- Development of Gonads and Germ Cells
- Sperm Preparation Techniques
- Setting up of an ART Center
- Classical CO2 Incubator—Heart and Soul of an IVF Laboratory
- Media in ART
- Gametogenesis and Microscopic Structure of Gametes and Early Embryo
- Tuberculosis and Laboratory Perspective
- The Importance of Water Quality in IVF Laboratories
- Oxidative Stress and ART
- ICMR Guidelines
- Troubleshooting in ART—Laboratory Perspective
Chapter Outline
- ▪ Endocrine and Paracrine Mechanism in Sexual Differentiation
- • Hormonal Sexual Differentiation
- • Sex Differentiation of the Hypothalamus
- • Psychosexual Differentiation
- ▪ Disorders of Gonadal Differentiation and Sex Determination
- • True Hermaphrodite
- • Female Pseudohermaphrodite
- • Male Pseudohermaphrodite
- ▪ Gametogenesis
- • Oogenesis
- • Spermatogenesis
- ▪ Capacitation
- • Sperm-Zona Binding
- • Acrosome Reaction
- ▪ Phases of Fertilization
- ▪ Cleavage
- • Nondisjunction of Chromosomes
- ▪ Spontaneous Abortions
Sex determination and differentiation are complex sequential events that involve establishment of genetic sex at the time of fertilization, sexualization of the brain, determination of gonadal sex and differentiation of internal and external genitalia and establishment of phenotypic sex. Thus, following establishment of gonadal/primary sex, sexual differentiation involves events subsequent to g onadal organogenesis. These events are regulated by over 40 genes on both autosomes and sex chromosomes. Initially, it was believed that it is only after determination of gonadal sex followed by hormonal stimulation there is differentiation of genitalia; however, Green R and Gahr M1–3 proposed that even before differentiation of gonadal sex from bipotential gonad there is differential expression of over 50 genes of the 12,000 genes expressed in brain. This was analyzed using DNA microarrays. This suggests that in mammals, male and female brain start traveling down different developmental paths from outset before even sex hormones are produced. The indifferent gonad has a tendency to develop into ovary unless there is expression of sex determining region on Y chromosome. SRY is the master testis determining region and is present on short arm of Y chromosome proximal to pseudoautosomal region (Fig. 1.1).
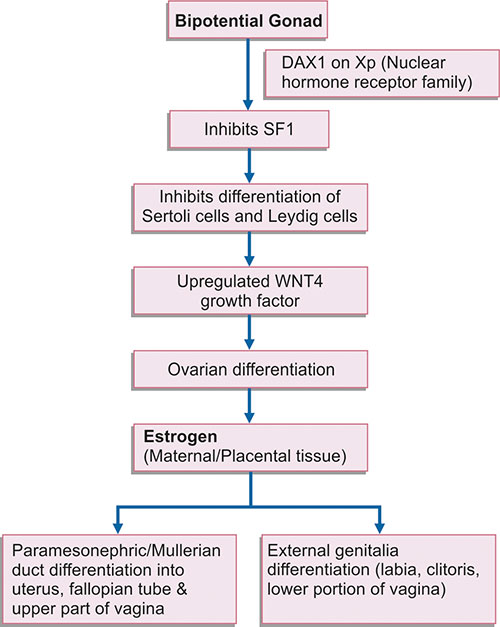
Development of female internal and external genitalia (Flow chart 1.1) occurs independently of gonadal hormones and in absence of testis. Under the influence of SRY gene which is expressed in mesenchymal cells, there is male differentiation despite an internal environment which is rich in estrogen and progesterone.
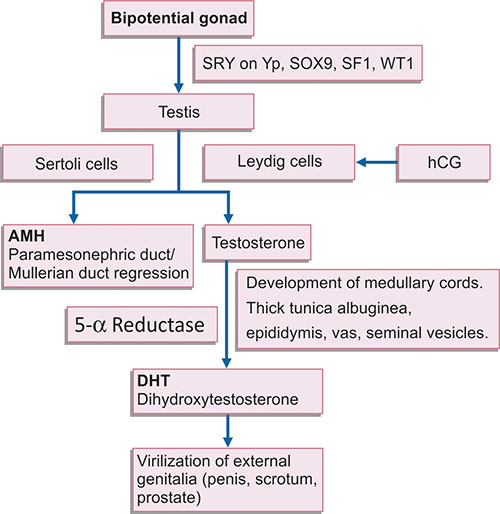
The mesenchymal cells differentiate into Sertoli cells, and are the first site of expression of SRY gene and several downstream sex determining genes like SOX9 and WTI (Flow chart 1.2). Thus, structurally and functionally intact and endocrinologically competent Sertoli cells are required for optimal SRY expression and testicular differentiation. Low SRY expression due to low Sertoli cell number may result in intersex conditions with ambiguous genitalia like cryptorchidism and hypospadias. Thus, sexual differentiation is actually the differentiation of Sertoli cells. Following this, the steel gene is expressed in Sertoli cells, which help in migration of germ cells by amoeboid movement from wall of yolk sac to the genital ridge by 6th week of development.
Thus, SRY gene codes for transcription factors that regulate expression of downstream autosomal genes (like steroidogenic factor SF1 and SOX9), which regulate Sertoli cell development and differentiation. Sertoli cells induce differentiation of Leydig cells, and secrete Mullerian inhibiting substance (MIS) or Anti-Mullerian hormone (AMH) which causes repression of Mullerian or paramesonephric duct differentiation. The action of hormones secreted by Leydig cells, i.e. testosterone and dihydroxy testosterone (DHT) induces male sexual differentiation of somatic structure, and development and differentiation of external and internal genitalia.
It was believed that sexual differentiation in females is by default mechanism that occurs in absence of Y chromosome.
Flow chart 1.2: Role of different genes and hormones in testicular differentiation, and development and differentiation of external and internal genitourinary structures
But recent studies have shown that ovarian differentiation occurs under the influence of DAX1 gene on short arm of X chromosome. DAX1 downregulates steroidogenic factor SF1 and inhibits differentiation of mesenchymal cells on genital ridges into Sertoli cells and Leydig cells. Growth factor WNT4 also helps in ovarian development. But under the influence of DAX1, ovarian differentiation occurs only if germ cells are present and resume in absence of MIS secreted by Sertoli cells. The paramesonephric ducts develop under the influence of estrogen into uterus, fallopian tube and upper part of vagina.
When primordial germ cells fail to migrate, the gonads remain indifferent or are absent. But in case of testis, it is found that testis may develop even in the absence of primordial germ cells. This is due to the fact that SRY gene on Yp only regulates testicular differentiation, but growth, development and differentiation of male germ cells (spermatogenesis) and spermiogenesis is under the control of azoospermia factor (AZF) on long arm of Y chromosome (Fig. 1.1). This loci consists of 3 subloci AZF a, b and c which regulate specific stages of spermatogenesis and deletion of each loci results in partial or complete spermatogenic arrest.4–6 So, XX SRY + cases show testicular differentiation due to the presence of SRY, but are azoospermic due to absence of AZF.
There is a striking difference between sexes in timing of gonadal differentiation. Testis organization begins at 6–7 weeks, but ovaries emerge from indifferent stage only at 12 weeks when the earliest sign, the beginning of meiosis appears as evidenced by maturation of oogonia into oocytes.
ENDOCRINE AND PARACRINE MECHANISM IN SEXUAL DIFFERENTIATION
Chemical messengers aid in regulation of sexual differentiation and act via endocrine and paracrine mechanisms. By the endocrine mechanism, Leydig cell which is a unicellular endocrine gland and testosterone secreted by the Leydig cells act on the external genitalia and urogenital sinus. hCG secreted by syncytiotrophoblast acts on Leydig cells to stimulate testosterone production.
Paracrine control is the second type of regulation in sexual differentiation. This involves the local dissemination of a hormone or a chemical messenger from its site of synthesis to target cells. There are several chemical messengers which act in the paracrine mechanism. For example in males, meiosis inhibiting substance secreted by Sertoli cells act on surrounding germ cells and arrests their proliferation and differentiation till puberty.
Anti-Mullerian hormone produced by Sertoli cells acts on Mullerian ducts and causes their regression. In the female, the chemical messengers which act in a paracrine mechanism are ovary inducing factor and meiosis inducing factor. Ovary inducing factor is produced by rete ovarii and it initiates meiosis in oogonia and granulosa cells producing meiosis inhibiting factor, which acts on oocytes and arrests them in dictyotene stage of prophase of meiosis I.
Hormonal Sexual Differentiation
Complete sexual differentiation occurs only when sexual characters have developed, fertility is attained and reproduction is possible. So, puberty is a series of complex maturational changes which is brought about by elevated levels of gonadal steroids and maturation of gonads.
Attainment of full gonadal function requires an intact hypothalamo-pituitary-gonadal axis. This axis functions in the fetus, but is suppressed to very low levels of activity during childhood and gets reactivated during puberty.
Sex Differentiation of the Hypothalamus
Gonadotropin secretion pattern is different in males and females. In females, there is a sustained tonic release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) and is characterized by preovulatory gonadotropic surge that leads to ovulation. Thus, the pituitary becomes differentiated according to the nature of gonads.7
Harris GW8 in 1964 suggested that hypothalamus or higher neural centers function differently in two sexes. Studies on rat showed that once the male pattern is imprinted on sex centers in the hypothalamus under the action of androgens, the potential for cyclical activity of FSH and LH release by pituitary is lost. However, this testosterone induced differentiation of gonadotropins regulatory mechanism is not seen in humans.
Psychosexual Differentiation
Sexually dimorphic human behavior includes: (i) cognitive differences; (ii) gender orientation; (iii) gender role and (iv) gender identity. Several hormones influence sexually dimorphic behavior in humans. The debate over the outcome of nature versus nurture emphasizes that important element in gender identity are assigned sex of rearing and reinforcement, this gender assignment is received during infancy and childhood.
DISORDERS OF GONADAL DIFFERENTIATION AND SEX DETERMINATION
True Hermaphrodite
Though the external genitalia show ambisexual development, but gonads show both functional ovarian and testicular tissue in either same or opposite gonad. The most common chromosomal complement is 46,XX (70%) followed by 46,XX/46,XY (13%), but 46,XY (12%) is rare. The external genitalia may be either male or female, but are often ambiguous. True hermaphroditism could result from sex chromosome mosaicism, Y/autosome translocation, X/Y aberrant recombination or mutation of X-linked or autosomal genes involved in sex differentiation.
Female Pseudohermaphrodite
In this condition, the chromosomal sex is 46,XX and gonadal sex is female (ovary); however, the external genitalia is ambiguous and virilized. Congenital adrenal hyperplasia accounts for most cases of female pseudohermaphroditism.6
Male Pseudohermaphrodite
In this the chromosomal sex is normal 46,XY as is typical of a male. The gonads are testis, but the genitalia are ambiguous due to incomplete masculinization of genital ducts and external genitalia, and varies from men having female external genitalia to hypospadias and cryptorchidism.
GAMETOGENESIS
Gametes are formed in the epiblast during 2nd week of development and migrate during 4th week by amoeboid movement to the genital ridges (developing gonad) where they reach by the 5th week. Germ cells undergo gametogenesis, which includes both mitosis and meiosis, and cytodifferentiation. For detailed explanation of these processes, the readers are requested to refer to chapter 6.
Oogenesis
Once germ cells arrive in the gonad of genetic female, they differentiate into oogonia. Before describing the process of oogenesis, the author will briefly discuss the structure of ovary.
Ovaries are oval (3 × 2 cm) almond shaped structures which lie in ovarian fossa against the lateral pelvic wall. The ovary has a cortex, which in turn, contains germ cells, and a medulla consisting of stroma and blood vessels. In the cortex the ova grow in a relatively hypoxic environment, which is favorable for developing germ cells and prevents free radical induced oxidative damage to germ cells. The stroma consists of connective tissue cells, contractile cells and androgen secreting interstitial cells. There are four types of interstitial cells, primary, secondary, thecal and hilar. These cells have features of steroid secreting cells. These hilar cells contain crystalloids of Reinke and morphologically resemble interstitial cells of Leydig and like Leydig cells secrete testosterone.
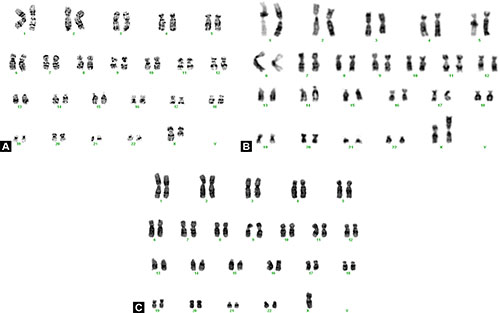
After arrival of primordial germ cells (PGC) in the bipotential gonad of a genetic female, there is differentiation of the gonad into ovary. Early in development, the ovary is close to mesonephros which influences gonadal differentiation and is essential for ovarian development. The ovary is not distinguishable till 10th–11th week though fetal testis can be identified by 7th week and PGC forms the oogonia. These oogonia divide rapidly by mitotic division and reach maximum number of 5–7 million by 5th month of gestation. However due to atresia, the number depletes to 1 million till birth and only 400,000 remain at menarche. In women with 45,XO chromosomal complement or 46, XX (Xq deletion), there is accelerated germ cell atresia due to haploinsufficiency of genes on long arm of X-chromosome. The long arm of X chromosome contains two genes (POF 1 and POF 2) which regulate ova growth and differentiation, and are critical for normal ovarian function. Thus two structurally and functionally intact X chromosomes are required for normal ovarian function and optimal oogenesis.9 Deletion of long arm of X chromosome 46, XXq del (Fig. 1.2A); 46,XX Iso X [monosomy Xp and trisomy Xq (Fig. 1.2B)] or X monosomy 45,XO (Fig. 1.2C) results in premature ovarian failure and manifests as primary and secondary amenorrhea. In women with 45,XO chromosomal complement, there are streak gonads at birth. In females the mesonephros regresses slowly and ovarian-mesonephros dissociation persists during early ovarian differentiation and Byskov in 198610 and Byskov and associates in 198511 reported that the ovary is invaded by mesonephric cells in the medullary region and they force the germ cells to periphery/ovarian cortex. The advantage of germ cell differentiation occurring in ovarian cortical region is that the developing germ cells are exposed to low oxygen tension and thus are not exposed to high reactive oxygen species (ROS) levels during development. This is in contrast to male germ cells which exist in a state of oxygen paradox and are exposed to high ROS levels due to aerobic metabolism,12,13 and thus tend to accumulate mitochondrial nucleotide alterations. In a recent study, the authors have shown mitochondrial dysfunction and oxidative stress in cases with premature ovarian insufficiency which leads to accelerated atresia of the germ cells and manifests as premature ovarian failure.
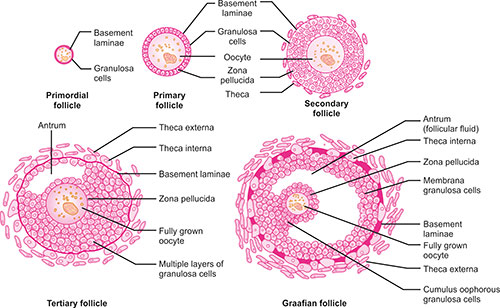
The oogonia divide rapidly until they differentiate to primary oocytes beginning at 8th–10th week of gestation. The primary oocytes begin meiosis, but are arrested at diplotene stage of meiosis I due to meiosis preventing substance or oocyte maturation inhibitor which is secreted by granulosa cells surrounding the oocyte. It is only at puberty that meiosis is induced by 2kD molecule known as meiosis inducing substance. A basement membrane separates the primordial follicle from the stromal cells.
So by 3rd month, the primary oocyte is arrested in diplotene stage of meiosis I and is surrounded by flat epithelial cells known as follicular cells. These flat follicular cells are separated from surrounding stroma by a basement membrane. The oocytes and the surrounding cells do not have direct blood supply and thus the basement membrane surrounding the follicular cells serves a vital function of transfer of nutrients from surrounding microenvironment. At puberty, there are a number of growing follicles that are maintained by the primordial follicles. Each month after menarche, about 15–20 primodial follicles begin to mature and differentiate into primary, secondary, tertiary and finally into mature ovarian follicles.7
The differentiation till stage of secondary follicle is controlled by intraovarian mechanism, and growth and differentiation to tertiary and mature follicle depends on gonadotropins.
Differentiation of Primordial Follicle
As primary oocytes grow (Fig. 1.3), the flat follicular cells become cuboidal and this stage of development is known as early primary follicle. In late primary follicle or secondary follicle, the cuboidal follicular cells divide by mitosis and become multilayered, and now follicular cells are known as granulosa cells. Granulosa cells are surrounded by a basement membrane that separates it from surrounding stromal cells which constitute the theca folliculi. Also granulosa cells and oocytes secrete a glycoprotein coat on the surface of oocyte known as zona pellucida. As the follicles grow, the theca folliculi differentiate into a thick cellular layer which has secretory cells and forms theca interna and an outer fibrous layer known as theca externa.
In tertiary follicles, there is appearance of fluid filled spaces among the granulosa cells which constitutes the antrum. The antral fluid consists of plasma transudate and secretory products of granulosa cells. Estrogens are found in a much higher concentration in antral fluid than in blood. The granulosa cells and cells of theca interna develop specialized contacts “gap junctions,” which allow small molecules to pass directly between interna and granulosa cells. This is because capillaries do not penetrate the basement membrane surrounding the granulosa cells and oocytes and granulosa cells remain avascular. These gap junctions also allow for synchronized and coordinated functioning of follicular cells.
Under the influence of gonadotropins, the follicle grows in size to form a mature follicle or a graafian follicle. The antral fluid increases and fluid filled spaces coalesce to form a single large antral cavity and the oocyte surrounded by granulosa cells (cumulus oophorus) occupies a polar and eccentric position within the follicle. The average time for development of a primary follicle to antral follicle is 12–14 days. These 15–20 follicles develop into a mature follicle, which is destined either to ovulate or degenerate. In each ovarian cycle, only one mature follicle undergoes ovulation.8
Just 3 hours prior to ovulation, the ova completes first meiotic division and one cell receives most of the cytoplasm and the first polar body receives practically none and forms a secondary oocyte. This secondary oocyte begins meiosis II, but arrests at metaphase stage and the process is only completed at fertilization. If fertilization does not occur, the cell degenerates in 20–24 hours.
The zona pellucida surrounding the oocytes is an acellular glycoprotein membrane secreted by both ova and granulosa cells. It mainly comprises zona protein (ZP); ZP1, ZP2 and ZP3 class of glycoproteins. The zona has species specific receptor sites, which allows only sperm of same species to enter the egg. This feature is very important in animals with external fertilization, but not of much significance in humans.
Following penetration of a single sperm, the zona undergoes certain changes which prevent polyspermy. The zona acts like a filter to allow certain secretory products and uterine milk or embryotroph to enter the zygote, morula and early blastocyst. It also prevents the premature implantation of blastocyst at an ectopic site like fallopian tube.
The zona pellucida subserves a very important function as it keeps the rapidly dividing/cleaving blastomeres together, and maximizes their contact and aids in conception. Compaction among blastomeres is also maintained by zonula occludens and E-cadherins. Theca interna is the inner highly vascularized layer of cuboidal secretory cells. These cells possess feature of steroid producing cells. Cells of theca interna possess LH receptors, and on response to LH they synthesize and secrete androgens. This layer has a lot of blood vessels as is typical of endocrine organs.
Theca interna contains collagen fibers and smooth muscles, and is the outer layer of connective tissue. There is a distinct boundary between theca interna and granulosa layer. This boundary forms a partition between the vascular theca interna and the avascular granulosa layer during the duration of follicular growth.
Several factors are required for oocyte and follicular growth like epidermal growth factor (EGF), insulin like growth factor 1 (ILGF 1), calcium ions and FSH.
- The follicular fluid is hyaluronan rich fluid called liquor follicle
- Cells of cumulus oophorus form corona radiata after ovulation
Ovulation results in release of secondary oocyte from graafian follicle. This occurs on 14th day of the 28 day cycle. The factors which lead to ovulation are:
- Activated plasminogen causes proteolysis of follicular wall
- Deposition of glycosaminoglycans between oocyte cumulus complex
- Follicular fluid increases in volume
- Prostaglandin mediated contraction of smooth muscle fiber in theca externa
Depending on when ovulation occurs, the primary oocyte in primordial follicle is arrested at diplotene stage of meiotic prophase for 12–50 years. During this long period the primary oocyte is exposed to several environmental factors/pollutants that can contribute to meiotic errors and lead to nondisjunction and anaphase lag, and may lead to numerical or structural anomalies like trisomy 21 (Down syndrome).
Spermatogenesis
Spermatogenesis is a complex series of events, which begins at puberty and by which spermatogonia develop into sperm. It occurs under the influence of elevated levels of FSH and LH at puberty and continues throughout life.
Spermatogenesis is divided into three phases:
- Spermatogonial phase (mitotic phase)—Spermatogonia divide into population of stem cells and also produce a population of committed spermatogonia that differentiate into primary spermatocytes.
- Spermatocyte phase (meiosis)—The primary spermatocyte undergoes meiosis I (reductional division) to half the chromosome number and meiosis II (equational division) to half the DNA content to form haploid spermatids.
- Spermiogenesis—The process by which spermatids differentiate into mature spermatozoa.
These three phases of germ cell development occur within the seminiferous tubules of the testis. The seminiferous epithelium of germ cells and a population of nonproliferating supporting sustentacular cells called Sertoli cells. Sertoli cell is a polarized cell and gives structural organization to seminiferous tubules and extends full thickness of seminiferous epithelium. Outside the seminiferous tubules, there is connective tissue stroma which contains interstitial cells of Leydig.
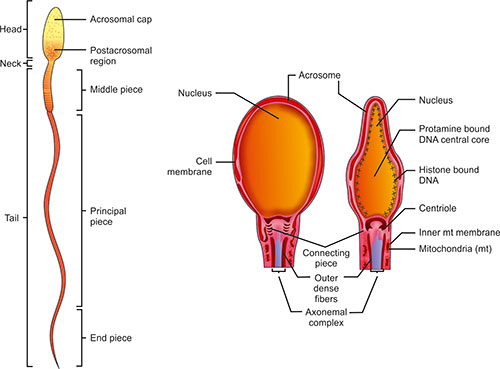
Sperm Morphology
The mature human sperm is a highly polarized cell and the only cell in the body which performs its function extracorporally (Fig. 1.4). Its head diameter is 2.5–3.2 μm and length is about 5-7 μm. The nucleus is covered on its anterior two-thirds by the acrosomal cap. The sperm tail comprises the neck, middle, principal and end piece. The neck contains the centrioles and origin of coarse fibers. The middle piece is 65–75 μm in length and comprises the tightly helically arranged mitochondria around the coarse fibers and axonemal complex. Each mitochondria (mt) contains a single copy of mitochondrial DNA. The sperm mt are organized around midpiece to provide energy for movement of sperm axoneme.
The principal piece is 38–40 μm in length and contains fibrous sheath external to coarse fibers and axonemal complex. The end piece comprises of only the axonemal complex.
The sperm released following spermiogenesis are immobile and only gain maturity in the epididymis.
Though sperm can survive several weeks in the epididymis, they survive only for 2–3 days in female reproductive tract. With shedding of majority of cytoplasm during spermiogenesis, sperms lose majority of their antioxidant enzymes and therefore are prone to oxidative injury. However, this is prevented by several enzymatic and nonenzymatic antioxidants in the seminal plasma. Venkatesh et al.14 in 2009 reported higher ROS levels and low total antioxidant capacity in semen of infertile men.15 Agarwal et al.13 in 2009 reported that infertile men with normal semen parameters have increased ROS levels. This is particularly important when semen samples are processed, washed and prepared for assisted reproductive technology (ART) or intracytoplasmic sperm injection (ICSI). In these cases in the washed semen samples, sperms are exposed to very high ROS levels as the antioxidant rich seminal plasma is removed.12 Thus it is recommended that ART culture media should be supplemented with antioxidants, though the dosage needs to be regulated so that normal physiological functions like acrosome reaction and capacitation are not impaired. Authors also found after density centrifugation, which segregates spermatozoa with normal morphology from sperms with abnormal morphology that sperm with abnormal morphology produce high levels of free radicals which are detrimental to sperm with normal morphology and thus there is a need to segregate the two population of germ cells after sperm wash in ART to prevent oxidative induced DNA damage in germ cells. They also found that the sperm with normal morphology and motility in semen of normozoospermic infertile men harboured significantly higher DNA damage [DNA fragmentation index (DFI) 23.8%] as compared to sperm of controls (19%). The authors have recently established cut off values of DFI in infertile men at 30% and in male partner of couples with idiopathic recurrent pregnancy loss at 24%.1610
In the spermatogonial phase, the spermatogonia undergo repeated mitosis and produce spermatogonial progeny. Human spermatogonia based on the appearance of nuclei can be grouped into three types:
- Dark Type A spermatogonia have ovoid nuclei with fine granular chromatin which is intensely basophilic. There is stem cell population in the seminiferous epithelium, which divide and give rise to dark type A stem cells and pale type A spermatogonia.
- Pale Type A spermatogonia have ovoid light staining nuclei with finely granular chromatin. These cells also undergo rapid mitotic division to increase their number and can undergo differentiation to produce type B spermatogonia.
- Type B spermatogonia have chromatin condensed into clumps attached to nuclear membrane.
For synchronous development of germ cells, all progeny of pale type A spermatogonia are connected by cytoplasmic bridges. For proliferation and cytodifferentiation, male germ require high testosterone concentration and the concentration in the testis is about 200 times than that in the blood.
After several cell divisions, the pale type A spermatogonia differentiate into type B spermatogonia. This is the last event in the spermatogonial phase in the basal compartment of seminiferous tubule.
Spermatocyte Meiotic Phase
Pale type B spermatogonia replicate their DNA so each primary spermatocyte contains twice the normal chromosomal number (4n) and double DNA content.
Meiosis I results in decreasing the chromosomal number to half (23) and meiosis II results in decreasing the DNA content to half.
During meiosis I, there is pairing of homologous chromosomes and formation of synaptonemal complex. This process ensures genetic diversity, as triads which have been modified by crossing over become dyads again. This is the step which distinguishes it from mitosis in which paired chromatids (one old and one newly synthesized) template separate. The movement of maternal and paternal homologous chromosomes to either pole is random and is another source of genetic diversity.
The primary spermatocytes undergo reductional division and form secondary spermatocytes, and shift to the adluminal compartment of the seminiferous tubules. The secondary spermatocyte immediately undergoes 11second meiotic division without entering S phase (synthesizing new DNA). At this stage, though the chromosome number is half there is diploid content of DNA. Thus secondary spermatocyte undergoes equational division to give rise to spermatids with haploid (1n) chromosome number and with single chromatid.
Spermatid (Spermiogenesis) Phase
During spermiogenesis, the spermatid undergoes extensive remodeling to give rise to mature sperm and consists of four phases:
- Golgi phase
- Cap phase
- Acrosomal phase
- Maturation phase
Golgi phase: During this phase, numerous proacrosomal granules accumulate in the Golgi complexes. These granules coalesce to form the acrosomal vesicle, which enlarges and covers a portion of the nucleus. The acrosomal vesicle determines the anterior pole of the sperm. The juxtanuclear centriole migrates to the posterior pole of the spermatid and initiates organization of microtubules in 9 + 2 arrangement which constitute cytoskeleton of the sperm axoneme.
Cap phase: The acrosomal vesicle caps the anterior half of the nucleus and now the vesicle is called acrosomal cap. There is also condensation of nuclear contents.
Acrosomal phase: During this phase, the spermatid nucleus further condenses, and becomes flat and elongated. The sperm head buries deeper in Sertoli cells so that head points toward seminiferous tubule basement membrane. The nucleus and acrosome come to lie first below the anterior plasma membranes displaying the cytoplasm posteriorly. The microtubules organize into a cylindrical sheath “manchette,” which extends from posterior end of acrosome to posterior poles of spermatid.
The posterior end of nucleus shows a groove which forms connecting piece/neck region. The centrioles migrate near posterior surface of nucleus. The coarse fibers develop from centriole to attach to nucleus and extend as outer dense fibers peripheral to the microtubules. These fibers unite nucleus with flagellum and thus get the name connecting piece.
The mt migrate from cytoplasm of head region and form a tight helical sheath around the coarse fibers. This is the middle piece. The mt are multitasking organelles and are site of ATP production. They also produce free radicals as by-products during ATP synthesis. As the electron transport chains are localized in the inner mitochondrial membrane, this is the site where ROS are produced and mitochondrial DNA in vicinity of inner mitochondrial membrane is the first site of ROS induced mitochondrial DNA damage. Thus during the course of evolution majority of genes on mitochondrial DNA have translocated to the nuclear genome and only those genes concerned with oxidative phosphorylation are located on the mitochondrial genome. The mitochondrial genome is not protected by histones, and has a very basic proofreading mechanism and a higher turnover rate, thus it tends to accumulate mutations at a much higher rate than the nuclear genome.15,16 Thus sperm mitochondrial DNA are reduced to disposable elements and are degraded post fertilization. In contrast, the ova develop in a hypoxic environment, and have a quiet metabolism and are not exposed to high ROS levels.
Distal to the middle piece, there are two longitudinal columns of numerous connecting ribs, which surround nine longitudinal fibers of the principal piece and extend till the end of flagellum. A short segment of tail distal to fibrous sheath is the end piece.
Maturation phase: This is the phase of removal of excess cytoplasm as the residual body. The intercellular bridge between cells is removed with residual body.
During this phase, there is condensation of sperm nucleus, removal of majority of histones (85%) and replacement of transition proteins by protamines (PRM). However, 15% of the genome is bound to histones and 4% of haploid DNA retains its nucleosomal structure. This histone bound portion is located in the periphery of the nucleus, and is less compact and transcriptionally active. It codes for various important developmental genes, which are required during gastrulation like HOX genes and several imprinted gene clusters. However, as this portion is peripherally located, and less compact and condensed it is more prone to oxidative injury.
The size of the nucleus is one-sixth to one-twentieth the size of somatic cell nucleus due to binding by PRM, which neutralizes the charge on DNA and packs it into a compact toroid. At this stage the cell is transcriptionally inactive due to condensation of nuclear chromatin and increased number of sulfide bonds between PRM. Thus, the sperm nucleus is highly compact, condensed and organized, and this protects it from various toxic physical, chemical and thermal insults. Though sperm is believed to be transcriptionally inert, but the peripheral histone bound fraction retains its nucleosomal structure and maintains imprints. Sperm also has certain RNA transcripts, which maintain nucleosomal structure and prevent protamination in specific regions of chromatin. Thus it is believed that RNA may facilitate structural maintenance of open chromatin structure and directly influences eventual gene activation. Also because of shedding of majority of cytoplasm and condensation of 12nucleus, the sperm is very cryostable and can be cryopreserved. However, it has been reported that there is a 30–40% loss of motility in cryopreserved spermatozoa. This is due to depolymerization of microtubules in the sperm axoneme. Microtubules exist in a state of dynamic instability constantly being polymerized and depolymerized, at low temperatures the equilibrium shifts to depolymerized form. On thawing sperm microtubules repolymerize, but are disorganized and this may explain for loss of motility following cryopreservation.17
In a study done in the author's laboratory, they have found that sperm from infertile men harbor mitochondrial nucleotide alterations. This was seen after whole genome amplification of mitochondrial DNA. These changes were maximal in cases with oligoasthenoteratospermia as compared to cases with just low sperm count. The presence of mutated mitochondrial DNA beyond a threshold count explains for low sperm count due to hypospermatogenesis, and production of sperms with abnormal morphology and impaired motility.12 However, such infertile men harbouring mitochondrial DNA mutations carry a good prognosis on ART as paternal mitochondrial DNA are not transmitted to offspring; however, such mutated mitochondrial DNA produces increased levels of ROS, which can cause pronuclear block or may impair cleavage.18
Mitochondria with mutated mitochondrial DNA produce lower ATP levels, but higher ROS levels. High ROS levels induce oxidative stress and damage both mitochondrial and nuclear genome.
In the epididymis, the sperm acquires motility and the ability to fertilize the egg under the influence of androgens. The head of sperm is modified by addition of glycol conjugates, the surface associated decapacitation factor (SDF). This process is known as decapacitation and inhibits the fertilizing ability in a reversible manner.
CAPACITATION
Capacitation is a very complex phenomenon involving several biochemical changes, which results in functional reprogramming of the sperm. During capacitation, the SDF is shed. This is a period of conditioning in the female reproductive tract. During capacitation, structural and functional changes occur in spermatozoa resulting in increased affinity to zona pellucida. Hyperactivation of sperm with vigorous whiplash beating of flagella indicates that sperm has undergone capacitation. During capacitation there are alterations in membrane composition, membrane potential, intracellular pH, calcium levels, cAMP and in the tyrosine phosphorylation state. There is also release of seminal fluid glycoconjugates from sperm head surface. The functional implication of capacitation is the increased capacity to bind the zona receptors, attain hyperactivated motility and signal transduction pathway activation that is required for acrosomal reaction. Capacitation is complete only when sperm binds to zona pellucida receptors.
Sperm-Zona Binding
The zona pellucida is a glycoprotein layer which is secreted both by the ovum and the granulosa cells. Molecular factors present on sperm and zona aid in mutual species specific recognition. ZP3 is the major glycoprotein involved in sperm zona binding. Sperm protein β-1,4-galactosyltransferase is the major protein that binds to ZP3 receptor. The surface of sperm contains sperm adhesion molecule 1 (SPAM-1) formerly named PH-20, which binds to zona pellucida and has hyaluronidase activity.
Acrosome Reaction
Sperm-zona binding triggers the acrosome reaction (AR). The acrosomal cap which covers anterior two-thirds of sperm nucleus contains hyaluronidase, neuraminidase, acid phosphatase and acrosin and trypsin like proteases. The AR involves changes in sperm plasma membrane and acrosomal membrane, and release of acrosomal enzymes which aid in penetration into zona pellucida. Various signals in the ionophore A23187 causes increase in intracellular calcium levels, which can trigger AR. Physiological inducers are ZP3 and progesterone present in high concentrations in follicular fluid and cumulus oophorus. The AR has a dual function; it not only aids in penetrating zona pellucida, but also exposes the sperm membrane just behind equatorial region that fuses with oocyte plasma membrane. Fertilin α (ADAM1) and fertilin β (ADAM 2) aid in sperm binding to oocyte plasma membrane.
Once a sperm binds with oocyte plasma membrane there are rapid and slow changes in oocyte which prevent polyspermy. These include depolarization of the oolemma, which results in electrical block to prevent multiple sperm entry. There occur changes in the oolemma polarity due to release of calcium (cortical reaction). The contents of cortical granules are released into perivitelline space.
The cortical granules also release proteases that cleave and degrade oolemma receptors, but also focus perivitelline barrier by cross-linking proteins on the surface of zona pellucida. This is known as zona reaction.
During cryopreservation, there is hardening of the zona pellucida and thus it is difficult to use such ova for ART. But this can be overcome by use of ICSI. Cryopreservation 13also can result in alteration of shape and fracture of zona pellucida and thus can result in polyspermy.17
PHASES OF FERTILIZATION
Fertilization is a complex multistep process that leads to union of a single sperm nucleus with female pronucleus of an activated oocyte. It involves:
- Passage of sperm through corona radiata, the cells surrounding the oocyte and zona pellucida.
- Penetration into zona pellucida (explained already).
- Fusion of oolemma and sperm plasma membrane—The head and tail of sperm enter the oocyte, but sperm plasma membrane remains behind. The sperm not only carries DNA, but also transfers oocyte activation factor, centrosome and mRNA.
- Completion of second meiotic division—with the entry of sperm in the oocyte, the oocyte which is arrested in metaphase stage completes second meiotic division and forms mature oocyte and second polar body. In humans, the first polar body does not divide. This is followed by decondensation of maternal chromosomes and formation of female pronucleus.
- Formation of male pronucleus—the highly condensed compact sperm nucleus decondenses due to glutathione in ova, which cleaves the disulfide bonds and sperm nucleus enlarges to form male pronucleus. The sperm tail degenerates. The mitochondrial DNA which enters is tagged by ubiquitin and is degraded. During growth of pronucleus, they replicate their DNA (each chromosome has 2 chromatids). The oocyte containing two haploid pronuclei is known as ootid.
- As the pronuclei fuse, a single diploid aggregation of chromosomes, the ootid becomes a zygote.
Thus fertilization helps to:
- Determine the genetic sex
- Helps in restoration of diploid chromosome number
- Results in variation of human species through mingling of maternal and paternal chromosomes. So, the zygote contains a new combination of chromosomes which is different from that in either parent.
- It also causes metabolic activation of the ootid and initiates cleavage
Successful fertilization depends upon inherent qualities of the oocyte. However with the advent of ART/ICSI, role of sperm factors in fertilization and embryogenesis is being realized.19–21 It has been reported that 40% of failed fertilization after ICSI are due to failure of egg to activate. Failed fertilization may be due to centrosomal dysfunction, which leads to disorderly organization of microtubules and to arrest of fertilization. It is also important to realize the importance of structural integrity of intact fertilizing sperm and its contribution to normal human embryogenesis. A high rate of mosaicism was observed is embryos with disrupted sperm.
CLEAVAGE
Cleavage is a series of rapid repeated mitotic divisions in which the cell size decreases with each division. In humans, the cleavage is rotational and asynchronous as all blastomeres do not divide at the same time. During cleavage, the zygote is in the zona pellucida. Cleavage of zygote begins 30 hours after fertilization. After three divisions, the cells tightly align against each other with maximal contact between these cells. These cells also express E-cadherins on the surface, which aid in the process of compaction. Compaction is formation of a compact ball of cells after nine cell stage. Prior to this, the cells are loosely arranged. This permits greater cell to cell interaction. This compact ball of cells is known as morula, and is formed 3 days after fertilization and at this stage the cells are pluripotent and can form any embryonic or trophoblastic tissue.
The zona pellucida acts like a selective barrier and permits fluids from uterine tube and uterine milk/embryotroph secreted from uterine glands to enter the morula. As fluids enter the zona pellucida, it forms a cavity known as blastocoel and is now known as blastocyst. With the development of blastocoel, the blastomeres are segregated into an inner cell mass and a thin outer layer known as trophoblast. At this stage, the cells of inner cell mass are multipotent. These multipotent cells of inner cell mass can form any embryonic tissue but do not give rise to trophoblastic tissue or placenta.
As the blastocyst grows, there is thinning and stretching of zona pellucida and ultimately the blastocyst hatches out of the zona pellucida. Zona proteins prevent premature implantation into the uterine tube of this sticky ball of cells. With its removal, once the blastocyst reaches uterus, it rapidly grows in size and attaches to the uterine endometrium usually at its embryonic pole. This occurs 6 days after fertilization (Day 20–28 of cycle) and marks the beginning of implantation.
Nondisjunction of Chromosomes
If nondisjunction occurs during early cleavage division, it results in mosaicism—a condition in which there are two or more cell lines arising from the same zygote.
SPONTANEOUS ABORTIONS
The spontaneous abortion rate is about 45–50% and is considered to be the commonest complication of pregnancy; however, the rate of recurrent spontaneous abortions (RSA) is lower about 0.5–2%. The losses may be 14pre-embyronic if they occur before 5th week of pregnancy, or embryonic if it occurs between 5th and 10th week of pregnancy, or post-embryonic if it occurs after 10th week of pregnancy.22 Though there are several reasons which can lead to spontaneous abortion, one of the most common cause of spontaneous abortion is the presence of chromosomal abnormalities in the conceptus. This can result in loss of conceptus in preimplantation or postimplantation stage. Chromosomal abnormalities in the embryo/fetus are usually inherited from parental germ cells. This is a mechanism of natural screening of embryos, so that only chromosomally normal healthy embryos survive. Majority of men and women who harbor chromosomal abnormalities are infertile or experience RSA. Infertile couples may opt for ART and this can result in birth of offspring with major and minor congenital malformations.16 One of the major causes of RSA both following natural conception or ART/IVF/ICSI conceptions is sperm DNA damage. One of the major factors which causes nuclear DNA damage is oxidative stress. Sperm DNA damage beyond a critical thereshold (more than 30%) affects fetal viability and results in early pregnancy loss.21 In an ongoing study in the laboratory, authors found that Yq microdeletions were present in 2.1% men with spontaneous conception with RSA and 11% men opting for ART and experiencing recurrent early pregnancy losses. Sperm DNA damage not only impairs functional competence of sperm, but may result in RSA or lead to increased risk of genetic and epigenetic abnormalities, childhood cancers and perinatal morbidity. Thus all couples opting for ART or experiencing RSA following spontaneous or ART conception must undergo a comprehensive genetic diagnostic work-up (cytogenetic analysis of both partners, sperm Yq microdeletion screening, seminal ROS assessment and sperm DNA damage analysis). Thus the sperm is not a mere carrier of genetic information, but serves a dynamic role which extends clearly beyond fertilization.
REFERENCES
- Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behaviors. Microsc Res Tech. 2001; 55: 1–11.
- Gahr M. Male Japanese quails with female brains do not show male sexual behaviors. Proc Natl Acad Sci USA. 2003; 100: 7959–64.
- Green R. Birth order and ratio of brothers to sisters in trans-sexuals. Psychol Med. 2000; 30: 789–95.
- Vogt PH, Edelmann A, Kirsch S, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996; 5: 933–43.
- Dada R, Gupta NP, Kucheria K. Molecular screening for Yq microdeletion in men with idiopathic oligozoospermia and azoospermia. J Biosci. 2003; 28: 163–8.
- Dada R, Kumar R, Shamsi MB, et al. Genetic screening in couples experiencing recurrent assisted procreation failure. Indian J Biochem Biophys. 2008; 45: 116–20.
- Pfeiffer RL. Frans Cornelis Donders-Dutch Physiologist and Ophthalmologist. Bull N Y Acad Med. 1936; 12 (10): 566–81.
- Harris GW. Sex hormones, brain development and brain function. Endocrinology. 1964; 75: 627–48.
- Dada R, Kumar R, Shamsi MB, et al. Higher frequency of Yq microdeletions in sperm DNA as compared to DNA isolated from blood. Asian J Androl. 2007; 9: 720–2.
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986; 66: 71–117.
- Byskov AG, Hoyer PE, Westergaard L. Origin and differentiation of the endocrine cells of the ovary. J Reprod Fertil. 1985; 75: 299–306.
- Venkatesh S, Riyaz AM, Shamsi MB, et al. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia. 2009; 41: 251–6.
- Agarwal A, Desai NR, Makker K, et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil Steril. 2009; 92: 1318–25.
- Venkatesh S, Deecaraman M, Kumar R, et al. Role of reactive oxygen species in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. Indian J Med Res. 2009; 129 (2): 127–37.
- Shamsi MB, Venkatesh S, Tanwar M, et al. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat Res. 2009; 665: 29–36.
- Kumar R, Tanwar M, Ammini AC, et al. Robertsonian translocation and their role in pathogenesis of recurrent in vitro fertilization failure. Med Sci Monit. 2008; 14 (12): CR617-20.
- Smith GD, Silva E, Silva CA. Developmental consequence of cryopreservation of mammalian oocytes and embryos. Reprod Biomed Online. 2004; 9 (2): 171–8.
- Aitken RJ, De Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online. 2007; 14: 727–33.
- Giwercman A, Lindstedt L, Larsson M, et al. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl. 2010; 33 (1): 221–7.
- Zini A, Kamal K, Phang D, et al. Biologic variability of sperm DNA denaturation in infertile men. Urology. 2001; 58 (2): 258–61.
- Dada R, Mahfouz Z, Kumar R, et al. A comprehensive work up for an asthenozoospermic man with repeated intracyto-plasmic sperm injection (ICSI) failure. Andrologia. (In press).
- Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1991; 10: 255–266.