INTRODUCTION
Cancers of the peritoneal cavity are a unique subset of malignancies. They can behave different from other malignancies, particularly in regards to propagation and systemic spread. Once considered universally fatal with few treatment options, multimodality therapies have been shown to palliate symptoms which improve quality of life as well as achieve significant lengths of survival. An understanding of the anatomic, physiologic and distinctive malignant properties is important to properly evaluate and treat patients with peritoneal-based cancers.
THE PERITONEAL CAVITY
Embryologic Origins
The primitive origin of the peritoneal cavity originates in the third week of gestation. During this time the intraembryonic mesoderm differentiates into two layers: (1) the somatic and (2) the splanchnic mesoderm layers. The somatic mesoderm layer forms the parietal peritoneum, which lines the abdominal and pelvic walls. The splanchnic mesodermal lining forms the visceral peritoneum. Early in development, these two layers of the peritoneum are connected to each other via the dorsal mesentery, which suspends the entire primordial gut from the midline of the posterior abdominal wall1,2 (Fig. 1).
The primitive mesentery of the foregut, midgut and hindgut has both ventral and dorsal divisions; initially has a broad attachment to the posterior abdominal wall. Early in development, the liver grows into the ventral mesentery; essentially dividing it into two peritoneal attachments: the falciform ligament and the lesser omentum. The dorsal mesentery is identified as the greater omentum in the adult. Initially formed as an omental bursa or sac in front of the transverse mesocolon, a portion of its anterior and posterior layers eventually join to form a single plane. This omental layer then fuses with the transverse colon and its mesentery to form the gastrocolic ligament.1,3
Anatomy
Although no true compartmentalization of the peritoneal cavity exists, it is helpful to divide the space into compartments, particularly to emphasize technical considerations of operating in each area. The greater peritoneal sac encompasses the main anterior portion of the peritoneal cavity. The lesser sac (omental bursa) lies posterior to the stomach. Its superior recess extends up to the diaphragm and its inferior recess is a sac-like structure that extends caudally between the two layers of the greater omentum. The greater and lesser sacs communicate via the foramen of Winslow, located on the posterior edge of the hepatoduodenal ligament.2
The transverse mesocolon effectively divides the peritoneal cavity into supracolic and infracolic compartments. The supracolic compartment contains the stomach, liver and spleen, and can further be divided between the infrahepatic and the suprahepatic spaces. The infracolic compartment contains the small and large bowel, and is lateralized to left and right subdivisions by the small bowel mesentery.4
Figure 1: Embryologic origin of the peritoneal cavity[Source: Reprinted with permission from Sadler TW. Langman's Medical Embryology, 9th edition. 20041]
The left paracolic gutter is completely contained within the left infracolic compartment by the phrenocolic ligament. This ligament does not exist on the right, thereby allowing free communication between the right paracolic gutter and the supracolic compartment. Inferiorly, fluid, infection or malignant cells can find a pathway into the pelvis along the paracolic gutters.2,3
Of additional clinical importance is the fact that, in males, the peritoneal cavity is a closed system. The peritoneum protrudes into the rectovesical pouch in males, creating a separation between the rectum and bladder. In females, however, the peritoneal cavity is contiguous with the pelvic organs (fimbria, ovaries, fallopian tubes, uterus and vagina), creating yet another pathway for the spread of malignancy. Additionally, the pelvic peritoneum creates two pouches in women: (1) the rectouterine pouch (separates bladder from rectum) and (2) the vesicouterine pouch (separates bladder from uterus).
The arterial supply and venous drainage of parietal peritoneum occur via branches of the abdominal wall vessels. These include the superior and the inferior epigastric arteries, lumbar vessels, musculophrenic artery and deep circumflex arteries. The visceral peritoneum derives its blood supply from branches of the viscera it covers. Nerve supply to the parietal and visceral peritoneum follows a similar pattern, with adjacent visceral nerves supplying the visceral peritoneum, and those of the abdominal wall (and diaphragm) supplying the parietal peritoneum.
The majority of lymphatic drainage from the peritoneal cavity occurs via the subdiaphragmatic lymphatic system. Intercommunicating plexuses on either side of the diaphragm are found in variable distribution within the muscular portion of the diaphragm. Lymphatic fluid is absorbed at specific sites on the diaphragm (lymphatic lacuna) and then travels through large substernal collecting ducts associated with the internal mammary vessels, eventually reaching the anterior mediastinal lymph nodes. A small portion of the lymphatic drainage from the diaphragm may go to the bronchial lymph nodes or proceed caudally into the retroperitoneal fat lymph nodes and eventually, the cisterna chyli. The main thoracic duct plays a very small role in lymphatic drainage of the peritoneal cavity.4
Physiologic and Immune Function
The mesothelial cell lining of the peritoneal cavity has an active role in the physiologic functioning of the peritoneal cavity and forms the “plasma peritoneal barrier”. One role of these cells is the secretion of surfactant that works as a lubricant to decrease friction between the abdominal organs.5 Mesothelial cells are actively involved in the initiation and regulation of peritoneal cavity's immune functions. These cells secrete inflammatory mediators and play a gatekeeper role for leukocyte extravasation into the peritoneal cavity at times of infection. This is part of the peritoneal cavity's complex system to resist microbial proliferation, which consists of both physical barriers and immune-mediated functions. First-line defenses against infectious insults include lymphatic uptake of particulate matter at the diaphragm and opsonization of microorganisms with subsequent macrophage-associated phagocytosis.6,75
Figure 2: Activation of peritoneal macrophages following bacterial contamination, and role of mesothelium in local host defenses[Source: Reprinted with permission from Brulez HF, Verbrugh HA. Perit Dial Int. 1995;15(7 Suppl):S24-33]7
The peritoneal fluid contains a variety of resident and migratory immunogenic cells, factors and peptides, even in the uninfected patient. Of the leukocytes, monocytes/macrophages are the most dominant. However, the fraction of any individual cell type on peritoneal fluid cell differential is highly variable. The percentage of leukocytes that are macrophages may range 20–95%, with lymphocytes having similar variability.7,8 Neutrophils are present in only small amounts in the normal state of health; however, their concentration and activity dramatically increases during times of active infection.8
Macrophages are also found in perivascular and submesothelial areas of the peritoneum.7 They secrete a variety of inflammatory factors including cytokines, prostaglandins, chemoattractants and leukotrienes6 (Fig. 2). Additionally, perivascular lymphoid aggregates have been identified in various tissues including the omentum and pouch of Douglas. These aggregates termed “milky spots”, harbor macrophages as well as lymphocytes, which can be released into the peritoneal cavity following inflammatory stimuli.9
Malignancies of the Peritoneal Cavity
Primary Peritoneal Malignancies
Primary de novo malignancy of the peritoneal cavity is exceedingly rare. A study examining 24 population-based registries in the United States from 1995 to 2004 found the incidence of primary peritoneal cancer to be 6.78 per million.10 White women accounted for the largest cohort of patients, with the disease least likely to affect black women. Mean age at diagnosis was 67 years. Subtypes of primary peritoneal malignancies and their important features are outlined in Table 1.
Secondary Peritoneal Malignancies
The majority of peritoneal malignancies are due to secondary spread of cancer cells. Appendiceal, ovarian and colorectal cancers are common sources of intraperitoneal disease. Gastric, endometrial, small bowel, pancreatic, breast cancers, sarcomas and pulmonary mesothelioma can also demonstrate peritoneal spread.
Appendiceal: Appendiceal cancers are exceedingly rare, and can encompass varying histopathologic subtypes including adenocarcinoma (with or without mucin production), signet ring cell adenocarcinoma, carcinoid, and adenocarcinoid. One study that evaluated 15 years of patient data from the National Cancer Institute's Surveillance, Epidemiology and End-Results (SEER) database found the annual incidence of all types of appendiceal cancer to be 0.12 per million people.23 Mucinous adenocarcinoma was the most common subtype reported in this study.6
| |||||||||||||||||||
Epithelial appendiceal cancers which are associated with peritoneal disease, are very difficult to diagnose preoperatively; the majority of these cancers are diagnosed during postoperative histopathological review.24
Ovarian: Ovarian epithelial cancer is an aggressive disease that often demonstrates intraperitoneal spread. The current standard for therapy includes cytoreductive surgery and adjuvant chemotherapy (platinum-based agent plus taxane). Only half of all patients obtain a complete clinical response with this regimen, a state which is maintained at the 5-year mark in less than 50% of the patients.25,26 These cancers commonly become chemotherapy-resistant following an initial response,7 further complicating the treatment algorithm. Long-term survival is poor.
Colorectal: Colorectal cancers spread to the peritoneal cavity with relative frequency, second only to the liver. This will often lead to peritoneal carcinomatosis. At the time of primary surgery, peritoneal disease is already detectable in approximately 7% of patients with colon cancer.27 Historically, peritoneal carcinomatosis was regarded as distant metastatic disease (M1/stage IV), and offered a grave prognosis. Patients were typically offered only palliative chemotherapy. When offered, surgery too was usually palliative in nature (e.g. intestinal bypass for obstruction).
In population-based studies, the improved application of palliative chemotherapy regimens over the past 15 years appears to have increased the overall survival of patients with colorectal carcinomatosis from approximately 35 weeks to 66 weeks.28 Within this cohort, patients with isolated peritoneal disease who were treated with systemic chemotherapy had the longest median survival. This “peritoneal-only” group of patients with colorectal carcinoma is thought to make up approximately 25% of all the patients with colorectal carcinomatosis. Recently, it has been suggested that these patients may be better served by treating their disease as locoregional extension as opposed to systemic metastases.
Pseudomyxoma peritonei: Pseudomyxoma peritonei (PMP) is a clinical syndrome of gelatinous mucinous ascites with a characteristic pattern of peritoneal and omental implants, resulting from peritoneal contamination from a mucinous tumor, commonly of appendiceal or ovarian etiology. Interestingly, these tumor cells lack the adhesion molecules necessary to arbitrarily bind to any tissue and are, therefore, often just “redistributed” around the peritoneal cavity until they reach specific areas of the peritoneum rich in lymphatic stomata. It is at these sites that tumor adherence is most likely to occur in the early stages of disease.29,30
The mucin produced by these tumors is a glycoprotein encoded for by the MUC family of genes, which MUC gene a cell expresses is dependent on location (e.g. MUC-2 is expressed by small intestine and colonic goblet cells; MUC-5AC is expressed by the stomach and respiratory tract). Mucin is produced by epithelial cells and has both a membrane-associated and secreted form.30
The exact origin of PMP in women has been debated in the literature. The question has been raised as to whether an ovarian malignancy is responsible for PMP, with secondary spread to the peritoneal cavity and appendix. The possibility of multifocal neoplasia or multiple primary tumors coexisting has also been raised.31,32 Studies examining the clinical and immunohistochemical properties of these tumors, however, have demonstrated convincing evidence that ovarian tumors seen in PMP are from a primary appendiceal malignancy; therefore, representing regional solid organ metastases.33,34
Tumor Biology of Peritoneal Malignancies
Biology of a Cancer Cell in the Peritoneal Cavity
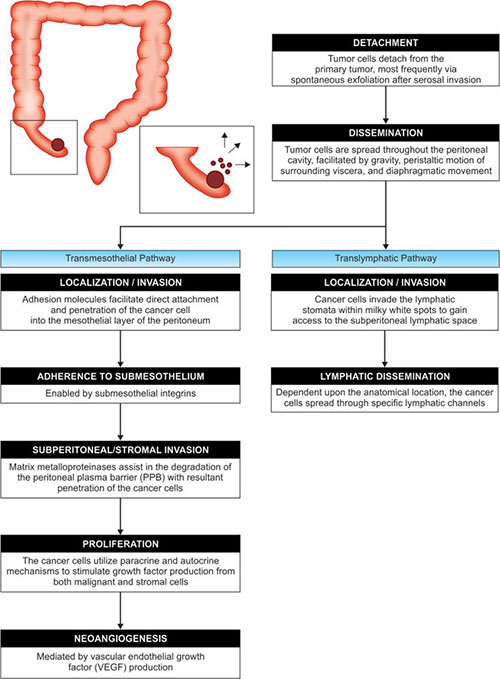
Kusumara et al. described three mechanisms for development of peritoneal carcinomatosis. First, as previously discussed, primary peritoneal malignancies can arise spontaneously or following carcinogenic exposure, e.g. asbestos (Fig. 2).30
Second, peritoneal carcinomatosis can occur independently in the setting of another primary tumor. This phenomenon of individual tumors with polyclonal multifocal origin is seen with peritoneal disease in the setting of ovarian tumors of low malignant potential (OTLMP) or peritoneal serous papillary carcinoma (PSPC). In this scenario the tumors appear histopathologically similar but behave differently from a pathophysiological standpoint. The independent origin of these tumors can be demonstrated by analyzing their patterns of X-chromosome inactivation. Early in development, normal somatic cells in women randomly undergo inactivation of one of their X chromosomes, resulting in mosaicism amongst the somatic cells. This pattern of inactivation remains constant throughout the life of the cell. Tumors of monoclonal origin would be expected to have identical patterns of X chromosome inactivation. In cases of peritoneal disease and OTLMP (or PSPC), polyclonicity (i.e. different patterns of X chromosome inactivation) has been demonstrated.30,35,36
Finally, tumor cells of a gastrointestinal or gynecological primary tumor can gain entry into the peritoneal cavity via a number of routes. A serosal invading malignancy may “shed” tumor cells directly into the peritoneal cavity. These cells can also gain entry into the peritoneal cavity following tumor rupture, as is commonly the case in appendiceal-derived mucinous malignancies. Intraoperatively, contamination of the peritoneal cavity can occur by disruption of vessels or lymphatics, with subsequent dispersal of tumor cells within the blood or lymphatic fluid. Additionally, manipulation of the tumor 8itself during the course of resection can introduce tumor cells into the peritoneal cavity.
Once in the peritoneal cavity, these rogue cells are then spread throughout with relative ease, aided by gravity and intra-abdominal movement as a consequence of intestinal peristalsis and respiration-generated changes in intra-abdominal pressure.30 Attachment and proliferation of these cells is outlined in Figure 3.
Following tumor detachment and dissemination within the peritoneal cavity, tumor cells may follow the transmesothelial pathway, translymphatic pathyway (e.g. PMP), or utilize both mechanisms for metastasis (e.g. colon cancer).30
Treatment of Peritoneal Malignancies
In general, carcinomatosis portends a shortened survival and poor quality of life. There are many treatment options available but not one alone has been shown to have any or minimal affect on survival. Most of these patients have a survival of 6−12 months depending on the disease and therapy provided. This has been a major problem in treating cancer patients for hundreds of years. The past 50 years has shown great promise in this field with improved chemotherapy, palliative treatments and novel multimodality therapy.
Systemic Chemotherapy
Systemic chemotherapeutic agents employ their cytotoxic effects through a variety of mechanisms, from generalized inhibition of DNA/RNA synthesis to more targeted pathways for specific proteins and enzymes. Systemic therapy, as compared to locoregional application of chemotherapy, is advantageous in that it can potentially reach undetectable distant malignant cells. However, because these therapies reach both malignant and healthy tissues, they are accompanied by the potential cytotoxic damage to healthy tissue leading to a variety of often undesirable, sometimes dose-limiting and, occasionally, life-threatening side effects.
In addition to potential side effects, the benefits of systemic chemotherapy for peritoneal-based malignancies have thus far had a very limited role. In fact, for appendiceal malignancies, currently available regimens of neoadjuvant systemic chemotherapy may have deleterious effects on outcomes.37 The reason why systemic chemotherapy is so ineffective for peritoneal-based disease is its multifactorial nature.
The “kinetic model” for drug resistance proposes that slow growing cancers are more likely to be drug resistant because the “window” during which time the cell is in a mitotic state, and is susceptible to cytotoxicity from chemotherapy occurs less frequently (longer intervals between cell division) as compared to a fast-growing malignancy. This theory is limited by a considerable number of exceptions (e.g. the blast cell phase of chronic myelogenous leukemia, which is often extremely fast growing but refractory to chemotherapy) but its principles may be contributory in some cancers, such as peritoneal mucinous tumors, which are often slow growing.38,39
Another mechanism that may help to describe the inadequacy of systemic chemotherapy for peritoneal malignancies is the “multicellular spheroid model” or diffusion model. This proposes that high drug concentrations cannot be obtained throughout large tumors due to inadequate drug diffusion. That is, as tumor depth increases, concentration of drug and associated cytotoxic effects decrease. Given that peritoneal malignancies often develop into bulky disease by the time they are diagnosed, it would be plausible that this theory may indeed apply to the inherit drug resistance seen in these cancers. In practice, however, it is often the higher molecular weight agents that impart superior malignant degradation, somewhat discrediting this theory as one would expect lower diffusion capabilities of these drugs.40,44
The “Goldie-Coldman” model for drug resistance is a useful tool to explain the overall poor performance of systemic chemotherapy for the treatment of peritoneal cancers. This mathematical model takes into account the generally accepted theory that resistance to chemotherapy is, at least in part, due to the generation of phenotypic drug resistance. Studies which investigated single tumor cell suspensions found that those cells responded to cytotoxic agents in a manner consistent with the clinical response of the larger tumor from which they were harvested, suggesting that it is the individual cell characteristics that dictate drug resistance.41,42,44
The tumor cell can develop this drug resistance through random mutations in its genetic code (“genetic instability theory”), which alters the gene products, and thereby changes the phenotypic drug responsiveness.44 A single mutation can result in significant drug resistance by changing cell membrane permeability to specific compounds (“pleiotropic drug resistance”). The “fluctuation test of Lunia and Delbruck” is a method of mathematically predicting the frequency of these random mutations.43,449
Figure 4: Goldie-Coldman mathematical model (probability of resistance for a given tumor size/mutation rate). Plot of the function p = θ–αN for two different mutation rates, A and B; where A > B.[Source: Reprinted with permission from Goldie JH, Coldman AJ. Cancer Res. 1984;44:3643-53]44
The function defines the probability of therebeing zero resistant cells present for any given value of the tumor size, and the mutation rate to resistance. Where treatment is capable of eradicating all nonresistant cells, then this probability approaches the value of potential cure. For tumor burdens distributed over the steep portion of the curve, relatively small increases in tumor mass will have a disproportionate effect on reducing curability.
Taking these principles of genetic variability into account, the Goldie-Coldman model demonstrates that the larger the tumor, the greater the likelihood of a mutant cell type arising.44 That is, the likelihood of a cure (p) = θ–αN, where “α” represents mutation rate per cell generation and “N” is the number of cells.44
This theory can be applied to most tumors with the exception of those that are highly curable or incurable as these lie on the flat part of the sigmoid curve (Fig. 4).44 For peritoneal cancers, which are often large, bulky and at the time of diagnosis, the Goldie-Coldman model would predict a high likelihood of phenotypic drug resistance. Additionally, this theory essentially supports the role of cytoreductive surgery prior to chemotherapy, which decreases the concentration of tumor cells thereby shifting the curve to the left (decreased probability of drug resistance).
Surgery
Surgical resection is the mainstay of treatment for many types and stages of gastrointestinal and gynecological malignancy. Surgical cytoreduction to the point of no measurable malignant cells by postoperative peritoneal cytology can be achieved and is associated with improved outcomes as compared to incomplete debulking.45
Unfortunately, however, gastrointestinal malignancies have a high rate of local recurrence at anastomotic regions and within the peritoneal cavity, even when complete resection (negative surgical margins and no gross disease) were achieved.46 The proposed mechanism for this recurrence is centered on the concept of “tumor cell entrapment”, which begins when tumor microemboli are disseminated into the peritoneal cavity during surgical resection.47 Intraoperative blood loss provides the opportunity for malignant cells in the venous blood to spread throughout the peritoneal cavity. Additionally, disruption of both lymphatic channels and tumor margins allow for tumor cell dispersal. These events can occur in even the most meticulously executed open and laparoscopic surgical procedures. Fibrin deposition, which occurs as part of the normal postsurgical inflammatory process, then entraps these cells, and essentially protects them as they propagate, while also providing a vascular framework further fostering tumor growth.
Intraperitoneal Chemotherapy
The peritoneal plasma barrier (PPB) allows for higher concentrations of cytotoxic agents to be administered intraperitoneally as compared to systemic administration. For many drugs, the PPB contributes to slower diffusion, thereby maintaining higher intraperitoneal concentrations over time. The regional exchange rate for pharmacologic agents introduced into the peritoneal cavity is 5–25 milliliters per minute, depending on the properties of the drug.48
Bioavailability refers to the extent to which a drug reaches the systemic circulation. The bioavailability of intraperitoneal chemotherapy is significantly lower than that of intravenous or oral chemotherapy due to first-pass hepatic metabolism. It has been suggested that the majority of intraperitoneally administered chemotherapy is absorbed by the visceral peritoneum.49 As discussed previously, the vascular drainage of this portion of the peritoneum follows that of the viscera, and its mesentery (i.e. the mesenteric veins to the portal circulation), subject to hepatic metabolism. Conversely, the parietal peritoneum, which follows the venous drainage of the anterior abdominal wall via the epigastric vessels, bypasses the portal system. The predilection for portal venous drainage of the peritoneal cavity dramatically decreases the amount of drug that reaches the systemic circulation.
The substantial drug concentrations attainable via peritoneal-based chemotherapy can be demonstrated 11by measuring the area under the curve (AUC) following drug administration. The AUC is a measure of drug exposure, calculated by taking the integral of plasma concentration versus time—∫([drug, plasma] × Dt). Sugarbaker et al. calculated AUC for intraperitoneal delivery of Mitomycin C (MMC), and 5-fluorouracil (5FU) in patients undergoing early postoperative intraperitoneal chemotherapy (EPIC).55 In this group of patients, it was found that the AUC for peritoneal fluid drug concentration versus plasma drug concentration was 117:1 for 5FU, and 21.6 for MMC. This allows for a dose intensive regimen of locoregional cytotoxic agents with a general avoidance of systemic toxicity. Additionally, this study demonstrated that intraperitoneal 5FU exhibited a high rate of first-pass hepatic metabolism (i.e. high portal venous drug concentration, and low systemic plasma drug concentration). The slow rate of peritoneal 5FU absorption and, therefore, the avoidance of hepatic over saturation contributed to the liver's capacity to metabolize the drug.
Unfortunately, in the absence of operative resection, intraperitoneal chemotherapy as a monotherapy or in conjunction with systemic chemotherapy has not been shown to impart significant disease-free or overall survival for peritoneal malignancies. Likely contributing to this failure is the observation that 90% of the concentration advantage that intraperitoneal chemotherapy provides (as compared to systemic therapy) is only maintained to a depth of up to one millimeter (100 cell layers).50
Hyperthermia
Hyperthermia—administered as tumor-directed therapy, locoregionally, or to the whole body—can be used as an adjunct to chemotherapy and radiation therapy, where it has been shown to have a synergistic effect in certain clinical situations. To some degree, elevated temperature can also independently induce tumor cell death or necrosis.
The scope of damage elicited by heat on cells was summarized by Hildebrandt et al. (Table 2). Malignant cells are more susceptible—as compared to normal tissues—to hyperthermic damage for a number of reasons. The microenvironment of tumor cells contributes to their temperature sensitivity. Tumor growth often occurs at a rate unsupported by their vascular supply, resulting in nutritional deprivation. This also generates acidic and hypoxic conditions, making these cells more susceptible to the cytotoxic effects of hyperthermia. The application of heat likely only further impairs blood flow to the tumor. Interestingly, tumor cells may indeed “get hotter” and “stay hotter” when compared to non-malignant cells due to their chaotic and insufficient vascular supply, which does not reliably cool the cells (and itself is more susceptible to heat-induced damage).51,52
|
Cell death from hyperthermia is a function of both temperature and length of exposure. It occurs in two stages. First, at the start of the heat exposure, linear growth arrest occurs (reversible). This is followed by an exponential rate of cell death. While a selective tumor killing being effective is exhibited within a range of 40°C to 44°C, research has shown that the ability to progress to the exponential phase of cell death is decreased considerably if temperatures are less than 43°C. In clinical practice, 43°C is a target temperature for therapy. The “thermal isoeffect dose” (TID) allows for the conversion of other thermal therapies into “units” of equivalent heating time at 43°C.53,57
Sugarbaker et al. found that heat has synergistic properties with some chemotherapeutic agents including MMC, doxorubicin and Cisplatin.54 These drugs have an increased “thermal enhancement ratio”, a ratio of percentage of cell death from an agent at normothermia and hyperthermia. The exact etiology of this synergism is unclear, and it is not demonstrated universally among cytoxic drugs. It is likely that heat-induced chemosensitization is a multifactorial process, involving changes in the tumor vascular supply, membrane sensitivity or 12permeability to specific agents, and local changes in the tumors microenvironment. Interestingly, heat may have an additional advantage of helping to overcome drug resistance with MMC, cisplatin, doxorubicin and nitrosureas.59
In practice, hyperthermia has been found to be safe when applied to the peritoneal cavity. In a small prospective study examining 16 patients with gastric cancer who underwent open instillation of intraperitoneal chemotherapy (without peritonectomy), hyperthermia by itself was not found to induce any additional peritoneal injury or inflammation, as measured by postoperative ascitic amount and protein concentration.55 Additionally, safe and effective methods for the intraoperative application of heated chemoperfusate have been developed. Spratt et al. studied intracavitary hyperthermia in an animal model, forming a basis for the development of a perfusion system.56
An important feature of hyperthermia in the treatment of malignancy is that prior cellular exposure to hyperthermia may have a deleterious effect on sensitivity—referred to as “thermal tolerance”. This is at least partially due to the development of heat-shock proteins following initial exposure to elevated temperature.57 These proteins are expressed on the surface of malignant cells following non-lethal application of heat, but are not produced by normal cells.59 This is an important concept to consider when anticipating time to optimal temperature intraoperatively and also for situations of reoperative surgery.
Multimodality Treatment: The Role of Cytoreductive Surgery with Intraoperative Hyperthermic Chemotherapy
In summary, systemic chemotherapy, surgery, locoregional chemotherapy and hyperthermia have properties important in the treatment of peritoneal malignancy. Unfortunately, as monotherapy, each of these therapies fails in regards to long-term achievement of a disease-free state and overall lengths of survival.
A case series examining patient data from M.D. Anderson Hospital during the mid-1950s to late-1970s, revealed an actuarial survival rate of 54% and 18% at 5 and 10 years respectively, in a cohort of 38 patients with ovarian or appendiceal PMP treated with cytoreductive surgery and adjuvant chemotherapy.58 Appendiceal origin was associated with significantly worse outcomes in this study. Data from 56 patients treated at the Mayo Clinic during a similar time period found 1-, 5- and 10-year survival rates at 98%, 53% and 32% respectively.59 Thirteen percent of these patients underwent normothermic intraperitoneal chemotherapy. Interestingly, the only adverse predictor of outcome in this group was administration of systemic adjuvant chemotherapy, which 27% of the patients received. Recurrence rate was 50%, irrespective of degree of operative cytoreduction.
These results have been improved by combining surgical resection with intraoperative application of regional hyperthermia and intraperitoneal chemotherapy, with or without adjuvant chemotherapy. This has become the mainstay of treatment for certain peritoneal-based malignancies, and is becoming a more commonly accepted treatment for other locoregionally advanced gastrointestinal malignancies.
Cytoreductive surgery and intraoperative hyperthermic chemotherapy (CRS-HIPEC) have been employed in the treatment of some primary peritoneal malignancies with promising results. Median survival of patients with malignant mesothelioma is approximately one year when treated with systemic chemotherapy alone.60,61 However, multiple studies investigating the use of CRS-HIPEC for this disease have demonstrated a median survival in the range of 28–35 months.62,63 Yano et al. performed CRS-HIPEC with doxorubicin and cisplatin (followed by early postoperative chemotherapy in select patients), and achieved a median survival of 3.7 years (range 0.7–6.9 years) for eight patients who were optimally debulked.64
Many studies have investigated the use of CRS- HIPEC for PMP and appendiceal cancers. Often times, however, the data for various pathologic origins of peritoneal disease (e.g. colorectal, gastric, appendiceal) are pooled, making assessment of outcomes based on tumor type difficult. Table 3 presents findings from some larger studies that examined CRS-HIPEC for PMP and appendiceal cancers. Of the pathological subtypes of appendiceal carcinoma, disseminated peritoneal adenomucinosis (DPAM) imparts a more favorable prognosis when compared to peritoneal mucinous carcinomatosis (PMCA), and those mucinous tumors with intermediate or discordant features.43 However, there is a benefit of treatment with CRS-HIPEC for even high-grade tumors.65
While historically the focus of treatment with CRS-HIPEC was appendiceal-related malignancies and PMP, its use for ovarian, colorectal and gastric malignancies has shown promising results. Glehen et al. performed a multi-institutional study of 1,290 patients with various peritoneal-based cancers including PMP, peritoneal mesothelioma, gastrointestinal adenocarcinomas (colorectal, gastric, appendiceal, small bowel), primary peritoneal serous carcinoma and peritoneal sarcomatosis.6813
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
About 91.8% of these patients underwent optimal surgical debulking (CC0-1) followed by either HIPEC (85.8%) and/or EPIC (16.6%). Median follow-up was 45.4 months (range 20.3–90.0 months), and overall median survival of 34 months was achieved for all patients. Further analysis demonstrated median survivals of 30 months, 9 months, 41 months and 77 months for peritoneal carcinomatosis from colorectal, gastric, peritoneal mesothelioma and appendiceal adenocarcinoma respectively. Median survival of PMP patients was not reached.
In a Phase III randomized trial by Verwaal et al., that compared CRS-HIPEC plus adjuvant chemotherapy with palliative surgery followed by systemic chemotherapy, a statistically significant survival advantage was observed in the CRS-HIPEC group with median survival of 22.3 months, compared with 12.6 months in the palliative arm.69 Of additional interest are studies comparing patients with peritoneal disease from colorectal cancer with those who have isolated hepatic colorectal metastases.70,71 Following optimal cytoreduction of the peritoneal disease or margin-negative hepatic metastasectomy, these patients had similar 1- and 5-year survival rates. This again makes a strong argument that isolated 14peritoneal metastases should be regarded as locoregional extension, and not systemic disease.
Additionally, there is some data to suggest that HIPEC may play a palliative role in patients who undergo suboptimal debulking, whereby it has reduced ascites and improved quality of life.72
What accounts for the significant survival and disease-free progression period when therapies are combined? First, effective cytoreductive surgery removes any pre-existing adhesive tissue, and allows exposure of all aspects of the peritoneal cavity. Surgical mobilization and peritonectomy “prime” the peritoneal tissues for responsiveness to cytotoxic agents by exposing the submesothelium. Additionally, with the abdomen already exposed, insertion of intraperitoneal catheters and application of chemoperfusate in the operating suite is relatively simple.
Second, the probability of chemotherapy resistance amongst the remaining tumor cells following cytoreduction is dramatically decreased, as demonstrated by Goldie-Coldman model, where the likelihood of chemotherapy resistance is proportionate to tumor cell concentration. Additionally, surgical resection stimulates the tumor cells to reenter the proliferative phase of the cell cycle, making them more susceptible to cytotoxic agents.30 In practice, intraperitoneal chemotherapy has been shown to be significantly more effective when utilized in the intraoperative or early postoperative period. The reason for this is multifold. As previously discussed, adhesive tissues that develop in the postoperative period are nidus for implantation by the rogue remnant tumor cells that remain following surgery. They provide a skeletal framework for attachment and subsequent malignant proliferation. These same adhesive tissues also cause compartmentalization of the peritoneal cavity which can prevent complete distribution of cytotoxic agents introduced into the peritoneal space.
Interestingly, the pharmacokinetics of intraperitoneal drug absorption—physiology of the plasma peritoneal barrier—seem to change over time. Evidence suggests that when cytotoxic agents are administered on consecutive days in the postoperative period, their clearance increases over time; in that the plasma concentrations of a cytotoxic agent are significantly higher following repetitive exposure and duration following surgery.73 This supports the argument for one-time intraoperative intraperitoneal chemotherapy. Additionally, a linear relationship between cell death and intraperitoneal exposure has been suggested, even with limited exposure time.74
The treatment of this dynamic group of intraperitoneal malignancies continues to evolve. Improvements in our understanding of the pathophysiology of these tumors will hopefully shed light on new and improved therapies. Operative cytoreduction and intraperitoneal chemotherapy will likely continue to be a staple in the treatment of peritoneal malignancies. There are challenges to overcome, however, which include the need for standardization of the operative procedure, improvements in the diagnostic and preoperative evaluation of disease, burden in this cohort of patients and increasing the availability of CRS-HIPEC to a larger number of patients. More effective systemic adjuvant therapies are also needed.
REFERENCES
- Sadler TW. Langman's Medical Embryology, 9th edition. Lippincott Williams & Wilkins; Philadelphia: 2004.
- Moore KL, Dalley AF. Clinically Oriented Anatomy, 4th edition. Lippincott Williams & Wilkins; New York: 1999.
- Skandalakis JE. Anatomical Complication in General Surgery. McGraw-Hill; Texas: 1983.
- Khanna R, Mactier R, Twardowski ZJ, et al. Peritoneal cavity lymphatics. Perit Dial Int. 1986;6:113–21.
- Dobbie JW, Anderson JD. Ultrastructure, distribution, and density of lamellar bodies in human peritoneum. Perit Dial Int. 1996;16(5):482–7.
- Faull RJ. Peritoneal defenses against infection: winning the battle but losing the war? Semin Dial. 2000;13(1):47–53.
- Brulez HF, Verbrugh HA. First-line defense mechanisms in the peritoneal cavity during peritoneal dialysis. Perit Dial Int. 1995;15(7 Suppl.):S24-33.
- Lewis S, Holmes C. Host defense mechanisms in the peritoneal cavity of continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1991;11(1):14–21.
- Krist LF, Eestermans IL, Steenbergen JJ, et al. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241(2):163–74.
- Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev. 2009;18(1):132–9.
- Bloss JD, Liao SY, Buller RE, et al. Extraovarian peritoneal serous papillary carcinoma: a case-control retrospective comparison to papillary adenocarcinoma of the ovary. Gynecol Oncol. 1993;50(3):347–51.
- Tobacman JK, Greene MH, Tucker MA, et al. Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet. 1982;2(8302):795–7.
- Killackey MA, Davis DR. Papillary serous carcinoma of the peritoneal surface: matched-case comparison with papillary serous ovarian carcinoma. Gynecol Oncol. 1993;51(2):171–4.
- Pickhardt PJ, Bhalla S. Primary neoplasms of peritoneal and sub-peritoneal origin: CT findings. Radiographics. 2005;25(4): 983–95.
- Chua TC, Yan TD, Morris DL. Surgical biology for the clinician: peritoneal mesothelioma: current understanding and management. Can J Surg. 2009;52(1):59–64.
- Daya D, McCaughey WT. Well-differentiated papillary mesothelioma of the peritoneum. A clinicopathologic study of 22 cases. Cancer. 1990;65(2):292–6.
- Tzanakakis G, McCully KS, Vezeridis MP. Benign papillary mesothelioma of the peritoneum: a consideration in the differential diagnosis of peritoneal implants. South Med J. 1989;82(12):1579–80.
- Chetty R. Well differentiated (benign) papillary mesothelioma of the tunica vaginalis. J ClinPathol. 1992;45(11):1029–30.
- Ordóñez NG, el-Naggar AK, Ro JY, et al. Intra-abdominal desmoplastic small cell tumor: a light microscopic, immunocytochemical, ultrastructural, and flow cytometric study. Hum Pathol. 1993;24(8):850–65.
- Tison V, Cerasoli S, Morigi F, et al. Intracranial desmoplastic small-cell tumor. Report of a case. Am J Surg Pathol. 1996;20(1):112–7.
- Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15(6):499–513.
- Ibarguen E, Sharp HL, Snyder CL, et al. Hemangiomatosis of the colon and peritoneum: case report and management discussion. Clin Pediatr (Phila). 1988;27(9):425–30.
- McCusker ME, Cote TR, Clegg LX, et al. Primary malignant neoplasms of the appendix: a population-based study from the Surveillance, Epidemiology and End-Results program, 1973–1998. Cancer. 2002;94(12):3307–12.
- Deans GT, Spence RA. Neoplastic lesions of the appendix. Br J Surg. 1995;82(3):299–306.
- Conte PF, Gadducci A, Cianci C. Second-line treatment and consolidation therapies in advanced ovarian cancer. Int J Gynecol Cancer. 2001;11(Suppl. 1):52–6.
- Diaz-Montes TP, Bristow RE. Secondary cytoreduction for patients with recurrent ovarian cancer. Curr Oncol Rep. 2005;7(6):451–8.
- Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–22.
- Klaver YL, Lemmens VE, Creemers GJ, et al. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Ann Oncol. 2011.
- Kusamura S, Baratti D, Zaffaroni N, et al. Pathophysiology and biology of peritoneal carcinomatosis. World J Gastrointest Oncol. 2010;2(1):12–8.
- Sugarbaker PH. Pseudomyxomaperitonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219(2):109–11.
- Seidman JD, Elsayed AM, Sobin LH, et al. Association of mucinous tumors of the ovary and appendix. A clinicopathologic study of 25 cases. Am J Surg Pathol. 1993;17(1):22–34.
- Kahn MA, Demopoulos RI. Mucinous ovarian tumors with pseudomyxoma peritonei: a clinicopathological study. Int J Gynecol Pathol. 1992;11(1):15–23.
- Kaern J, Tropé CG, Abeler VM. A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer. 1993;71(5):1810–20.
- Chuaqui RF, Zhuang Z, Emmert-Buck MR, et al. Genetic analysis of synchronous mucinous tumors of the ovary and appendix. Hum Pathol. 1996;27(2):165–71.
- Young RH, Gilks CB, Scully RE. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxomaperitonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991;15(5):415–29.
- Prayson RA, Hart WR, Petras RE. Pseudomyxoma peritonei. A clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol. 1994;18(6):591–603.
- Ronnett BM, Kurman RJ, Zahn CM, et al. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995;26(5):509–24.
- Ronnett BM, Shmookler BM, Diener-West M, et al. Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol. 1997;16(1):1–9.
- Cuatrecasas M, Matias-Guiu X, Prat J. Synchronousmucinous tumors of the appendix and the ovary associated with pseudomyxomaperitonei. A clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol. 1996;20(6):739–46.
- Gu J, Roth LM, Younger C, et al. Molecular evidence for the independent origin of extra-ovarian papillary serous tumors of low malignant potential. J Natl Cancer Inst. 2001;93(15): 1147–52.
- Muto MG, Welch WR, Mok SC, et al. Evidence for a multifocal origin of papillary serous carcinoma of the peritoneum. Cancer Res. 1995;55(3):490–2.
- Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxomaperitonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 2008;15(2):526–34.
- Goldie JH, Coldman AJ. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984;44(9):3643–53.
- Shackney SE, McCormack GW, Cuchural, Jr GJ. Growth rate patterns of solid tumors and their relation to responsiveness to therapy: an analytical review. Ann Intern Med. 1978;89(1):107–21.
- Buick RN, Mackillop WJ. Measurement of self-renewal in culture of clonogenic cells from human ovarian carcinoma. Br J Cancer. 1981;44(3):349–55.
- Salmon SE, Alberts DS, Meyskens Jr. FL, et al. Clinical correlations of in vitro drug sensitivity. In: Salmon SE (Ed). Cloning of Human Tumor Stem Cells. Alan R. Liss; New York: 1980. pp. 223–46.
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976;7(1):1–11.
- Law LW. Origin of the resistance of leukemic cells to folic acid antagonists. Nature. 1952;169(4302):628–9.
- Loggie BW, Fleming RA, Geisinger KR. Cytologic assessment before and after intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Acta Cytol. 1996;40(6):1154–8.
- Minsky BD, Mies C, Rich TA, et al. Potentially curative surgery of colon cancer: patterns of failure and survival. J Clin Oncol. 1988;6(1):106–18.
- Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16(4 Suppl. 6):83–97.
- Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2(5):498–504.
- Sugarbaker PH, Graves T, De Bruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res. 1990;50(18):5790–4.
- Gianni L, Jenkins JF, Greene RF, et al. Pharmacokinetics of the hypoxic radio sensitizers misonidazole and demethylmisonidazole after intraperitoneal administration in humans. Cancer Res. 1983;43(2):913–6.
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56.
- Bleehen NM. Hyperthermia—a treatment method for cancer? J R Soc Med. 1981;74(12):865–7.
- van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–84.
- Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14(3):254–61.
- Shido A, Ohmura S, Yamamoto K, et al. Does hyperthermia induce peritoneal damage in continuous hyperthermic peritoneal perfusion? World J Surg. 2000;24(5):507–11.
- Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980;40(2): 253–5.
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44(17):2546–54.
- Fernandez RN, Daly JM. Pseudomyxomaperitonei. Arch Surg. 1980;115(4):409–14.
- Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxomaperitonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219(2):112–9.
- Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol. 2008;19(2):370–3.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–44.
- Sebbag G, Yan H, Shmookler BM, et al. Results of treatment of 33 patients with peritoneal mesothelioma. Br J Surg. 2000;87(11):1587–93.
- Loggie BW, Fleming RA, McQuellon RP, et al. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67(10):999–1003.
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21(24): 4560–7.
- Deraco M, De Simone M, Rossi CR, et al. An Italian Multicentric Phase II study on peritonectomy and intraperitoneal hyperthermic perfusion (IPHP) to treat patients with peritoneal mesothelioma. J Exp Clin Cancer Res. 2003;22(4 Suppl):41–5.
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol 2006;13(3):405–12.
- Yano H, Moran BJ, Cecil TD, et al. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur J Surg Oncol. 2009;35(9):980–5.
- Omohwo C, Nieroda CA, Studeman KD, et al. Complete cytoreduction offers long-term survival in patients with peritoneal carcinomatosis from appendiceal tumors of unfavorable histology. J Am Coll Surg. 2009;209(3):308–12.
- Cioppa T, Vaira M, Bing C, et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol. 2008;14(44):6817–23.
- Deraco M, Baratti D, Inglese MG, et al. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol. 2004;11(4):393–8.
- Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36(5):456–62.
- Loungnarath R, Causeret S, Bossard N, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum. 2005;48(7):1372–9.
- Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6(8):727–31.
- Stewart 4th JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13(5):624–34.
- Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245(1):104–9.
- Witkamp AJ, de Bree E, Kaag MM, et al. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxomaperitonei. Br J Surg. 2001;88(3):458–63.
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116(24):5608–18.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43.
- Shen P, Thai K, Stewart JH, et al. Peritoneal surface disease from colorectal cancer: comparison with the hepatic metastases surgical paradigm in optimally resected patients. Ann Surg Oncol. 2008;15(12):3422–32.
- Cao CQ, Yan TD, Liauw W, et al. Comparison of optimally resected hepatectomy and peritonectomy patients with colorectal cancer metastasis. J Surg Oncol. 2009;100(7):529–33.
- Deraco M, Rossi CR, Pennacchioli E, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion in the treatment of recurrent epithelial ovarian cancer: a phase II clinical study. Tumori. 2001;87(3):120–6.
- Sugarbaker PH, Klecker RW, Gianola FJ, et al. Prolonged treatment schedules with intraperitoneal 5-fluorouracil diminish the local-regional nature of drug distribution. Am J Clin Oncol. 1986;9(1):1–7.
- Jol C, Kuppem P, Leeflang PA, et al. In vitro human ovarian cancer. In: Taguchi T, Andryse O (Eds). New Trends in Cancer Chemotherapy with Mitomycin C. Excerpta Medica; Amsterdam: 1987. pp. 181–91.