Chapter Outline
- • Genital Flap Selection
- • Penoscrotal Fascial Anatomy
- • Penile Vascular Anatomy
- – Arterial
- – Venous
- • Scrotal Vascular Anatomy
- – Arterial
- – Venous
- • Surgical Pearls
- – Arterial Anatomy and Implications for Urethral Stricture Repair
- – Venous Anatomy
- – Urethral Elasticity
- – Urethral Location within Corpus Spongiosum
INTRODUCTION
It is vital that anyone undertaking urethral reconstruction should have a thorough understanding of the blood supply that allows flap to survive, grafts to take and the urethra to heal. The male genitalia are well-vascularized with a redundant blood supply that has implications for reconstructive procedures, as detailed below.
GENITAL FLAP SELECTION
Genital skin and subcutaneous tissues are crucial for successful urethral reconstruction. A detailed understanding of the vascular supply can help to ensure long-term success of the repaired urethra. The ideal flap will have a vascular pedicle that is reliable and robust. As outlined below, the vascular anatomy is predictable and can be utilized to ensure a well-vascularized urethral repair.
PENOSCROTAL FASCIAL ANATOMY
The penile skin is elastic and has no adipose layer. Deep to the skin is the Dartos fascia, a loose areolar layer. The Dartos is also free of adipose tissue and freely moves over the underlying Buck's fascia or fascia penis. The 2Dartos layer is continuous with the Scarpa's fascial layer of abdominal wall and continues into scrotum. In the perineum, it continues as Colles’ fascia. Within this layer runs the superficial nerves and lymphovascular system. Buck's fascia covers the tunica albuginea of both corpora cavernosa as well as the corpus spongiosum. Buck's fascia is continuous with the external spermatic fascia in the scrotum (Fig. 1.1).
The clinical utility of these tissue planes is evident when developing fasciocutaneous penile skin island flaps, as in an Orandi (vertical) or McAninch1/Quartey2 (circular transverse) flap. Dissection in the correct plane will ensure well-vascularized flaps. It is debated which label is given to these correct planes (i.e. whether the anterior lamella of Buck's fascia or the tunica Dartos). Regardless, a plane can be developed between the skin and the Dartos-related fascial vascular structures, and another plane can be developed between these and the deep penile structures which allows for creation of a well-vascularized penile skin flap.
Fig. 1.1: Penoscrotal fascial anatomySource: McAninch JW, Morey AF. Penile circular fasciocutaneous skin flap in 1-stage reconstruction of complex anterior urethral strictures. J Urol. 1998;159(4):1209–13.
PENILE VASCULAR ANATOMY
Arterial
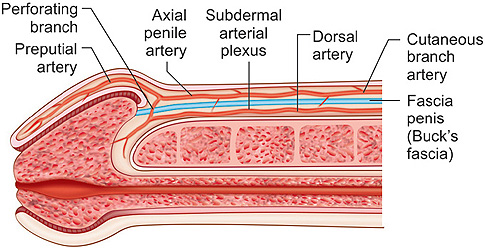
The blood supply to the penile skin derives from femoral arteries. There are two main blood supplies, the superficial and deep external pudendal arteries. Superficial to Scarpa's fascia, these arteries run from lateral to medial, and enter base of the penis. There, they give off scrotal branches and then divide into dorsolateral and ventrolateral axial penile arteries that run superficial to the anterior lamella of Buck's fascia and within the tunica Dartos fascia (Fig. 1.2).3 These axial penile arteries then give off superficial branches to form the subdermal plexus, which supplies the penile skin (Figs 1.3A and B). The perforators that connect the subcutaneous and subdermal arterial plexuses are so small that an avascular plane can be developed between the skin and Dartos. The axial penile arteries continue to the foreskin as preputial arteries and also send perforating branches through Buck's fascia to provide collateral circulation with the dorsal arteries (Fig. 1.4). It is important to keep in mind that the circulation comes from lateral part and base of the penis when developing axial penile skin flaps so that they can be mobilized and transposed appropriately.
Fig. 1.2: Penile vascular anatomySource: Jordan GH, Stack RS. General concepts concerning the use of genital skin islands for anterior urethral reconstruction. Atlas Urol Clin North Am. 1997;5:23–44.
Figs 1.3A and B: Dartos vascular anatomy. (A) Arterial; (B) VenousSource: Quartey JK. Microcirculation of the penile and scrotal skin. Atlas Urol Clin North Am. 1997;5:23.
Fig. 1.4: Penile arterial microcirculationSource: Quartey JK. Microcirculation of the penile and scrotal skin. Atlas Urol Clin North Am. 1997;5:23.
Venous
The glans and corpus spongiosum drain into the retrobalanic venous plexus, which lies in the space between proximal part of the glans and the distal corpora cavernosa. From there it divides into the superficial and deep dorsal median veins. The deep dorsal median vein runs proximally down the penis deep to the anterior lamella of Buck's fascia, eventually travels through the suspensory ligament of the penis and finally empties into dorsal vein complex plexus beneath the pubic symphysis (Figs 1.5 and 1.6).
The superficial dorsal median vein receives blood from the retrobalanic plexus or can take off directly from the deep dorsal median vein. Regardless, it pierces Buck's fascia to travel superficially within the tunica Dartos to base of the penis. There may be multiple superficial veins in a variable pattern that drain ultimately through base of the penis and into the external pudendal veins. The preputial veins may drain directly through base of the penis or may combine with the other superficial veins (Figs 1.2 and 1.3).
Fig. 1.5: Penile venous microcirculationSource: Quartey JK. Microcirculation of the penile and scrotal skin. Atlas Urol Clin North Am. 1997;5:23.
Other venous drainage includes the subdermal venous plexus which drains through many large veins at base of the penis and venae comitantes which run with the axial penile arteries (Fig. 1.7). There are not generally connections between the subdermal plexus and the Dartos-based venous drainage, although there is an occasional large communication.
Fig. 1.6: Penile venous anatomySource: Quartey JK. Microcirculation of the penile and scrotal skin. Atlas Urol Clin North Am. 1997;5:23.
Fig. 1.7: Superficial venous penile drainageSource: Quartey JK. Microcirculation of the penile and scrotal skin. Atlas Urol Clin North Am. 1997;5:23.
SCROTAL VASCULAR ANATOMY
Arterial
The scrotum's arterial blood supply is from two main sources, the femoral artery via the external pudendal arteries and the internal iliac artery via the internal pudendal artery.
The anterior scrotum is nourished by the anterior scrotal arteries, branches of the superficial and deep external pudendal arteries. These arteries are variable in course. Within the scrotum, near the penoscrotal junction, superficial branches supply a subdermal plexus that travel toward apex of the scrotum to anastomose with the posterior circulation (Fig. 1.8A).4
Figs 1.8A and B: (A) Anterior; (B) Posterior scrotal circulation—crosshatched area is anterior and dotted area is posterior circulationSource: Jordan GH, Stack RS. General concepts concerning the use of genital skin islands for anterior urethral reconstruction. Atlas Urol Clin North Am. 1997;5:23–44.
In the perineum, the internal iliac artery continues as the internal pudendal artery. After emerging from Alcock's canal, it travels through the perineal membrane to continue as the perineal artery (Fig. 1.8B). The course of this artery is between ischiocavernosus and bulbospongiosus muscles, in the membranous layer of the superficial perineal fascia. From there, posterior scrotal arteries are given off in a variable pattern and travel toward apex of the scrotum. Interconnections are made with the anterior circulation, as well as across the central septum combining left and right-sided circulations. In addition, it has been shown (Figs 1.9A and B) that the scrotal raphe receives branches through a separate branch of the perineal artery.5
Scrotal skin island flaps that are based on the Dartos fascia can bring a well-vascularized flap to the bulbar urethra, but is often not long enough to reach the anterior urethra. A Blandy flap for perineal urethrostomy can also be fashioned from the posterior circulation by making an inverted “U”-shaped incision.
Venous
The anterior scrotum is drained by veins in the subdermal plexus and then come together at the base of scrotum. From there they travel to the external pudendal veins and ultimately to the femoral veins.
Figs 1.9A and B: Scrotal raphe branches of posterior scrotal artery. (A) Anterior scrotum; (B) Posterior scrotum (Red—anterior or posterior scrotal branches, Yellow—median raphe branches)Source: Carrera A, Gil-Vernet A, Forcada P, et al. Arteries of the scrotum: a microvascular study and its application to urethral reconstruction with scrotal flaps. BJU Int. 2008:103;820–4.
The posterior scrotum is emptied by coalescence of subdermal plexus veins into the perineal vein and finally the internal pudendal vein, mirroring course of the arterial system (Figs 1.8A and B).
SURGICAL PEARLS
Arterial Anatomy and Implications for Urethral Stricture Repair
One of the unique properties of the urethra that is crucial to reconstruction is its dual blood supply. This allows extensive mobilization and division of the urethra without compromising distal vascular flow. The distal and proximal urethra are maintained by two related blood supplies, but with the proximal being nourished in an antegrade fashion and the distal, retrograde fashion (Fig. 1.10).6
The arterial supply is from the internal iliac to the internal pudendal artery. After giving off perineal, posterior scrotal and central scrotal arteries, the internal pudendal continues as the common penile artery. The common penile artery (Fig. 1.11) first gives off bulbar and urethral artery and then continues to split into the central cavernosal artery and the dorsal artery of penis. The bulbar and urethral arteries supply the proximal corpus spongiosum. The urethral artery continues within the spongy tissue in a variable location (Figs 1.12A and B).7
Fig. 1.10: Bidirectional arterial flow to the urethra (cp: Common penile; da: Dorsal artery of the penis; cc: Central cavernosal; u: Urethral artery and b: Bulbar artery)Source: Brandes SB. Urethral Reconstructive Surgery. Totowa, NJ: Humana Press; 2008.
Fig. 1.11: Arterial anatomy of the urethraSource: Jordan GH, Stack RS. General concepts concerning the use of genital skin islands for anterior urethral reconstruction. Atlas Urol Clin North Am. 1997;5:23–44.
The paired dorsal arteries travel down shaft of the penis beneath the anterior lamella of Buck's fascia and ultimately form multiple small branches that supply the glans penis. Blood then flows in a retrograde fashion proximally down the corpus spongiosum. Along the way, the dorsal artery also gives off a variable number of circumflex arteries that travel ventrolaterally and help to supply the corpus spongiosum.
Figs 1.12A and B: Urethral arterial location. (A) Bulbar urethra; (B) Pendulous urethraSource: Redrawn from Chiou RK, Donovan JM, Anderson JC, et al. Color Doppler ultrasound assessment of urethral artery location: potential implication for technique of visual internal urethrotomy. J Urol. 1998;159(3):796–9.
Another source of blood supply to the spongy tissue is via perforators between the corpora cavernosa (central cavernosal arteries) and the corpus spongiosum. However, the distribution of these small vessels is neither constant nor reliable.
Urethrotomy
During anastomotic urethroplasty, maintaining adequate blood flow is crucial. As stated, the urethral arteries travel in a variable location, not always at the 3- and 9 o'clock position as previously thought. Rather, the arteries are distributed almost equally throughout the corpus spongiosum (Figs 1.12A and B). These arteries can be superficial and near the urethral lumen. The location and depth of the urethral arteries can be seen by ultrasound. While this can help in guiding the location of urethrotomy, it is more important to cut deep enough to allow the urothelium to open without being too deep into the spongiosum (which can bleed aggressively).
Anterior Urethroplasty/Excision and Primary Anastomosis
Mobilization of the urethra in conditions with poor distal retrograde blood flow in patients with severe hypospadias and deficient distal spongiosum can lead to bulbar urethral ischemia (Figs 1.13A to D).8 In cases like this, anastomotic urethroplasty can lead to complications such as necrosis or restricture.9 These patients may be better served with substitution urethroplasty, flap onlay or even a staged approach. In patients with poor bipedal blood supply, it is important to maintain blood supply through the bulbar arteries and perforating vessels (Fig. 1.14).
Figs 1.13A to D: Bipedal blood flow in anastomotic urethroplasty. (A and B) With loss of bulbar artery inflow, the proximal bulbar urethra relies on retrograde flow; (C and D) If the retrograde supply is deficient by hypospadias, spongiofibrosis or mobilization, ischemia can developSource: Yu G, Miller HC. Critical Operative Maneuvers in Urologic Surgery. St Louis: Mosby; 1996.
Fig. 1.14: With bilateral internal pudendal inflow loss (X marks), there can be ischemia of the proximal urethra after excision/primary anastomosis (hatch marks)Source: Brandes SB. Urethral Reconstructive Surgery. Totowa, NJ: Humana Press; 2008.
Posterior Urethroplasty
Posterior urethroplasty is usually required after posterior urethral distraction defects from pelvic trauma. The pelvic trauma can cause concomitant vascular injury, of which bilateral internal pudendal arterial injury or iatrogenic thrombosis is of particular importance to the reconstructive urologist. To ensure that patients scheduled for posterior urethroplasty have a good outcome without excessive risk of ischemia-related restricture, selected patients require a vascular work-up. Jordan et al. noted that children, the elderly, patients with bilateral internal pudendal injury, failed prior urethroplasty, decreased erections, or a cold or insensate glans, were at increased risk for posterior urethral ischemia and stenosis. Patients with risk factors are studied as per Flow chart 1.1, and revascularized 3–6 months prior to urethroplasty, if necessary.
Postprostatectomy Stricture
Patients that are postprostatectomy and have both a urethral stricture and sphincter-deficiency incontinence can also be a challenge regarding the maintenance of adequate urethral blood flow. When stress incontinence is treated with artificial urinary sphincter after anastomotic urethroplasty, the arterial inflow can be compromised by the circumferential compression from the cuff, leading to cuff erosion. It is recommended that the bulbar arteries should be maintained here for anastomotic urethroplasty in order to help the blood supply proximal to the cuff, which might otherwise be dependent on the retrograde blood flow from the distal urethra.
Venous Anatomy
The corpus spongiosum has a venous drainage pattern that is essentially the same as that of the glans penis and other deep structures. This is usually through the periurethral veins, circumflex veins emptying into the paired para-arterial veins that run with the dorsal arteries, and the deep and superficial dorsal veins. The corpora cavernosa are drained by emissary veins which feed cavernosal veins and travel to base of the penis to drain independently into the dorsal vein complex. There are free communications between all of the veins, which eventually end in the superficial and deep dorsal median veins. The deep dorsal median vein is deep to Buck's fascia and ultimately drains through the dorsal vein complex. There may be multiple superficial dorsal median veins, and they are superficial to Buck's fascia and drain to the right or left superficial external pudendal vein (Figs 1.6 and 1.15).10
Flow chart 1.1: Evaluation of arterial inflow prior to posterior urethroplastySource: Jordan GH. Reconstruction for urethral stricture. Atlas Urol Clin North Am. 1997;5(1).
Urethral Elasticity
Excision and primary anastomosis (EPA) relies upon the natural elasticity of the corpus spongiosum and urethra to allow bridging of the area excised with a tension-free anastomosis of normal urethra.
Figs 1.15A and B: Deep venous anatomy of the penisSource: Hsu GL, Hsieh CH, Wen HS, et al. Penile venous anatomy: An additional description and its clinical implication. J Androl. 2003;24(6):921–7.
Sampaio et al. demonstrated the expected extensibility of the urethra and penis in fresh cadavers. There were two main findings:
Fig. 1.16: Extensibility by age. Top line is urethral extensibility and bottom line is penile extensibility. Age is on the x-axis and shows decreased extensibility with increasing ageSource: Da Silva EA, Sampaio FJ. Urethral extensibility applied to reconstructive surgery. J Urol. 2002;167(5):2042–5.
- The urethral extensibility of the urethra exceeds that of the penis (Fig. 1.16).
- The urethral and penile extensibility decrease with age (Table 1.1). Based upon these findings, the estimated amount of urethral mobilization, needed for EPA, can be determined (on average about 1:4, but varies with age as per Figure 1.16.11 Knowledge of these principles can lead to decreased complications such as chordee, ischemia-related restenosis, dehiscence and meatal retraction.
Figs 1.17A to D: Anatomic location of urethra within the corpus spongiosum by cross-section. (A) The bulbous urethra; (B) Penile shaft; (C) Coronal margin; (D) GlansSource: Jordan GH, Virasoro R, Eltahawy EA. Reconstruction and management of posterior urethral and straddle injuries of the urethra. Urol Clin North Am. 2006;33:97–109.
Urethral Location within Corpus Spongiosum
In the penis, the urethra is very central, so there is generally insufficient spongiosum ventrally to perform an adequate spongioplasty for a ventral graft (buccal or skin). For this reason, penile grafts are placed within the split urethral plate (as in the Asopa technique) or placed dorsally, quilted to the corpora cavernosa. Moving proximally, the spongy tissue is thicker and the urethra is located more eccentric and dorsal (Figs 1.17A to D).12 Thus, the bulb makes an excellent location for a ventral onlay graft with spongioplasty, while the penis does not. The disadvantage of making a ventral urethrotomy for graft placement is that the spongiosum is very thick here and can bleed profusely. For this reason, we prefer a dorsal urethrotomy and dorsal graft placement, in order to avoid brisk bleeding.
REFERENCES
- McAninch JW, Morey AF. Penile circular fasciocutaneous skin flap in 1-stage reconstruction of complex anterior urethral strictures. J Urol. 1998;159(4):1209–13.
- Quartey JK. Microcirculation of the penile and scrotal skin. Atlas Urol Clin North Am. 1997;5:23.
- Jordan GH, Stack RS. General concepts concerning the use of genital skin islands for anterior urethral reconstruction. Atlas Urol Clin North Am. 1997;5:23–44.
- Carrera A, Gil-Vernet A, Forcada P, et al. Arteries of the scrotum: a microvascular study and its application to urethral reconstruction with scrotal flaps. BJU Int. 2008;103:820–4.
- Brandes SB. Urethral Reconstructive Surgery. Humana Press; Totowa, NJ: 2008.
- Chiou RK, Donovan JM, Anderson JC, et al. Color Doppler ultrasound assessment of urethral artery location: potential implication for technique of visual internal urethrotomy. J Urol. 1998;159(3):796–9.
- Yu G, Miller HC. Critical operative maneuvers in urologic surgery. Mosby; St Louis: 1996.
- Complications of interventional techniques for urethral stricture. In: Carson CC (Ed). Complications of Interventional Techniques. Igaku-Shoim; New York: 1996.p.89.
- Hsu GL, Hsieh CH, Wen HS, et al. Penile venous anatomy: an additional description and its clinical implication. J Androl. 2003;24(6):921–7.
- Da Silva EA, Sampaio FJ. Urethral extensibility applied to reconstructive surgery. J Urol. 2002;167(5):2042–5.
- Jordan GH, Virasoro R, Eltahawy EA. Reconstruction and management of posterior urethral and straddle injuries of the urethra. Urol Clin North Am. 2006;33:97–109.