Abstract
Fungal keratitis is an important cause of ocular morbidity in the developing world. It has replaced bacteria as the most common cause of suppurative keratitis. Characteristic clinical features include feathery edges, raised dry surface, satellite lesions, and a cheesy hypopyon. Microbiological investigations, such as KOH, Gram's stain and Sabouraud's dextrose agar culture confirm the diagnosis. Natamycin is the drug of choice for the treatment of fungal keratitis, while other drugs include voriconazole, econazole, itraconazole, and amphotericin B. Deeper lesions require a keratoplasty.
Introduction
The incidence of fungal keratitis has shown a dramatic increase in the recent years. In fact, in countries like India, fungi have nearly replaced bacteria as the most common cause of infectious suppurative keratitis. This increase is thought to be due to a combination of various factors, namely increased clinical suspicion, advances in diagnostic techniques, and paradoxically, the advancement in the field of antibacterial therapy, which has proportionately reduced the incidence of bacterial keratitis. Morbidity in fungal infections tends to be greater than that in bacterial keratitis, because the diagnosis is often delayed and the available drugs are not very effective.
Epidemiology
Fungal keratitis is most often encountered in rural populations, where agricultural workers are exposed to corneal injury contaminated with organic matter much more frequently than population at large.1 It is relatively 2uncommon in the western world. Even, when it happens, it is usually caused by Candida. Filamentary fungi, especially Aspergillus and Fusarium are the common causes of mycotic keratitis in most of the developing world.
Classification of Fungi
Fungi are eukaryotic and heterotrophic organisms. Although numerous fungi have been implicated, the pathogenic fungi, which cause significant keratitis, can be divided into filamentous, yeast, and dimorphic forms.
FILAMENTOUS FUNGI
Filamentous fungi are also known as moulds. They occur as long filaments, called hyphe, which grow by apical extension and produce feathery aerial colonies above the culture media. They can be further divided into septate and nonseptate organisms. The septate filamentary fungi are the most common cause of keratomycosis. They are divided into nonpigmented monilial (which include Fusarium sp., Aspergillus sp., and Acremonium sp.) and pigmented dematiaceous (Curvularia sp., and Lasiodiplodia sp.) varieties. The nonseptate filamentary fungi (Mucor, Absidia and Rhizopus sp.) are important causes of orbital disease and endogenous endophthalmitis but do not commonly produce corneal disease.
YEASTS
Yeasts are fungi with usual and dominant growth as unicellular organisms and form creamy, pasty colonies, which may be mistaken for staphylococcal colonies. They divide by asexual budding, forming pseudohyphae and do not form mycelium in culture. The most common fungi in this category are the Candida sp. and Cryptococcus sp., which are part of the normal flora of skin, respiratory tract, and conjunctiva and act as opportunistic pathogens.
DIMORPHIC FUNGI
Dimorphic fungi have two distinct morphologic forms: the yeast phase, which occurs in tissues and a mycelial phase, which occurs in media and natural surfaces. These fungi in this category are Blastomyces, Coccidiodes, Histoplasma, and Sporothrix, exhibit properties of molds, when cultivated at 25°C and of yeasts when grown at 37°C.3
Risk Factors
Fungi are ubiquitous organisms present almost everywhere in the environment. Although the eye is continuously exposed to these pathogens, the normal defense mechanisms, such as the eyelids, tear components, and the corneal epithelium provide adequate protection. An epithelial defect is a prerequisite for these organisms to set up an infection.
The importance of trauma that is often trivial and frequently associated with plant material has been well documented in the initiation of fungal infection caused by filamentous fungi.
Contact lens wear is an uncommon risk factor in fungal keratitis. These organisms have been shown to grow within the matrix of soft contact lenses.2 Filamentous fungi were more commonly associated with cosmetic lens wear and yeasts from therapeutic lens use.
Corticosteroids appear to activate and increase the virulence of fungi. The other factors uncommonly reported include vernal or allergic keratoconjunctivitis, neurotrophic ulcers, and penetrating keratoplasty. The predisposing factors for the development of fungal keratitis in patients after penetrating keratoplasty include suture problems, topical steroid therapy, chronic antibiotic use, contact lens wear, graft failure, and persistent epithelial defects. Fungal corneal ulcers have been reported following refractive surgical procedures like radial keratotomy, photorefractive keratotomy, and more recently following Laser in situ keratomileusis (LASIK) procedures, either in the immediate postoperative period (direct surgical contamination) or later (following trauma).
The principal risk factors for yeast or Candida keratitis are prolonged epithelial ulceration, penetrating keratoplasty, and therapeutic contact lens wear. In addition systemic diseases like Sjügrens syndrome, erythema multi—forme, immunodeficiency, endocrinopathy, diabetes, and hypovitaminosis A have been shown to predispose to candidal infection.
Certain investigators have highlighted the association between the prevailing climatic conditions and the incidence of fungal keratitis. In India the incidence is highest in the harvesting seasons (September-October). There is less seasonal variation with regard to yeast infections.
Pathogenesis
The filamentous fungi affect normal eyes of immunocompetent hosts after corneal abrasion or trauma, from some kind of vegetable matter. The yeasts usually cause keratitis in immunocompromised individuals. Their pathogenicity 4is related to a decrease in the systemic or local defense mechanisms either by a direct effect on the immune system (topical steroids) or by an alteration in the normal epithelial barriers (i.e. persistent epithelial defects, bandage contact lenses, neurotrophic keratitis, topical anesthetics, etc.) with predisposing systemic and/or eye disease. The dimorphic fungi are a rare cause of keratitis.
The exact mechanisms underlying the pathogenesis of fungal infections are unclear. As compared to bacteria, the fungi are relatively nonimmunogenic, partly, because of their large size, which prevents them from being engulfed by the neutrophils, and partly because they do not secrete chemotactic factors, which attract inflammatory cells. After entering through a corneal epithelial defect, the fungi elaborate toxic substances and enzymes, such as proteases, hemolysins, and exotoxins. Fusarium sp, especially is known to possess specific cellular and molecular attributes, which aid to cause virulent reaction. They can adhere to biopolymers and have the ability to produce toxins and elaborate enzymes.3
A few strains of Asperigillus produce aflatoxins and ochratoxins.4 The conidia of Aspergillus fumigatus have been shown to bind to and degrade basement membrane laminin, an extracellular matrix glycoprotein found in basement membranes. They have the capacity to penetrate an intact Descemet's membrane. The resultant host inflammatory response subsequently contributes to a part of the tissue damage. Activation of the complement system leads to concentration of polymorphonuclear inflammatory cells in the corneal cells that liberate proteolytic enzymes. Encapsulated Cryptococcus neoformans, Candida albicans, and the conidia of A. fumigatus, all activate the complement system by either classic or alternative pathways.
Fungi can penetrate deep into stroma and through an intact Descemet's membrane. It is thought that once fungus gains access into anterior chamber or to the iris and lens, eradication of the organism becomes extremely difficult. Likewise, organisms that extend from cornea into sclera become difficult to control.
Candida albicans strains have also been known to produce a variety of proteolytic enzymes. The most important of these is an aspartyl acid protease that can act on a wide variety of tissue proteins and is thought to contribute to the invasiveness of the organism. In addition, various virulence factors, such as a phospholipase A and lysophospholipase have been identified with Candida sp.5
Clinical Features
FILAMENTARY FUNGI
Unlike bacterial infections, there is less pain, conjunctival congestion, discharge and chemosis early in the course of fungal infection and the symptoms are far less than what is expected of the size of the ulcer.
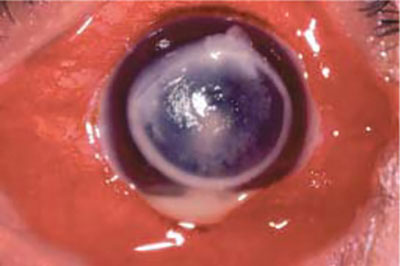
The earliest finding may be a small nonspecific stromal infiltrate with a surrounding unhealthy looking epithelium, in which case it is indistinguishable from a bacterial infection. Commonly the patient presents with a central or a paracentral ulcer with feathery stromal margins (Fig.1.1). The common misdiagnosis, which might be made at this stage, may be a dendritic keratitis caused by herpes simplex. These fungal pseudodendritic lesions are shorter, stockier and are associated with surrounding stromal infiltration. In addition, a mirror image of the pseudodendritic lesion in the deeper stromal layers can also be seen. The adjacent Descemet's membrane may be thrown into folds.
With time, the ulcer starts to become larger and elevated above the level of the corneal surface. The edges of the ulcer looks serrated, somewhat looking like a can opener capsulotomy. The surface looks dirty white and dry and has a rough texture. The serrated edges and the dry elevated rough surface can be considered pathognomonic of a fungal lesion (Fig.1.2). The stromal lesions are present beyond the size of the epithelial defect and have a feathery pattern. In rare instances, the lesion may be entirely in the posterior aspect of the corneal stroma without an accompanying epithelial defect. In these cases, the posterior stromal lesions also have a feathery edge like paint sprayed over a wall.6
Foci of infiltration can be seen several millimeters away from the main area of involvement. These are called satellite lesions, and they may remain isolated from the main lesion or may be connected with the main ulcer by a thin line of stromal infiltration (Fig. 1.3). The epithelium can be intact over the infiltrate. An endothelial plaque may also be present. Like in many other keratitis, a ring infiltrate may surround the primary lesion, most likely representing an antibody response to fungal antigen (Fig. 1.4).
Hypopyon can be present in varying proportions and the amount is not directly proportional to the size of the ulcer.
As the lesions progress, the pain gets intense and the ulcer involves almost the whole of the cornea and starts to lose its characteristic pattern. The lesions look more suppurative and soupy.7
The edges of the ulcer may start becoming rounded like a bacterial ulcer, but the edges of the deeper stromal lesions have the characteristic feathery pattern until the final course of the disease. As the size of the ulcer becomes larger, it becomes more flushed with the surface and assumes a smooth surface. A significant majority of the deeper stromal ulcers perforate over time.
In the ulcers caused by the dematiceous fungi, there may be macroscopic pigment deposition over the surface. The color may vary from a thick blackish-brown pigmentary lesion to a less mottled greenish-gray pigment dispersed over a whitish base (Fig. 1.5). The denser lesion can be mistaken for a uveal prolapse. The lesion caused by dematiceous fungi are more 8elevated than those caused by nonpigmentary filamentous fungi and has a characteristic mushroom head appearance. These lesions can be dissected from the surface using a Bard Parker knife and has a tough, leathery attachment to the ulcer surface.
In contrast to the filamentary fungal keratitis, the stromal keratitis caused by Candida may be more localized and have a collar-button configuration, often with a small ulcer and an expanding infiltrate. They are usually superimposed on a chronic debilitated ocular condition. The lesions tend to be oval, plaque like, and elevated. They are well outlined and surrounded by stromal edema and more closely resemble the bacterial keratitis. If untreated, the keratitis evolves to produce dense suppuration and necrosis of the deep stroma.
It should be emphasized that the differentiation between an advanced keratitis caused by bacteria and fungi is difficult to distinguish by clinical features alone. Future developments in the field of confocal microscopy may become a useful clinical tool for the diagnosis of fungal keratitis. The lack of experience and the cost of the instrument may be the limiting constraints for its widespread use.
Histopathology
Non-replicating fungi, and their mycotoxins, proteolytic enzymes and soluble fungal antigens are capable of inducing severe inflammatory reactions. These agents can result in necrosis of the corneal lamellae, and incite an antigenic response with immune ring formation and hypopyon. The inflammatory response tends to be less marked than seen in bacterial keratitis though the epithelium is often remain intact over the infective lesion. The classical histopathologic findings in fungal keratitis include fungal hyphal elements oriented perpendicular to the normal corneal lamellae and the tendency for the hyphal elements apparently to penetrate Descemet's membrane and spread into the anterior chamber (these two features are considered to be suggestive of progressive pathogenicity). When this occurs, it is seen as retrocorneal or anterior chamber inflammatory mass adjacent to an area of deep keratitis.
Laboratory Diagnosis
A standard microbiologic baseline work-up should be performed in every case of suspected infectious keratitis. This is even more crucial in the regions of the world, where fungi and bacteria cause keratitis, almost in equal proportions. In addition, combined infections with bacteria and fungi, or even with multiple fungi, can be detected through this routine examination.9
Scrapings are made from the base and the edges of the ulcer under topical anesthesia using a Bard Parker knife or a Kimura spatula. The lesions caused by fungi often feel gritty and have a leathery attachment to the base of the ulcer. Calcium alginate swabs have been reported to increase the recovery rate of the organisms. In deeper lesions, a corneal biopsy may be required for obtaining adequate specimen.
Direct microscopic evaluation is the most valuable and rapid diagnostic tool for the detection of fungal elements in corneal scrapings. The initial smears can be examined using a potassium hydroxide mount (KOH) preparation and a Gram's stain. The KOH preparation is a simple, reliable and reproducible screening method for identifying filamentary fungi. KOH has been used as a 10–20% suspension, either plain or with ink or with lactophenol cotton blue. Proteinaceous components, such as host cells are partially digested by the alkali, leaving intact the polysaccharide containing fungal cell walls (Fig. 1.6). However, the sensitivity of this method is highly variable ranging between 33 and 94%. A recent study done by Sharma et al aimed to look at the sensitivity, specificity and predictive values of KOH preparation and to compare its efficacy with other methods of corneal scraping examinations used in the diagnosis of mycotic keratitis.5 KOH is an excellent and simple tool and has a definite place in the armamentarium of diagnostic techniques.
Giemsa and Gram's stain techniques are equally sensitive in detecting fungal elements. The Gram's stain does not stain the cell wall or septa of hyphal fragments but is absorbed by the protoplasm (Fig. 1.7). Yeasts typically stain dark blue and can be distinguished from bacteria, foreign material, precipitate and other artifacts.10
Hyphal fragments and yeasts appear dark blue or purple in Giemsa stain. Gomoris methenamine silver and periodic acid Schiff stains are other methods used when conventional staining techniques yield no results. However, these methods are time consuming, tedious and difficult to interpret.
Though the use of calcofluor white, acridine orange and fluorescein conjugated lectins yield rapid results, special infrastructural requirements make them inapplicable in most situations. However, these are the stains of choice for the detection of yeasts.
The findings of direct examination can be confirmed by culture. Sabourauds dextrose agar and brain-heart infusion broth are the most commonly used primary isolation media for fungi. Cycloheximide should never be incorporated in Sabourauds, because it inhibits the saprophytic fungi predominantly responsible for keratitis. The media are kept at room temperature (25°C) for the isolation of fungi. Since bacterial pathogens are always a consideration in the differential diagnosis of suppurative keratitis, additional material is inoculated in a row of C streaks on fresh sheep blood agar or chocolate agar media. Most fungi show mycelial growth on blood agar within 24–72 hours. Yeasts grow on these media at 30°C and are visible in 18–24 hours.
In our laboratory, we prefer to use potato dextrose agar as the principal culture media to isolate fungi, since this medium is thought to have a profound effect on the conidiogenesis and the surface character of Fusarium colonies. Colonies of Fusarium are usually white in the early stages of development, but often show reverse pigmentation. The classification is based on 11microscopic features of conidia that form on specialized hyphae called conidiophores.
Colonies of Aspergillus fumigatus are white at first, but as spores are produced, they become velvet green owing to the pigmentation of the conidia. A. niger colonies are also white during the initial growth phase but turn completely black on sporulation. The typical colony of Candida sp. on Sabourauds dextrose agar has a dirty white color. It is opaque with a smooth, flat, round contour and a pasty soft consistency. When grown on blood agar 35°C, they are slow-growing and nonhemolytic. Candida colonies have a distinctive fruity and yeasty odor.
Identification of C. albicans is usually based on the formation of germ tubes in vitro and further speciation is based on sugar fermentation. Classification of noncandidial yeasts is based on biochemical testing. Since most of the ocular fungal isolates grow quicker, it may not be absolutely necessary for the incubation period to be prolonged than a week.
Scraping also provides for debridement of organisms and the epithelium which may provide a barrier to antifungal drug penetration. When scrapings are negative, a diagnostic superficial keratectomy or corneal biopsy may be performed and sent for smear, culture and histopathological examination. In some cases of deep keratitis, a 27 G hypodermic needle or a 6-0 silk suture can be introduced into the infiltrate to obtain a specimen for culture.
A polymerase chain reaction (PCR) has been used in an animal model to develop a sensitive, specific and rapid test to diagnose Fusarium keratitis. Even as the availability of in vitro susceptibility testing for fungi have been reported, there is yet no agreement on the standardization and applicability of these tests and currently do not enjoy widespread usage.
Medical Treatment
All the available antifungal agents are fungistatic and not fungicidal. The penetration of the drugs is poor and has to be aided by repeated debridement, which acts by debulking of the pathogenic organism. The treatment schedules are usually prolonged and often leading to a poor compliance with medical therapy.
Most of the antifungal drugs exhibit their effect through their actions on the fungal cell membrane. In addition to its barrier function, the fungal cell membrane controls the movement of electrolytes and thereby controls the internal homeostasis of the cell. Ergosterol is the predominant sterol unique to the fungal cell membrane, while mammalian cell membranes are composed of cholesterol. Most antifungals capitalize on this important difference in plasma membrane constituents to damage the fungal cells, 12while minimizing damage to the host cells. There are three major classes of antifungal drugs available:
- Polyenes.
- Imidazoles, and
- Fluorinated pyrimidines.
POLYENES
Polynes constitute the first line of the antifungal agents. They bind preferentially to ergosterol in the fungal plasma membrane, thereby, altering membrane permeability and disrupting the fungal cell. Larger polyenes (such as amphotericin B and nystatin) create channels that span the cell membranes and allow electrolyte movement. Small polyenes, such as natamycin are too small to bridge the width of the cell membrane and causes localized membrane disruptions thus altering permeability.6
Natamycin
Natamycin is a tetraene antibiotic. Discovered in 1958, it has proved itself to be the most valuable ocular antifungal agent available to date. The drug is available as a 5% suspension and must be shaken well before use. Natamycin has a broad spectrum of activity against filamentous fungi. When applied topically, it adheres to the ulcer site like a rope, which may act as a reservoir of drug. In general, topical natamycin is well tolerated and its corneal toxicity is rare. It may cause conjunctival necrosis when given subconjunctivally and, therefore, this route is not preferred.
Amphotericin B
Amphotericin B is a heptaene polyene. It is still considered the drug of choice for treatment of life threatening infections. It is also the drug of choice for candidal keratitis and Cryptococcus. The drug is variably active against filamentary fungi and is not recommended as the first line treatment for these organisms. Rifampin reportedly increases the effectiveness of amphotericin B against Aspergillus sp. but not against Candida sp. The drug is insoluble in water, unstable at 37°C and degrades rapidly if exposed to light. However, solubility can be improved by ad——dition of deoxycholate. It is relatively toxic and cannot penetrate intact epithe-lium. The adverse effects of the topical application include a sting—ing sensation, chemosis and punctate epithelial keratitis. They are mostly though to be due to the presence of bile salt used to stabilize the drug in solution.13
Amphotericin B is dispensed in 20 ml vials for intravenous use containing 50 mg powder. The powder is initially reconstituted to a concentration of 5 mg/ml in 10 ml of sterile water for injection. It should be prepared in distilled water because it precipitates in sodium chloride solution. An initial dose of 0.15% is applied for every five minutes for half an hour as a loading dose and then hourly. Subconjunctival injections can cause conjunctival necrosis and should be avoided. Systemic administration of amphotericin B is nephrotoxic and is ineffective in the treatment of keratitis.
Occasionally, intracameral injection of amphotericin B (5–10 μg in 0.1ml) can be a useful alternative treatment of severe fungal keratitis with intraocular extension that is resistant to conventional therapy.7
Nystatin
Nystatin is another polyene antifungal agent that is used as an ointment or formulated eyedrops (50,000 units /ml) and can be used for the treatment of superficial candidal keratitis. The ointment preparation causes severe stinging sensation and patient compliance is poor for prolonged usage. It is too toxic for parenteral administration.
IMIDAZOLES
This group constitutes the imidazoles and the structurally related N-substituted triazole. The imidazoles include clotrimazole, miconazole, econazole, and ketoconazole. The triazoles include fluconazole and itraconazole. These drugs exhibit their antifungal activity by having two mechanisms of action. In lower concentration, they are fungistatic by inhibiting sterol 14 alpha demythylase; a microsomal P-450 related enzyme, which is required in the demythylation of lanosterol in the synthesis of ergosterol.8 At higher concentrations, they are fungicidal which is due to direct membrane damage to the phospholipids present in the fungal cell wall. However, they are never able to achieve fungicidal concentration in the human cornea
Clotrimazole
Clotrimazole is used extensively topically for the treatment of skin and genital candidal infection. A topical ophthalmic preparation of clotrimazole can be made with 1% clotrimazole in arachis oil. A dermatological cream containing clotrimazole 1% is well tolerated when applied to the eye. The drug is too toxic for systemic use. Though not preferred, it can be used in the treatment of Aspergillus keratitis.14
Miconazole
Miconazole can be used through various routes' topically, subconjunctivally, or intracamerally. It can be prepared for topical application as a 1% drop in arachis oil or a 2% cream. The commercially available intravenous preparation can also be administered topically (10 mg/ml) or by sub—conjunctival injection (5–10 mg). The drug is relatively unstable in solution and has to be reformulated weekly. It is active against mainly yeasts and is variably active against filamentary fungi.
Econazole
Econazole is a dichlorimidazole and which exhibits a wide spectrum of activity against filamentous fungi. A topical preparation of econazole 1% can be prepared and is well tolerated.
Ketoconazole
Ketoconazole is more water-soluble and is better absorbed after systemic administration than other imidazoles. It is available as 200 mg tablets. The usual dose for adults is 200–400 mg/day, which can be increased to 800 mg or more. It can also be administered as a topical preparation in concentrations ranging from 1 to 5%. Clinically, the agent has been shown to be effective against Candida, Aspergillus, Fusarium and Curvularia sps in particular.9
The most common side effects are dose-dependent nausea, anorexia, and vomiting. Ketoconazole inhibits steroid biosynthesis in fungi. Several endocrinological abnormalities like decreased libido and testosterone levels, menstrual irregularities and suppression of the ACTH-stimulated plasma cortisol response have been reported. It blocks the hepatic micro-somal system, interfering with the metabolism of several drugs, such as cyclosporine.
Fluconazole
Fluconazole, a water-soluble triazole is available in an oral dose of 100 mg capsules and intravenous solution (sterile solution in 0.9% sodium chloride, 2 mg/ml: 100 ml unit). Although the MICs of fluconazole are higher than other azoles in most susceptibility test systems, the in vitro activity of fluconazole does not parallel the efficacy in infections in animals and in clinical trials in humans.10 The recommended dose of the drug is 200– 400 mg/day and is the same for oral and intravenous route. It is useful in Candida keratits.15
Itraconazole
The newer oral triazole antifungal agent itraconazole may also be a helpful adjunctive agent in the treatment of fungal keratitis, based on in vitro activity, pharmacokinetic properties and limited clinical trials in nonocular infections. However, its drawback is that it is quite hydrophobic, and being 90% protein bound in the serum, it does not permeate the tissues as well as fluconazole.
Voriconazole
Voriconaxole is one of the recent drugs useful in the management of fungal keratitis.11,12 It is a synthetic derivative of fluconazole in which the triazole group is substituted with a fluoropyrimidine moiety and has an increased potency and in vivo efficacy. Addition of a methyl group to the propyl backbone increases the affinity of the drug for the target enzyme (14-α-sterol demethylase). It shows a broader spectrum of activity against Fusarium, Aspergillus, Scedosporium, Paecilomyces, and Candida. As with other triazole antifungal agents, voriconazole exerts its affect primarily through inhibition of cytochome P-450 dependent 14-sterol demethylase, an enzyme responsible for conversion of lanosterol to 14-demethyl lanosterol in ergosterol biosynthetic pathway.
Voriconazole is available in vials containing 30 mg lyophilized powder. It has excellent corneal penetration because of its low molecular weight (349 g/mol) and its lipophilic properties. It can be used topically in the form of 1% eyedrops and can be titrated based on epithelial healing. In deeper lesions, intrastromal voriconazole may be given in the dose of 50 μg/0.1 ml. Oral voriconazole may be supplemented in the dose of 5 mg/kg body weight. A baseline liver function test should always be performed before starting oral voriconazole therapy.
The adverse effects with systemic voriconazole therapy include transient visual disturbances (photopsias, photophobia, or color vision defects), formed visual hallucinations, and elevated liver enzymes.
FLUORINATED PYRIMIDINES
Flucytosine
Flucytosine is a fluorinated pyrimidine and is selectively taken by susceptible fungi and deaminated to fluorouracil which blocks thymidine synthesis. Since mammalian cells do not normally metabolize flucytosine, it does not inhibit metabolic processes in the mammalian cells. It is effective against Candida and Cryptococcus, and certain strains of Aspergillus, but is not effective against Fusarium and Cephalosporium. However, some fungal 16strains lack the specific permease to transport the drug into the cells and they are resistant to flucytosine. Topical flucytosine is used as drops in a 1% solution every hour and is well tolerated. Therapeutic levels can also be achieved in adults with the administration of a dose of 50–150 mg/kg /day in divided doses. Flucytosine should not be given alone in the treatment of keratomycosis for fear of inducing resistance. Its topical form is relatively nontoxic to the eye. When administered systemically, it may cause a transient depression of the bone marrow and diarrhea.
Principles of Therapy
As mentioned earlier, the treatment for fungal keratitis is usually prolonged. Improvement may not be visible for several days. Lack of progression of the stromal infiltrate is the first sign that the antifungal drug is effective. This is followed by a rounding of the feathery margins, blunting of the perimeters and reduction in cellular infiltrate and edema in surrounding stroma. Prolonged conjunctival injection, protracted epithelial ulceration, punctate corneal epithelial erosions and diffuse stromal haze imply drug toxicity. The appearance of a transient hypopyon in the face of an improving corneal picture has been attributed to hypersensitivity or a toxic reaction.
The initiation of treatment can be performed as soon as the results of the KOH mount and the Gram's stain are obtained. These tests can readily be performed at the office of the physician itself. Studies have questioned the reliability of the positivity of culture as the gold standard and have recommended that all smear positive cases of mycotic keratitis be treated accordingly despite negative culture reports.13 If the smears and the culture results are negative, but the clinical features are strongly in favor of fungal keratitis, we recommend initiation of topical antifungal therapy, especially in countries where there is a high prevalence. Culture results are useful in certain situations and can play an effective role in identification of the species, which is useful for epidemiological purposes.
For suspected filamentous fungal keratitis, natamycin 5% drops should be administered hourly for the initial 24–48 hours and at 2-hour intervals. The dosage can then be gradually reduced depending upon the response. If natamycin is unavailable, then 1% econazole or 1% miconazole can be used.
For yeast keratitis, topical amphotercin B 0.1% to 0.25% drops are initally applied at 15 minute intervals for the first 24–48 hours and then frequency of application is gradually reduced for the next 4–6 days. If initial therapy was topical natamycin or amphotercin B drops alone and the stromal suppuration is progressing, oral ketoconazole or fluconazole should be added 17for either filamentous fungal or yeast keratitis. Subconjunctival miconazole (5 mg) or fluconazole (1 mg) can be given.
Prolonged treatments with systemic antifungals are reserved for deep keratitis associated with scleritis and endophthalmitis. The most frequently used oral antifungal has been ketoconazole. Recent evidence suggests that fluconazole may penetrate better into the cornea after systemic administration than some other azoles and associated with fewer side effects.14 The other drugs, which have been tried for the treatment of fungal keratitis in past include silver sulfadiazine and sulfacetamide.
The issue of synergy or antagonism when the antifungal drugs are combined has given varying results. A combination of flucytosine and miconazole or natamycin was found to be synergistic in vitro against Aspergillus keratitis.15 Certain investigators routinely use flucytosine topical (1% solution) and systemic (oral 150 mg/kg) in combination with amphotericin B in the management of Candida keratitis.16 Antagonism has been reported when amphotericin B and the imidazoles have been used systemically.17
Supplementary therapy of keratomycosis includes a cycloplegic-mydriatic agent, such as atropine 1% twice a day, and topical beta blocker or oral carbonic anhydrase inhibitor to control secondary ocular hypertension. Corticosteroids do not have any role in the treatment regimen of fungal keratitis and is not recommended in any stage of the disease.
Surgical Treatment
Regular debridement of the ulcer using a scalpel or a Kimura spatula is an invaluable step to ensure adequate therapeutic levels of the antifungals into the deeper stromal layers. Some authors feel that surgery is seldom needed during the acute spread of the infection.18 Studies conducted at the Bascolm Palmer Institute indicate that therapeutic keratoplasty was required seven times more frequently in fungal keratitis than in bacterial keratitis. We also feel that the surgical modality of excisional keratoplasty has an important role to play in the treatment of fungal keratitis and perhaps in some parts of the world, it may well be the primary choice of treatment.
Therapeutic keratoplasty has to be contemplated when the ulcer progresses despite specific antifungal therapy. The progression is characterized by increased area and depth of stromal suppuration, stromal ulceration to the point of extreme thinning of the cornea, dense fibrinos exudate in the anterior chamber and frank perforation. However, a small perforation developing during the course of the healing can be managed by the use of a tissue adhesive.
The goals of the therapeutic keratoplasty are to remove the infected part of the cornea, and restore the integrity of the globe. A recurrence of infection 18in a graft is more difficult to treat than a graft rejection. Even if one of these transplants is rejected, a second keratoplasty for optical rehabilitation can be performed at a later date. A large-sized graft should be used without any fear of rejection.
Local anesthesia may suffice in a majority of the cases. In these cases, O Brien's facial nerve block is given first prior to the ciliary block to prevent squeezing of the eyelid and resultant inadvertent perforation. The initial incision is performed using a hollow trephine needle. The anterior chamber is entered using a sharp razor blade. If the area of suppuration is large and asymmetric, decenteration of the trephination or a free hand dissection to encompass the infected areas should be done. The size of the trephination should preferably leave 1 to 1.5 mm clear zone of the uninvolved cornea to reduce the possibility of residual infective organisms peripheral to the trephination. Thicker fibrous membranes covering the surface of the iris or the lens can be peeled off using a forceps or in some cases excised using scissors. A peripheral iridectomy should be performed in all cases to prevent secondary glaucoma. If involvement of the posterior segment or endophthalmitis is suspected, an antifungal agent such as amphotericin B (5 microorganisms per 0.1 ml) is injected. The intact lens should not be removed as much as possible and localized cataracts should warrant an extraction at a later date in a separate sitting. The graft is then secured with 10 nylon interrupted sutures. The advantage of these interrupted sutures is that they can be removed selectively if future infiltrations develop. Results of therapeutic keratoplasty are often encouraging (Fig. 1.8). The recurrence of the infection in the graft starts from the periphery.
Postoperatively, topical natamycin 5% is continued for a period of 1–2 months. During this time, the usage of topical steroids is not recommended and supplementary topical nonsteroidal anti-inflammatory drugs may be used. Steroids can be used cautiously after the first postoperative month follow-up.19
Conclusion
In conclusion, fungal keratitis is fast becoming a silent epidemic, especially in developing countries. A lot of research is clearly needed in the field of antifungal pharmacology to limit the morbidity caused by these infections. Until then, early surgical intervention may be the preferred mode to eliminate the infection.
References
- Forster RK. Fungal keratitis and conjunctivitis. Clinical disease. In Smolin G, Thoft RA, (Eds): The Cornea: Scientific foundation and clinical practice. Bostom Little Brown; 1994:229–52.
- Churner R, Cunningham RD. Fungal contaminated soft contact lenses. Am J Ophthalmol 1988;106:708–14.
- Nelson PE, Dignani MD, Anaissie EJ. Taxonomy, biology and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504.
- Bennett JE. Mycoses: Aspergillus. In Mandell GL, Douglas Jr. RG Bennett JE (Eds): Principles and practice of infectious disease. Churchill Livingstone; New York: 1990. p. 1958–62.
- Sharma S Silverberg M, Mehta P, et al. Early diagnosis of mycotic keratitis: predictive value of potassium hydroxide preparation. Ind J Ophthalmol 1998;46(1):31–5.
- Medoff G, Kobayaski GS. Strategies in the treatment of systemic fungal infections. NEJM. 1980;302:145–55.
- Saye Yilmaz, Ture M, Maden A. Efficacy of Intracameral Amphotericin B in the management of refractory keratomycosis and endophthalmitis. Cornea 2007;26:398–402.
- Sud IJ, Feingold DS. Mechanisms of action of the antimycotic imidazoles. J Invest Dermatol. 1981;6:438–41.
- Thienpont D, Van Cutsem J, Van Gerren F, et al. Ketoconazole, a new broad spectrum orally active antimycotic. Experimentia. 1979;35:606–9.
- Saag MS, Dismukes WE. Azole antifungal agents: Emphasis on new triazoles. Antimicrob Agents Chemothe. 1988;32:1.
- Hariprasad SM, Mieler WF, Lin TK, et al: Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol. 2008;92:871–8.
- Sponsel W, Chen N, et al. Topical voriconazole as a novel treatment for fungal keratitis. Antimicrob Agents Chemother. 2006;50:262–8.
- Jones DB. Initial therapy of suspected microbial corneal ulcers. II. Specific antibiotic therapy based on corneal smears. Surv Ophthalmol. 1979;24:97–116.
- O'Day DM. Orally administered antifungal therapy for experimental keratomycoses. Trans Am Ophthalmol Soc. 1990;88:685–725.
- Johns KJ, O'Day DM: Pharmacologic management of keratomycosis. Surv Ophthalmol 1998;33:178.
- Abad JC, Foster SC. Fungal keratitis. International Ophthalmol Clin. 1998;36 3:1–15.
- Brajtburg J, Kobayashi D, Medoff G, Kobayashi GS. Antifungal action of amphotericin B in combination with other polyenes or imidazole antibiotics. J Infect Dis 1982;146:138–46.
- O'Day DM. Fungal keratitis. In Pepose JS, Holland GN, Wilhelmus KR (Eds): Ocular infection and immunity. St. Louis, Mosby. 1996;1048–1061.