurinol treatment. Eye 1988; 2: 600–6.
- Lovastatin Study Group II. Therapeutic response to lovastatin (mevolin) in non-familial hypercholesterolemia. JAMA 1988; 260: 359–66.
- Lovastatin Study Group III. A multicenter comparison of lovastatin and cholestyramine therapy for severe primary hypercholesterolemia. JAMA 1988; 260: 359–66.
- Lovastatin Study group IV. A multicenter comparison of lovastatin and probucol for treatment of severe primary hypercholesterolemia. Am J Cardiol 1990; 66: 22B–30B.
- Lubkin VL. Steroid cataract—a review and conclusion. J Asthma Res 1977; 14: 55–9.
- Lubsen NH, Renwickj FL, Tsui LC, et al. A locus for a human hereditary cataract is closely linked to the gamma-crystallin gene family. Proc NatI Acad Sri USA 1987; 84: 489–92.
- Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidants. FASEB J 1987; 1: 441–5.
- Marais JS. The Cape Coloured People 1652 to 1932 (1st edn) 1-31. Witwatersrand University Press: Johannesburg, 1957.
- Mares-Perlman JA, Brady WE, Klein BEK, et al. Diet and nuclear lens opacities. Am J Epidemiol 1995b; 141: 322–34.
- Marinho A, Neves MC, Pinto MC, et al. Posterior chamber silicone phakic intraocular lens. J Refract Surg 1997 13 (3): 219–22.
- Marmot MG, Kogevinas M, Elston MA. Social/economic status and disease. Ann Rev Public Health 1987; 8: 111–35.
- Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 1991; 337: 1387–93.
- Matthews KA, Kelsy SF, Meilahn EN, et al. Educational attainment and behavioral and biologic risk factors for coronary heart disease in middle-aged women. Am J Epidemiol 1989; 129: 1132–44.
- McCarty CA, Mukesh BN, Fu CL, et al. The epidemiology of cataract in Australia. Am J of Ophthal 1999; 128 (4): 446–65.
- McCornsick AQ. Transient cataract in prensature infants:a new clinical entity. Can J Ophthalmol 1968; 3: 302–8.
- Meltzer EO. Prevalence, economic, and medical impact of tobacco smoking. Ann Allergy 1994; 73: 381–91.
- Merin S, Crawbird S: The etiology of congenital cataract. Can J Ophthalmol 1971; 6: 1782–4.
- Merits S, Craw S. Hypoglycemia and infantile cataract. Arch Ophthalmol 1993; 86: 495–8.
- Micozzi MS, Beecher GR, Taylor HR, et al. Carotenoid analyses of selected raw and cooked foods associated with a lower risk for cancer. J Natl Cancer Inst 1990; 82: 282–5.
- Minassian DC, Mehra V, Jones BR. Dehidrational crisis from severe diarrhoea or heatstroke and risk factor for cataract. Lancet 1984; 1: 751–3.
- Minassian DC, Mehra V, Jones BR. Dehidrational crisis: a major risk factor in the risk of blinding cataract. Br J Ophthalmol 1989; 73: 100–5.
- Minchin RF, Kadlubar FF, Ilett KF. Role of acetylation in colorectal cancer. Mutat Res 1993; 290 (1): 35–42.
- Mitchell RN, Cotran RS: In Kumar V, Cotran RS, Robinson SL (Eds). Basic Pathology (6th edn): WB Saunders Company.
- Mohan M, Sperduto RD, Angra SK, et al. India US case control study of age-related cataract. Arch Ophthalmol 1989; 107: 670–6.
- Molgaard J, Lundh B, van Schenck H, et al. Long- term efficacy and safety of simvastatin alone and in combination therapy in treatment of hypercholesterolemia. Atherosclerosis 1991; 91: S21–24.
- Mosley ST, Kalinowski SS, Schafer BL, et al. Tissue-selective acute effects of inhibitors of 3-hydroxy-3-methyl- glutaryl coenzyme: a reductase on cholesterol biosynthesis in lens. J Lipid Res 1989; 50: 1411–20.
- Mostafa MSE, Teintamy S, EI-Gammal MY, et al. Genetic studies of congenital cataract. Metah Pediatr Ophthalmol 1981; 5: 233–42.
- Motten AG, Martinez LJ, Holt N, et al. Photophysical studies on antimalarial drugs. Photochem Photobiol 1999; 69 (3): 282.
- Mune M, Meydani M, Jahngen-Hodge J, et al. Effect of calorie restriction on liver and kidney glutathione in aging emory mice. AGE 1995; 18: 49.
- Munoz B, Tajchman U, Bochow T, et al. Alcohol use and risk of posterior subcapsular opacities. Arch Ophthalmol 1993; 111: 110–12.
- Nagata M, Hohmann TC, Nisihimura C, et al. Polyol and vacuole formation in cultured canine kens epithelial cells. Exp Eye Res 1989; 48: 667–77.
- Nakamura B, Nakamura O. Ufer das vitamin C in der linse und dem Kammerwasser der menschlichen katarakte. Graefes Arch Clin Exp Ophthalmol 1935; 134: 197–200.
- Neilson NV, Vinding T. The prevalence of cataract in insulin-dependent and non-insulin-dependent diabetes mellitus: an epidemiological study of diabetics treated with insulin and oral hypoglycaemic agents (OHA). Acta Ophthalmol 1984; 62: 591–602.
- O'Neil WM, Gilfix BM, Di Girolamo A, et al. N-acetylation among HIV-positive patients and patients with AIDS: when is fast, fast and slow, slow? Clin Pharmacol Ther 1997; 62 (3): 261–71.
- Orzechowska-Juzwenko K, Milejski P, Patkowski J, et al. Acetylator phenotype in patients with allergic diseases and its clinical significance. Int J Clin Pharmacol Ther Toxicol 1990; 28 (10): 420–5.
- Oshima Y, Emi K, Motokura M, et al. Survey of surgical indications and results of primary pars plana vitrectomy for rhegmatogenous retinal detachments. Jpn J Ophthalmol 1999; 43 (2): 120–6.
- Oxman TE, Berkman LF, Kasl S, et al. Social support and depres-sive symptoms in the elderly. Am J Epidemiol 1992; 135: 356–68.
- Pacurariu I, Marin C: Changes in the incidence of ocular disease in children and old people. Ofthalmologia (Buchuresti). 1973; 17: 289–308.
- Palmquist BM, Phillipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol 1984; 60: 113–7.
- Pande A, Gamer WH, Spector A. Glucosylation of human lens protein and cataractogenesis. Biochem Biophys Res Commun 1979; 89: 1260–6.
- Petrohelos MA: Chloroquine-induced ocular toxicity. Ann Ophthalmol 1974; 6 (6): 615.
- Pirie A, van Heyningen R. The effect of diabetes on the content of sorbitol, glucose, fructose and inositol in the human lens. Exp Eye Res 1964; 3: 124–31.
- Probst-Hensch NM, Haile RW, Ingles SA, et al. Acetylation polymorphism and prevalence of colorectal adenomas. Cancer Res (US) 1995; 55 (10): 2017–20.
- Pruett RC. Ritinitis pigmentosa: clinical observations and correlations. Trans Am Ophthalmol Soc 1983; 81: 693–35.
- Racz P, Erdohelyi A. Cadmium, lead and copper concentrations in normal and senile cataractous human lenses. Ophthalmic Res 1988; 20: 10–13.
- Rafferty iVS. Lens morphology. In Maisel H (Ed): The Ocular Lens: Structure, Function and Pathology. Marcel Dekker: New York 1985; 1–60.
- Ramsay RC, Barbosa JJ. The visual status of diabetic patients after renal transplantation. Am J Ophthalmol 1979; 87: 305–10.
- Reddy VN. Glutathione and its function in the lens—an overview. Exp Eye Res 1990; 150: 771–8.
- Renwick JH, Lawler SD. Probably linkage between a congenital cataract locus and the Duffy blood group locus. Ann Ham Genet 1963; 27: 67–84.
- Resnikoff S, Filliard G, Dell'Aquila B. Climatic droplet kera-topathy, exfoliation syndrome, and cataract. Br J Ophthalmol 1991; 75: 734–6.
- Risch A, Wallace DM, Bathers S, et al. Slow N-Acetylation genotype is a susceptibility factor in occupational and smoking related bladder cancer. Hum Mol Genet (Feb 1995); 4 (2): 231–6.
- Ritter LL, Klein EK, Klein R, et al. Alcohol use and lens opacities in the Beaver Dam Eye Study. Arch Ophthalmol 1993; 111: 113–17.
- Robertson J McD, Donner AP, Trevithick JR. Vitamin E intake and risk for cataract in humans. Ann NY Acad Sci 1989; 570: 372–82.
- Rubb RM. Cataract acquired following varicella infectims. Ault Ophthalmol 1972; 873–2254.
- Sabiston DW. Cataract, Dupuytren's contracture, and Alcohol Addiction. Am J Ophthalmol 1973; 76: 1005–7.
- Sacanove A. Pigmentation due to phenothiazines in high and prolonged dosage. JAMA 1965; 191: 263–8.
- Salive ME, Guralnik J, Christen W, et al. Functional blindness and viusal impairment in older adults from three communities. Ophthalmology 1992; 99: 1840–7.
- Salmon JF, Wallis CE, Murray ADN. Variable expressivity of autosomal dominant microcornea with cataract. Arch Ophthalmol 1988; 106: 505–10.
- Scales DK. Immunomodulatory agents. In Mauger TF, Craig EL (Eds): Haveners Ocular Pharmacology (Mosby-Yearbook: St Louis 1994; 402–14.
- Schein OD, West S, Mlnoz B, et al. Cortical lenticular opacification: distribution and location in a longitudinal study. Invest Ophthalmol Vis Sci 1994; 35: 363–6.
- Schocket SS, Esterson J, Bradford B, et al. Induction of cataract in mice by exposure to oxygen. Israel J Med 1972; 8: 1596–1601.
- Scott MR, Hejtmaucik F, Wozencraft LA, et al. Autosomal dominant congenital cataract; interocular phenotypic variability. Ophthalmol 1994; 101: 866–71.
- Shun Shin GA, Ratcliffe P, Bron AJ, et al. The lens after renal transplantation. Br J Ophthalmol 1990; 73: 522–27.
- Siddall JR. The ocular toxic findings with prolonged and high dosage chlorpromazine intake. Arch Ophthalmol 1965; 74: 460–4.
- Siegmund W, Fengler JD, Frane G, et al. N-Acetylation and debrisoquine hydroxylation polymorphisms in patients with Gilbert's syndrome. Br J Clin Pharmacol 1991; 32 (4): 467–72.
- Simonelli F, Nesti A, Pensa M, et al. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp Eye Res 1989; 49: 181–7.
- Simons LA. Interrelations of lipids and lipoproteins with coronary artery disease mortality in 19 countries. Am J Cardiol 1985; 57: 5G–10G.
- Sirtori CR. Tissue selectivity of hydroxymethylglutaryl co- enzyme A (HMG CoA) reductase inhibitors. Pharmacol Ther 1993; 60: 431–59.
- Solberg Y, Rosner M, Belkin M. The association between cigaret smoking and ocular diseases. Surv Ophthalmol 1998; 42: 535–57.
- Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res 33: 673–81, 1981.
- Sperduto RD, Hu T-S, Milton RC, et al. The Linxian Cataract Studies: two nutrition intervention trials. Arch Ophthalmol 1993; 111: 1246–53.
- Srivastava S, Ansari NH. Prevention of sugar induced cata-ractogenesis in rats by mutilated hydroxytoluene. Diabetes 1988; 37: 1505–8.
- Stambolian D. Galactose and cataract. Surv Ophthalmol 1988; 32: 333–49.
- Stayte M, Reeves B, Wortham C. Ocular and vision defects in preschool children. Br J Ophthalmol 1993; 77: 228–32.
- Steele G, Peters R. Persistent hyperplastic primary vitreous with myopia: a case study. J Am Optom Assoc 1999; 70 (9): 593–7.
- Stewart Brown SL, Raslum MN. Partial sight and blindness in children of the 1970 birth cohort at 10 years of age. J Epidemiol Community Health 1988; 42: 17–23.
- Stoll C, Alembik Y, Dott B, Roth MP. Epidemiology of con-genital eye malformations in 131,760 consecutive births. Ophthalmic Pediatr Genet 1993; 39: 433–5.
- Stryker WS, Kaplan LA, Stein EA, et al. The relation of diet, cigaret smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol 1988; 127: 283–296.
- Subar AF, Block G. Use of vitamin and mineral supplements: demographics and amounts of nutrients consumed—the 1987 Health Interview Survey. Am J Epidemiol 1990; 132: 1091–1101.
- Summers CG, Letson RD. Is the phakic eye normal in mo-nocular pediatric aphakia? J Pediatr Ophthalmol Strabismus 1992; 29: 324–7.
- Szmyd L Jr, Schwartz B. Association of systemic hypertension and diabetes mellitus with cataract extraction: A case-control study. Ophthalmology 1989; 96: 1248–52.
- Takemoto L, Takehana M, Horwitz J. Covalent changes in MIP 26K during aging of the human lens membrane. Invest Ophthalmol Vis Sci 1986; 27: 443–6.
- Tavani A, Negri E, La Vecchia C. Selected diseases and risk of cataract in Women. A case-control study from northern Italy. Ann Epidemiol 1995; 5 (3): 234–8.
- Taylor A, Jacques P, Nadler D, et al. Relationship in humans between ascorbic acid consumption and levels of total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr Eye Res 1997; 16: 857–64.
- Taylor A, Jacques PF, Nadler D et al. Relationship in humans between ascorbic acid consumption and levels of total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr Eye Res 1991; 10: 751–9.
- Taylor A, Jaques PF, Epstein EM. Relations among aging, antioxidant status, and cataract. Am J Clin Nutr 1995; 62 (Suppl): 1439S–47S.
- Taylor A. Nutritional and Environmental Influences on the Eye. CRC Press, London; 1999; 1–5.
- Taylor A. Nutritional and Environmental Influences on the Eye. CRC Press: London. 1999; 56–81.
- Taylor D, Rice NSC. Congenital cataract, a cause of preventable child blindness. Arch Dis Child 1982; 57: 165–7.
- Taylor HR, West S, Munoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol 1992; 110: 99–104.
- Taylor HR. The environment and the lens. Br J Ophthalmol 1980; 64: 303–10.
- Taylor HR. Ultraviolet radiation and the eye: an epidemiologic study. Trans Am Ophthalmol Soc 1989; 87: 802–53.
- Teramoto S, Fukuchi Y, Uejima Y. Influences of chronic tobacco smoke inhalation on ageing and oxidant-antioxidant balance in the senescence-accelerated mouse (SAM)-P/2. Exp Gerontol 1993; 28: 87–95.
- Thaler JS, Curinga R, Kiracofe G. Relation of graded ocular anterior chamber pigmentation to phenothiazine intake in schizophrenics: quantification procedures. Am J Optom Physiol Optics 1985; 62: 600–04.
- The Italian-American Cataract Study Group: Risk factors for age-related cortical, nuclear, and posterior subcapsular cataract. Am J Epidemiol 1991; 133: 541–53.
- Tielsch JM, Sommer A, Katz J, et al. Socioeconomic status and visual impairment among urban Americans. Arch Ophthalmol 1991; 109: 637–41.
- Tint, et al. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Ed 1994; 330: 107–13.
- Tobert JA. New developtments in lipid-lowering therapy: the role of inhibitors of hydroxymethylglutaryl-coenzyme A reductase. Circulation 1987; 76: 534–8.
- Traboulsi El, Weinberg RJ. Familial congenital cornea gut-tata with anterior polar cataract. Am J Ophthalmol 1989; 108: 123–5.
- Tsutomu Y, Mihori K, Yoshito H. Traumatic cataract with ruptured posterior capsule from a nonpenetrating ocular injury.
- Tuormaa TE: The adverse effects of tobacco smoking on reproductive and health: A review from the literature. Nutr Health 1995; 10: 105–120.
- Ughade SN, Zodpey SP, Khanolkar VA. Risk factors for cataract: a case control study.
- Urban RC (Jr), Cotlier E. Corticosteroid-induced cataract. Surv Ophthalmol 1986; 31: 102–10.
- Vadot E, Guibal JP. Pathogenic de la cataracte diabetique. Bull Soc Ophthalmol Fr 1982; 82: 1513–4.
- Vajpayee RB, Angra SK, Honavar SG, et al. Pre-existing posterior capsular breaks from penetrating ocular injuries. J Cataract Refract Surg 1994; 20: 991–94.
- Van Heyningen R, Harding JJ. A case-control study of cataract in Oxford: some risk factors. Br J Ophthalmol 1988; 72: 804–08.
- van Heyningen R, Harding JJ. Do aspirin-like analgesics protect against cataract? Lancet i: 1986; 1111–3.
- van Heyningen R. The human lens. I—a comparison of cataracts extracted in Oxford (England) and Shikarpur (W Pakistan). Exp Eye Res 1972; 13: 136–47.
- Varma S, Schocket SS, Richards RD. Implications of aldose reductase in cataract in human diabetes. Invest Ophthalmol Vis Sci 1979; 18: 237–41.
- Vesti E. Development of cataract after trabeculectomy. Acta Ophthalmol 1993; 71 (6): 777–81.
- Vidal P, Fernandez-Vigo J, Cabezas-Cerrato J. Low glycation level and browning in human cataract. Acta Ophthalmol 1988; 66: 220–22.
- Vitale S, West S, Hallfrisch J, et al. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiol 1994; 4: 195–203.
- Waxman SL, Bergen RL. Wagner's vitreoretinal degene-ration. Ann Ophthalmol 1980; 12 (10): 1150–51.
- Weale R: A note on a possible relation between refraction and a dis-position for senile nuclear cataract. Br J Ophthalmol 1980; 64: 311–4.
- Weber WW. Acetylation. Birth Defects Orig Artic Ser 1990; 26 (1): 43–65.
- Wensor MD, McCarty CA, Taylor HR. The prevalence and risk factors of myopia in Victoria, Australia. Arch Ophthalmol 1999; 117: 658–63.
- West S, Munoz B, Emmett EA, et al. Cigaret smoking and risk of nuclear cataract. Arch Ophthalmol 1989; 107: 1166–9.
- West S, Munoz B, Schein OD, et al. Cigaret smoking and risk for progression nuclear opacities. Arch Ophthalmol 1995; 113: 1377–80.
- Wiechens B, Winter M, Haigis W, et al. Bilateral cataract after phakic posterior chamber top hat-style silicone intraocular lens. J Refract Surg 1997; 13 (4): 392–7.
- Wilczek M, Zygulska-Machowa H. Zawartosc witaminy C W.roznych typackzaem. J Klin Oczna 1968; 38: 477–80.
- Winkleby MA, Fortmann SP, Barret DC. Social class disparities in risk factors for disease: eight-year prevalence patterns by level of education. Prev Med 1990; 19: 1–12.
- Wolff SM. The ocular manifestations of congenital rubella. Sri Am Ophthalmol Soc 1972; 70: 577–14.
- World Health Organization. Management of Cataract in Primary Health Care Services. WHO: Geneva 1990.
- Ye JJ, Zadunaisky JA. A Na+/H+ exchanger and its relation to oxidative effects in plasma membrane vesicles from lens fibers. Exp Eye Res 1992; 55: 251–60.
- Young RW. Optometry and the preservation of visual health. Optom Vis Sd 1993; 70: 255–62.
- Zadok D, Chayet A. Lens opacity after neodymium: YAG iridectomy for phakic intraocular lens implantation. J Cataract Refract Surg 1999; 25 (4): 592–3.
- Zelenka PS. Lens lipids. Curr Eye Res 1984; 3: 1337–59.