Chapter Contents
- Eukaryotic versus Prokaryotic Cells
- Cell Membrane
- Cytoplasmic Organelles
- Nucleus
- Deoxyribonucleic Acid
INTRODUCTION
Cell is the structural and functional unit of life. Human body is made of various types of cells. The fundamental characteristics of these cells are essentially the same. However, during the process of differentiation, the cell acquires many unique morphological and functional properties.
EUKARYOTIC VERSUS PROKARYOTIC CELLS
Cells are mainly classified as prokaryotic or eukaryotic cells. The cells of bacterial and other lower organisms are known as prokaryotic cell. In a prokaryotic cell, deoxyribonucleic acid (DNA) is present within the cytoplasm without any distinct nucleus. The higher animals are made of eukaryotic cells. In eukaryotic cells, DNA is enclosed within the nucleus. In addition, the cell contains mitochondria (MT) and other membrane bound vesicles (Table 1.1).
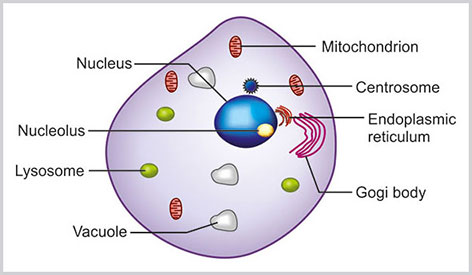
Unlike histology, in cytological examination detailed cell-to-cell relation is often lost. Cytologists study cluster of cells or a single cell for diagnosis. It is essential to know the detailed morphology and function of the cell to understand the alteration of its constituents in reaction to various external and internal stimuli. The various morphological constituents of a cell are highlighted in Box 1.1 and demonstrated in Figures 1.1 to 1.3. The co nstituents of a cell can be divided into cytoplasmic organelles and nucleus.
CELL MEMBRANE
Cell membrane is a selectively permeable biological membrane that separates the interior of a cell from the external environment.
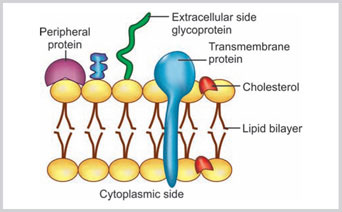
A fluid mosaic model of the cell membrane was first proposed by Singer SJ and Nicolson GL.1 According to this model, the plasma membrane is just like fluid. The membrane proteins are floating on discontinuous fluid like lipid bilayers. The proteins of the membrane are a set of heterogeneous globular molecules. The highly polar groups are protruding out of the membrane and the nonpolar groups are within the interior portion of the phospholipid membrane. The membrane is described as “mosaic” because it is composed of different types of molecules, such as phospholipids, glycolipids, cholesterol, and proteins (Fig. 1.4).2
Composition and Structure
The cell membrane is composed predominantly of bilayered phospholipid molecules, proteins, and carbohydrates (Box 1.2).
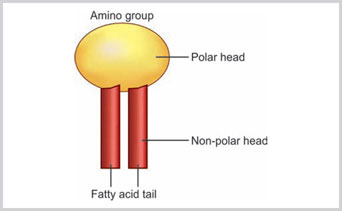
- Lipids: There are three classes of lipids: (1) phospholipid, (2) cholesterol and (3) glycolipid. The phospholipids are the predominant type of lipids noted in the cell membrane. There are four varieties of phospholipids: (1) phosphatidylcholine, (2) phosphatidylethanolamine, (3) phosphatidylserine and (4) sphingomyelin. Phospholipids have the hydrophilic or polar ends and hydrophobic or nonpolar ends. In the hydrophilic ends, usually the glycerol molecules combine with serine, choline, or ethanolamine, whereas, in the hydrophobic ends, the glycerol molecule is attached with the long chain fatty acids.Hydrophobic ends of the molecules are facing each other and they are away from the cytosol or external environment. Whereas, the hydrophilic ends are facing toward the cytosol (Fig. 1.5). At low temperature, the bilayered lipid is just like gel; however, at body temperature, the lipid bilayer is fluid and moving and can exchange their places.Good amount of cholesterol molecules are also present in the plasma membrane, and one cholesterol molecule is present for one phospholipid molecule. The cholesterol molecules are embedded within the phospholipid layers.
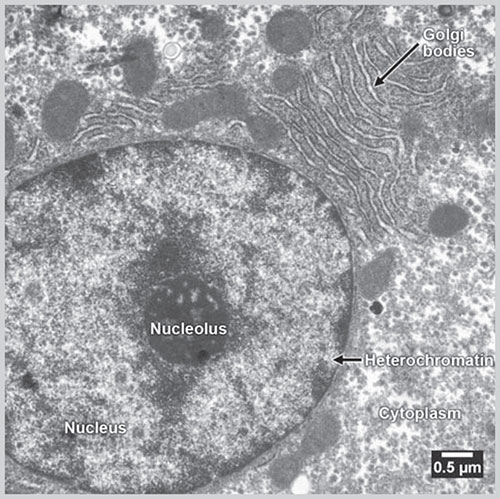
Fig. 1.2: Electron microscopic picture of a cell(Courtesy: Charan Singh Rayat, Department of Histopathology, PGIMER, Chandigarh, India)
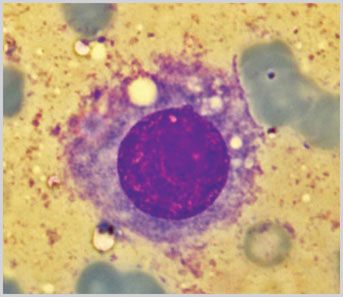
Fig 1.3: (May Grunwald Giemsa stain x1000)
Photomicrograph of a cell with central nucleus having a prominent nucleoli and moderate amount of cytoplasm
Fig 1.5:
Hydrophilic amino acid tail and hydrophobic fatty acid tail of phospholipid molecule in the lipid bilayered cell membrane
They prevent the mobility of the first few hydrocarbon molecules of the phospholipid and also prevent the crystallization of the hydrocarbon. Thus, cholesterol maintains the fluidity and stability of the membrane.
- Carbohydrate: They are present in the form of glycoproteins and glycolipids. Glycoprotein is the predominant type of carbohydrate and is generally noted on both sides of the membrane. It is involved in cell recognition and protection of the membrane.
- Proteins: Proteins constitute 50% of the membrane. Depending on their positions in the membrane, they may be labeled as: (1) integral protein, (2) transmembrane protein and (3) peripheral membrane protein. Integral proteins are incorporated within the membrane (Fig. 1.4). Integral proteins are incorporated within the membrane (Fig. 1.4). Transmembrane proteins are the type of integral proteins that traverse through the complete breadth of the membrane. Peripheral membrane proteins are present on the inner or outer surface of the membrane.
Functions
The plasma membrane is biologically active semipermeable membrane with many important functions (Box 1.3).
- Cell identity: Plasma membrane encircles the essential component of the cell and acts as a physical barrier between the cell and its surroundings.
- Transport: Plasma membrane is selectively permeable to various substances. As the membrane is hydrophobic in its interior, so it is impermeable to most polar molecules such as Na+, H+ and Cl-. However, the lipid bilayer is permeable to small nonpolar molecules, such as CO2 and O2. There are two types of transport that occur through the membrane:
- Active transport: Energy is needed for this type of transport.
- Passive transport: No additional energy is needed for this type of transport. This can be channel or transporter protein mediated, facilitated diffusion, or by osmosis. In osmosis, passive transport occurs across the concentration gradient. In case of facilitated diffusion, two types of protein take part in action:
- Transporter proteins: This type of protein alters the conformation of the solutes to be transported and sequentially transports them through the lipid bilayer.
- Channel protein: This protein forms an aqueous pore across the membrane.
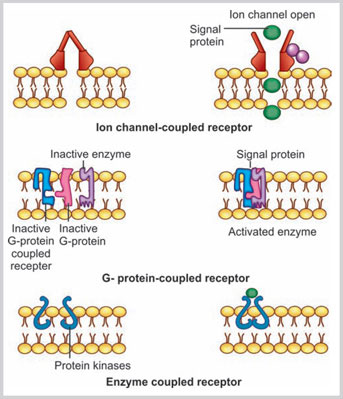
- Signal transduction: Plasma membrane contains many membrane-bound receptors. These receptors bind with signaling molecules and transport the information via the intracellular signaling proteins. The signaling molecules may be soluble, attached to the other cell or may be bound to the extracellular matrix. The three major classes of plasma membrane receptor proteins are involved in signal induction (Fig. 1.6):
- Ion channel-coupled receptors: These receptor proteins are involved in transient opening and closing of the ion channel after binding with the signaling molecules.
- G protein-coupled receptors (GPCR):3,4 These GPCRs mediate their actions by transiently binding with a trimeric Guanosine triphosphate (GTP)-binding protein that is also known as G protein (Fig. 1.6). The binding of GPCRs and G protein further activates an enzyme or changes the ion permeability of the plasma membrane. The receptor site of GPCR is located toward the extracellular space and the other long chain portion coils the plasma membrane several times.
- Enzyme-coupled receptors: These receptor proteins are predominantly protein kinases. In their activated form, they phosphorylate the specific types of proteins.
- Cell polarity (Box 1.4): Most of the cells in human body are polarized. Cell polarization is studied in the epithelial cell. The epithelial cells have distinct polar distribution, such as luminal surface and basolateral surface facing toward the basement membrane and side of the cell. Various membrane protein complexes are responsible for the polarity of the epithelial cells. Three types of polarity complex proteins are described in the membrane of the epithelial cells: (1) PAR (CDC42–PAR3–PAR6–aPKC), (2) crumbs (Crb–PALS–PATJ), and (3) scribbles (Scrib–Dlg–Lgl). PAR and crumb complexes are involved in the apical polarization and scribble complexes are responsible for basolateral polarization of the epithelial cells. They are also involved in the asymmetric cell division, cell proliferation and cell migration.5 Asymmetric cell division suppresses cell proliferation.6 Disruption of the polarity complexes are related with cell proliferation.7It is noted that the loss of epithelial cell polarity complexes is related with tumor progression and invasion.8 So in fact, the membrane polarity complexes behave as tumor suppressor elements.
Epithelial mesenchymal transition and cell polarity (Box 1.5): Epithelial mesenchymal transition (EMT) is an orchestrated series of events in which the epithelial cells loose many of their characteristics and gain the many typical properties of the mesenchymal cells. It is one of the key steps during embryonic development, chronic degenerative fibrosis and cancer metastasis.9 During the process of EMT, there is loss of polarity of the epithelial cells and the separation of the individual cells that help in gaining of cell motility followed by subsequent dispersion of the cells.10
Evidences suggest that the loss of cell polarity proteins is important in EMT.11 Decreased expression of cell polarity proteins weakens the apical junctional complexes and therefore induces EMT.12 E-cadherin is a transmembrane protein that regulates the establishment of the adherens junctions. The intracellular domain of the E-cadherin molecules binds cytosolic catenin and makes a link with actin cytoskeleton. In contrast to epithelial cells, mesenchymal cells do not have any stable intercellular junctions. The mesenchymal cells possess an elongated morphology with front-back symmetry. This facilitates motility and locomotion. Similarly, the loss of E-cadherin in epithelial cells enables them to detach easily and facilitates the dispersion of carcinoma cells from the primary site. In EMT, the transformed epithelial cells show increased expression of N-cadherin, vimentin, catenin, and matrix metalloproteinase.13,14
- Cell-cell recognition: Glycolipids and glycoproteins are responsible for mutual cell-cell recognition.
- Intercellular joining: One of the important functions of the plasma membrane is connection between two cells. This is discussed below.
- Attachment to the cytoskeleton and extracellular matrix.
Cilia and Flagella
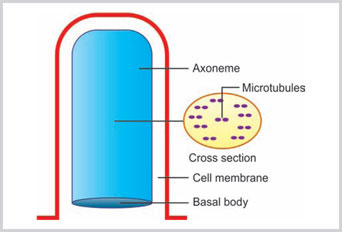
Cilia and flagella are the mobile extension from the surface of the cytoplasm (Box 1.6). Cilia are small, regular and multiple in number, whereas, flagellum is a single slender structure. Cilia are seen on the lining epithelium of the upper respiratory tract and fallopian tube. Each cilium is attached with the thick terminal plate near the apical surface of the cell. The ciliated cells are usually polar and are attached with the basement membrane. The central elongated portion of the cilium is known as axoneme. At the base of the cilium or flagellum is a basal body. The basal body is composed of microtubules. There are total 11 microtubules. In the center, there are two singlet microtubules surrounded by nine triplet microtubules. The shaft of the axoneme consists of nine peripheral doublet microtubules and two central singlet microtubules (Fig. 1.7).
Each of the outer peripheral doublet microtubules in the axoneme has a pair of dynein arms that are extended to the adjacent microtubules. These dynein arms help in the movement of the cilium and flagellum. Cilia are usually lost in the cancer cell originated from the bronchial epithelium.
Fig 1.7:
The structure of cilia is shown. The long shaft of axoneme is originated from the basal body. The cross section of axoneme shows nine doublet microtubules and two central singlet tubules
Therefore, the presence of cilia on the cell almost safely excludes the possibility of malignancy.
Function
Cilia help in the movement of the particles or organism in one direction.
Brush Border
The surface of certain specialized epithelial cells covered with multiple microvilli is known as brush border (Box 1.6). They are regular finger-like projections on the cell surface about 1 micron in length.
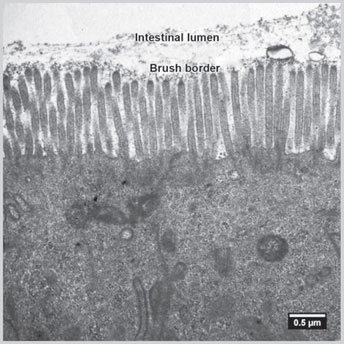
Fig 1.8: Electron microscopic picture of brush border of intestinal cell(Courtesy: Charan Singh Rayat, Department of Histopathology, PGIMER, Chandigarh, India)
Microvilli are commonly seen on the luminal surface of the intestinal epithelium (Fig. 1.8) and also on the proximal tubular epithelial cells of kidney. On light microscopy, the microvilli are seen as fuzzy appearance.
Function
The brush border increases the surface area of the cell and helps in better absorption of the substances from the large surface area.
Cell Junction
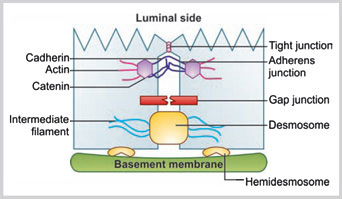
Cell junction can be classified depending on localization of the junction as follows (Fig. 1.9):
- Cell-to-cell
- Tight junction
- Adherens junction
- Desmosomes
- Gap junction
- Cell-to-matrix
- Hemidesmosomes.
Tight Junction
This is located in the apical region of the epithelial cells and almost completely seals the gap between the two epithelial cells toward the luminal site. The sealing strands of transmembrane adhesion proteins encircle the apical portion of the plasma membranes of the two cells and hold the membrane tightly. Claudins and occludins are two major transmembrane adhesion proteins.
Fig 1.9:
Various types of cell junctions are shown such as tight junction, adherens junction, gap junction, desmosome, and hemidesmosome
Function
There are two major functions of the tight junctions:
- Tight junction closes the gap between luminal side and the intercellular space. This helps in effective transport of substances from luminal side of the cell to the extracellular fluid compartment.
- Tight junction acts as a barrier and prevents the drift of the apical membrane proteins to the basal region and vice versa.
Adherens Junction
The adherens junction holds the two cells together and confers mechanical strength. Adherens junction is made of cadherin, catenin, and intracytoplasmic actin filaments. Altogether they form adhesion belt-like structure.
Function
To provide cell-to-cell adhesion and mechanical strength.
Desmosomes
These are button-like spots which connect the plasma membrane of two cells together. Desmosomes are linked to the intermediate filaments. The type of intermediate filament depends on the type of cell.
Function
The desmosomal junction provides tensile strength and rigidity of the tissue.
Gap Junctions
These are intercellular channels that connect two adjacent cells. In gap junctions, the two plasma membranes are connected by the transmembrane proteins known as connexins.
Function
There are continuous channels between the two adjacent cells and therefore the cells can rapidly share small molecules and ions. 9With the help of the gap junctions the action potential can rapidly travel among a group of cells without any neurotransmitter.
Hemidesmosomes
Hemidesmosomes connect the cell with the basal lamina. Hemidesmosomes are composed of keratin filaments, dystonin, plectin, integrin, collagen XVII, and laminin. In hemidesmosome, integrin binds with keratin in cytoplasmic side by dystonin and plectin. It also binds with collagen XVII and laminin toward basal lamina side.
Function
It attaches cell with the basal lamina.
CYTOPLASMIC ORGANELLES
Endoplasmic Reticulum
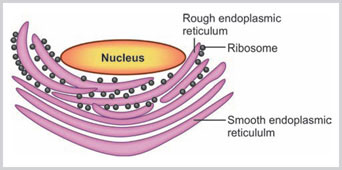
Endoplasmic reticulum (ER) is a tubular and cistern-like space and vesicular structure folded within the cytoplasm. An ER generally connected with cell membrane to nuclear membrane (Box 1.7). The cisterns are membrane-like long flat spaces which are straight, whereas tubules are irregularly branched structures. The ER contains fluid with many enzymes and proteins. There are two types of endoplasmic reticulum: (1) rough endoplasmic reticulum (RER) and (2) smooth endoplasmic reticulum (SER). The membrane of the RER is continuous with the membrane of SER (Figs 1.10 and 1.11).15,16
Rough Endoplasmic Reticulum
They are tightly packed parallel bundles of cistern-like spaces which are beaded in appearance due to ribosome particles attached to the surface of RER. The ribosomes are bound with RER by a receptor known as ribophorin.
Function
It is the site of synthesis of secretory protein synthesis and lysosomal enzymes.
Smooth Endoplasmic Reticulum
The SER predominantly contains tubules and vesicles. SER is connected with Golgi apparatus and plasma membrane.
Function
Synthesis of lipids.
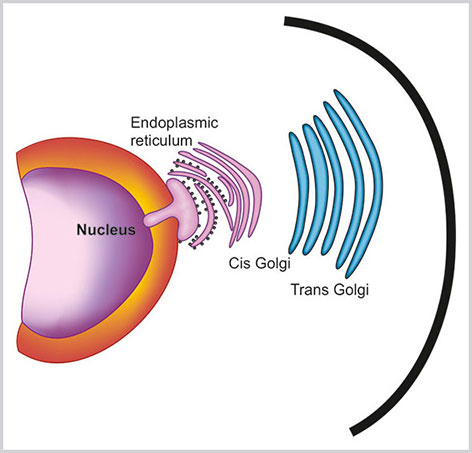
Golgi Complex
Golgi complex (GC) are stacks of membrane-bound cistern-like spaces within the cytoplasm arranged in a polarized fashion. Each stack of GC has four parts (Box 1.8, Figs 1.2 and 1.12):
Fig 1.10:
Rough endoplasmic reticulum is studded with ribosomes. The membrane of rough endoplasmic reticulum is continuous with outer layer of nuclear membrane
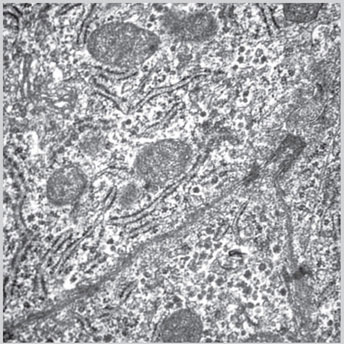
Fig 1.11: Electron microscopic picture of rough endoplasmic reticulum(Courtesy: Uma Nahar Saikia, Additional Professor, Department of Histopathology, PGIMER, Chandigarh, India)
Fig 1.12:
Golgi complex has cis, trans, endo and medial parts. Cis Golgi faces towards the nucleus and trans Golgi towards the cell membrane
- Cis Golgi network: Cis Golgi network is the concave surface of the stack of GC that faces towards the RER and small transfer vesicles. Cis Golgi network receives the initial protein from ER.
- Endo Golgi and medial Golgi: These are the middle parts of GC and most of the proteins are modified here.
- Trans-Golgi network: This is the convex surface of the stack of the GC. Trans-Golgi network is associated with large secretory vesicles and final transport of the protein.
Function
- The main function of the GC is chemical processing of the protein received from RER followed by packaging and transfer.
- Along with classical “protein trafficking”, there are many other novel functions of GC such as entry of the cell to mitotic check point, calcium homeostasis and cytoskeletal organization.17
- Protein modification: N-linked and O-linked glycosylation of protein and lipids occur in the GC.18
- Protein transport: GC receives the neosynthesized protein from the ER and transport to their respective destination. The cargo proteins are first modified and then they are transported by GC. The mechanism of transport of cargo proteins is not exactly known. However, there are two theories:
- Vesicular transport model theory: The cargo protein is transported by an anterograde way with the help of vesicles that bud from one cisterna and then fuse to the next one.
- Cisterna maturation model: In this model, it is assumed that the Golgi cisterns are formed de novo, progressively mature and finally dissipate.19
- Calcium storage: GC is the most important site of intracellular calcium storage and can also release Ca2+ in case of agonistic stimulation.20
- Platform of different cells signaling: GC acts as a platform of different signaling events within the cell. In addition to receiving initial signal, GC can also induce a cascade of signal transduction.
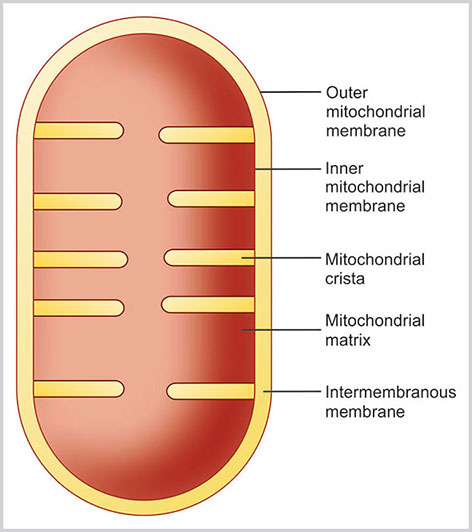
Mitochondria
These are 0.5–1 micron diameter organelle considered as the power house of the cell, as they are the major source of energy (Box 1.9). Mitochondria are richly localized in that part of the cell which requires energy, such as interfibrillar space in the striated muscle and in the middle part of the sperm.
Other than nuclear DNA, MT has its own independent DNA and this is the unique feature of MT.
Structure
Mitochondria are double membrane-bound structures, which consist of following parts (Figs 1.13 and 1.14):
- Outer membrane
- Inner membrane
- Intermembranous space
- Cristae
- Mitochondrial matrix.
- Outer membrane: This is composed of phospholipid bilayers. The outer membrane of MT is rich in porin, a variety of integral protein. This forms a through and through aqueous channel in the outer membrane. Therefore, the small ions and proteins can pass through the outer membrane easily. The outer membrane is also connected with ER by mitochondria- associated endoplasmic reticulum.
- Inner membrane: This is the inner phosphopolipid bilayer of the MT. The inner membrane is rich in double phospholipid known as cardiolipin. The cardiolipin possesses four fatty acids rather than two, and the presence of cardiolipin makes the inner membrane impermeable to proton, ion, and electrons. In certain regions, the outer and inner membrane joins together known as contact sites and makes a passage of the proteins and small molecules from the cytoplasm to the matrix space. The inner membrane contains the large number of lollipop-like structure with small stalk attached to the inner membrane and globular region in the matrix.These globular regions contain protein complex of ATP (Adenosine triphosphate) synthase. The inner membrane contains three types of enzymes: (1) ATP synthase (2) the respiratory chain protein complexes such as NADH dehydrogenase complexes, Cytochrome b-c1 and Cytochrome oxidase, and (3) transport protein complexes.
- Intermembranous space: This is the minute space between the inner and outer membrane of the MT. The concentration of small molecules is same in both cytoplasm and intermembranous space.
- Cristae: These are the small shelf-like folds of the inner membrane. This makes the larger space in the inner membrane to retain more enzymes.
- Matrix: It is the innermost space of MT encircled by the inner membrane. The matrix consists of dense fluid that is rich in viscosity. Matrix is rich in enzymes of citric acid cycle and also contains mitochondrial DNA. Mitochondrial DNA is unique to MT, and it is considered as separately developed during evolution. In sexual reproduction, MT is exclusively inherited from mother and so MT DNA is of maternal origin. MT DNA is circular DNA. This is responsible for the formation of selected MT protein, ribosomal ribonucleic acid (rRNA), and transfer RNA (tRNA). Replication of mitochondrial DNA is not limited to the S phase of the cell cycle, and this may occur in any phase of the cell cycle.
Function
Main function of MT is energy production in the form of ATP synthesis. However, it is also involved in other important functions, such as calcium storage, cell death, etc.21
- Citric acid cycle: The essential enzymes in citric acid cycles are located in the mitochondrial matrix, and the main reactions of citric acid cycle happen in the mitochondrial matrix. Initial oxidative breakdown of glucose occurs in the cytoplasm by the process known as glycolysis. The glucose is converted to pyruvate, which is transported to the mitochondrial matrix. This is further oxidized to acetyl coenzyme A (CoA). Fatty acid also enters into the mitochondrial matrix and is oxidized to acetyl CoA. Glucose to acetyl CoA is the basic ingredient of citric acid cycle. The main end-products of citric acid cycle are CO2, FADH2, and NADH. FADH2 and NADH further help in the production of ATP.
- Electron transport: During the oxidative phosphorylation, a series of electron transport reactions occur in the inner mitochondrial membrane. Electrons from NADH enter to flavin mononucleotide and then through a series of complex proteins to molecular oxygen. The energy released in these reactions is used to generate ATP from ADP.
- Calcium storage: Mitochondria can store calcium and play an important role in calcium homeostasis.22
- Cell cycle: Mitochondria play as a signaling platform for cell cycle progression. AMP-activated protein kinase is activated in the MT which helps in phosphorylation of serine 15 of p53 protein. This prevents the degradation of this protein p53 and, which subsequently helps in cell cycle arrest in DNA damage.23
- Apoptotic death: Mitochondria play a key role in apoptosis. During the process of apoptosis MT releases cytochrome c, which activates Apaf-1 and ultimately caspase 9 is activated. Activated Caspase 9 breaks DNA in small pieces causing apoptosis. Release of Cytochrome c from the MT membrane is induced by pro-apoptotic BCl-2 family members.24
Ribosome
Ribosome is the site of protein synthesis (Box 1.10).
Structure
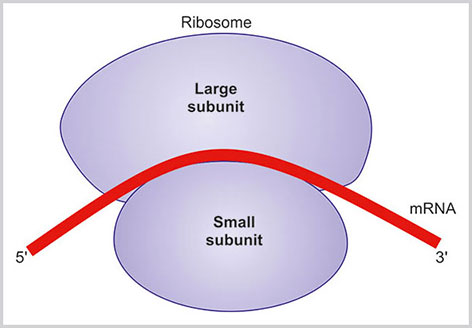
Ribosomes are small 25–30 nm particles present in the cytoplasm. They are present both as free ribosomes in cytosol and also in the membrane-bound form attached with ER and thus forming RER.
The ribosome is made up of rRNA and proteins. The ribosome is classified according to the sedimentation coefficient in ultracentrifugation. The eukaryotic ribosome has two units, a smaller 40S and a larger 60S subunit. In its inactivated form, the two subunits are detached; however, when the ribosome is engaged in protein synthesis both the units are attached together. Small subunit has the binding site for mRNA and tRNA. Some rRNA of the larger subunit has enzymatic activity to catalyze the peptide bond. These rRNA are known as the ribozyme (Fig. 1.15).
Function
Ribosomes play a vital role in protein synthesis by decoding information from mRNA and then help molecules of tRNA to assemble particular amino acids to make a protein.
Lysosomes
These are 0.2–0.4 micron small membrane-bound vesicles present in the cytoplasm (Box 1.11). They contain near about 40 acid hydrolytic enzymes. Lysosomal enzymes are synthesized in the ER and transported in GC. The lysosomal enzymes and membrane of the lysosome are finally synthesized in the trans- Golgi network and are carried to the endosome by clathrin-coated transport vesicles. The final lysosomal vesicles are synthesized in the late endosomal intermediate (also known as endolysosome).
There are two types of lysosomes: (1) primary lysosome (no morphological sign of hydrolytic enzymes) and (2) secondary lysosomes (when lysosome fuses with other phagocytic vesicles of an organism, it shows enzymatic activities, and this lysosome is known as secondary lysosomes).
Function
Lysosome contains acid hydrolytic enzymes such as lipase, amylase, protease and nuclease. These enzymes are activated in the acid environment.
The lysosomal enzymes digest the macromolecules, destroy the microbes and remove the other cytoplasmic organelle such as mitochondria. The foreign organisms enter the cytoplasm as phagocytic vesicles. Lysosome fuses with the phagosome and releases acid hydrolytic enzymes, which degrade the protein and carbohydrate components of the organism. The lipid component is more resistant to digestion and may remain as the residual body. At times, lysosome fuses with the nonfunctioning MT or fragments of RER to clear these substances from the autophagic vacuoles in the cytoplasm. When these autophagic vacuoles remain persistently in the cytoplasm, they accumulate pigment known as lipofuscin.
Peroxisome
These are tiny vesicles of 0.2–1 micron in size. They are synthesized from RER. Peroxisomes contain many oxidative enzymes. The enzymes in the peroxisome break down fatty acid by beta oxidation and generate acetyl CoA and H2O2. Acetyl CoA is involved in various energy producing metabolic processes. The hydrogen peroxide helps kill the various organisms. Excess hydrogen peroxide is further degraded by catalase enzyme of peroxisome into water and oxygen.
Cytoskeleton
Cytoskeleton is the meshwork of protein filaments within the cytoplasm of the cell that maintains the shape of the cell along with other important functions, such as cell movement, cell contraction and maintaining cell polarity (Box 1.12). There are three components of the cytoskeleton: (1) microfilament, (2) microtubules and (3) intermediate filaments.
Microfilament
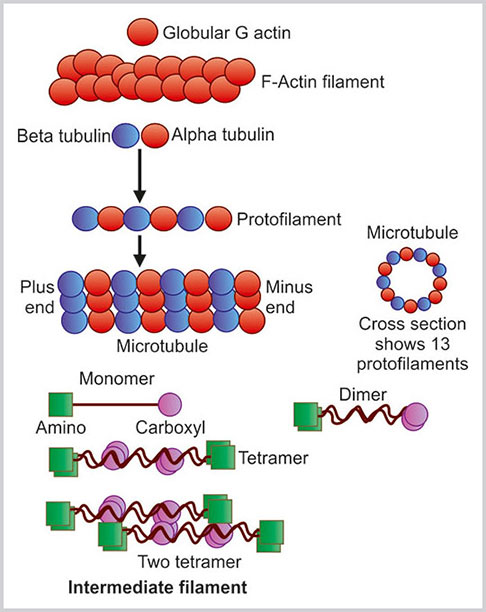
It is also known as actin filament. These are the thinnest filaments and present either as a bundle or as fine network within the cytoplasm. The actin filament is composed of globular G-actin which is polymerized and forms a long chain of F-actin (Fig. 1.16). Most of the G-actin is bound with small proteins such as profilin and thymosin.
Binding of these small proteins prevents the polymerization of G-actin. Actin filament binds with filamin and makes a robust supporting meshwork just underneath the plasma membrane known as cell cortex. There are three types of actin: (1) alpha actin, (2) beta actin and (3) gamma actin. Alpha actin is present in muscle, and other two forms of actin are present in nonmuscular cells.
Functions
- Actin maintains shape of the cell
- Actin binds to the myosin and helps in contraction of muscle fibers
- Actin can shorten its length and helps in the movement of the cell
- Phagocytosis or pinocytosis is helped by actin
- Actin helps in the transport of various vesicles within the cytoplasm.
Fig. 1.16:
Three types of cytoskeletal structures: actin filament, microtubules and intermediate filament
Microtubules
Microtubules are long, straight, hollow rigid tubules of 25 nm diameter. These are dynamic fibers, as they are always in the process of assembling and disassembling. The microtubules constitute mitotic spindles, centrioles, cilia and flagella. The basic constituents of the microtubules are alpha (α) and beta (β) tubulin. These tubulins are arranged alternatively to form a protofilament (Fig. 1.16). GTP is tightly bound with α tubulin and resistant to hydrolysis whereas it is loosely bound with β tubulin and can be separated by hydrolysis. The protofilament of tubulin is polar as one end is formed by β tubulin and other end is formed by α tubulin. The β end of the tubulin protofilament is plus end as the growth and shrinkage of this end is rapid. The opposite α tubulin end is known as the minus end. In each microfilament, there are a total of 13 protofilaments. These protofilaments are parallel in position. All the plus ends or growing ends of the protofilaments are in one direction.
Functions
The main functions of the microtubule are:
- Intracellular transport: Microtubules help in the transport of vesicles containing proteins from the GC to plasma membrane.
- Mitotic spindle movement: The mitotic spindles are formed by microtubules. The chromatids are separated and pulled to each daughter cell nucleus by the mitotic spindles formed by microtubules.
- Movements: Movements of cilia and flagella are done by the microtubules.
Centrosome
It is small round body located near the nucleus in the interphase cell. This is also known as microtubule organizing center. The microtubules are attached with the centrosome by their minus ends and they radiate from the centrosome in a star-shaped manner. Centrosome consists of a pair of centrioles arranged in L-shaped manner surrounded by the amorphous matrix material known as centrosome matrix or pericentriolar material. Centrosome matrix material takes the main role in the development of the microtubule. Centrioles are the basal bodies of cilia or flagella. During mitosis, the centrosome duplicates and each one contains one pair of centrioles. From each of the centrosomes, microtubules radiate and form a complete mitotic spindle.
Intermediate Filament
Intermediate filaments have an average diameter of 10 nm. The name of the intermediate filaments is such because the diameter of intermediate filaments is in between the microfilaments (7 nm) and microtubules (25 nm). The individual polypeptide of intermediate filaments is an alpha helix with 310–350 amino acids. It has N and C terminals. Two such alpha helix monomers coil with each other to form a dimer. Both the N and C terminals are in same direction in this monomer. Two dimmers then coil in a staggered antiparallel fashion to form a tetramer. Eight such tetramers twist in a rope-like manner to form an intermediate filament. Therefore, in a cross section of intermediate filament, there are 32 alpha helix coils.
Types25
There are a total six types of intermediate filament (Table 1.2):
- Type I and type II: Acidic and neutral basic keratin. Type I keratin is acidic and type II keratin is basic in nature. They include a good number of epithelial and hair keratins.
- Type III: There are four varieties of type III intermediate filaments. They are:
- Vimentin: This is widely expressed in mesenchymal cells and a variety of other cells such as leukocytes, vascular endothelial cells and some epithelial cells.
- Desmin: Desmin is noted in the skeletal and cardiac muscle fibers.
- Glial fibrillary acidic protein (GFAP): This is expressed in astrocytes and other glial cells.
- Peripherin: It is noted in the peripheral neurons, such as neurons of the dorsal root ganglia, sympathetic ganglia and cranial nerves.
- Type IV
- Neurofilaments: Neurofilaments are classified according to their sizes as NF-L (light, 62 kDa), NF-M (medium 102 KDa) and NF-H (heavy 112 kDa). They are expressed in the mature neurons.
- Alpha internexin: These are found in developing central nervous system.
- Type V
- Lamin: They are found in the nucleus of the cell as lamin A, lamin B and lamin C.
Table 1.2 Intermediate filaments TypeVarietiesLocationMolecular weight (Da)FunctionIAcidic keratin (11 epithelial keratin, four hair keratin)Epithelial cells40,000–70,000Tensile strengthIIBasic keratin (8 epithelial keratin and four hair keratin)Cells of Hair and nail40,000–70,000Tensile strengthIIIVimentinMesenchymal cells, leukocytes, vascular endothelial cells and some epithelial cells54,000Support the cytoplasmic membrane and helps in holding the various organelles in proper positionDesminSkeletal and cardiac muscle fibers53,000Helps in stabilizing sarcomeres of the contracting muscle cellsGlial fibrillary acidic proteinAstrocytes and other glial cells50,000Supports the glial cellsPeripherinNeurons of the dorsal root ganglia, sympathetic ganglia and cranial nerves56,000Supports the neuronsIVNeurofilaments (NF) NF—LightMature neurons62,000They form the cytoskeleton of dendrites and axons.NF—Medium102,000NF—High110,000VLamin A, lamin B, and lamin CNuclear envelope65,000–75,000Control of assembly of the nuclear envelope during mitotic event and chromatin organizationVINestinStem cells of the central nervous system and in developing skeletal muscle2,00,000Lamin is noted as a proteinaceous structural meshwork underneath the nuclear membrane and also found within the nucleoplasm. The meshwork of lamin underneath the nuclear membrane acts in chromatin organization and gene expression.
- Type VI
- Nestin: Nestin is expressed in proliferating stem cells of the central nervous system and in developing skeletal muscle.26
- Unclassified
- Filensin: It is expressed during the differentiation of the vertebrate lens epithelial cells.
Functions
- Supporting the cytoskeleton structure: Intermediate filaments are more stable than microtubules and microfilaments and provide good support and tensile strength to the cytoskeleton of the cell. Desmin links myofibrils of the striated muscles. GFAP supports the glial structure, and neurofilaments support the cytoskeleton of the axons and dendrites.
- Chromatin organization: Nuclear lamin plays an important role in chromatin organization of the nucleus. They also play a role in control of assembly of the nuclear envelope during the mitotic event.
NUCLEUS
Nucleus is the central processing unit of the cell and acts as the controlling center of the cell (Box 1.13). The important components of the nucleus are:
- Nuclear envelope and pore
- Nuclear matrix
- Nuclear chromatin
- Nucleoli.
Nuclear Envelope
Nuclear envelope is the barrier which separates the nucleus from the cytoplasm. It is composed of three parts:
- Nuclear membrane
- Nuclear pore
- Nuclear lamina.
Nuclear Membrane
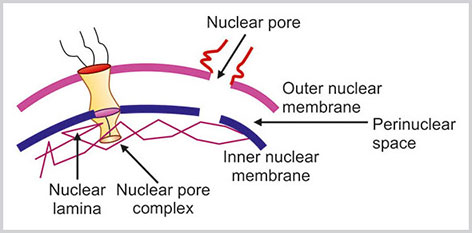
The nuclear membrane is further divided into outer nuclear membrane (ONM), inner nuclear membrane (INM), and perinuclear space (Figs 1.17 and 1.18).
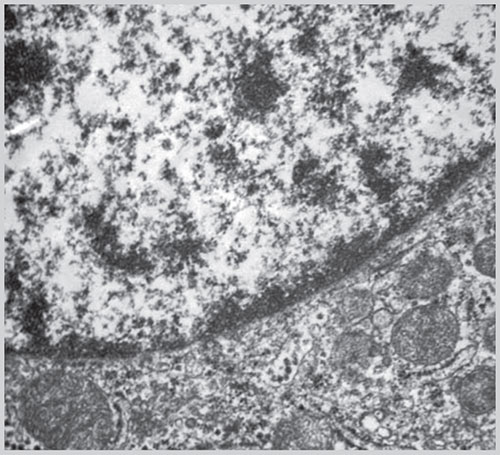
Fig. 1.18: Electron microscopic picture of nucleus and its membrane(Courtesy: Uma Nahar Saikia, Additional Professor, Department of Histopathology, PGIMER, Chandigarh, India)
Outer nuclear membrane is the outermost part of the nuclear membrane and is 6 nm thick. It is continuous with the endoplasmic reticulum. It is usually studded with multiple ribosomes on its cytoplasmic side that are involved in protein synthesis. The INM is parallel to ONM and is directly attached to the nuclear lamina. The space in between ONM and INM is known as perinuclear space. The width of this space is 50 nm. Both the ONM and INM are perforated by multiple nuclear pores.
The nuclear lamina is located in the nuclear side of the INM. It is made up of nuclear lamin that is intimately related to the cytoplasmic intermediate filaments. The lamins are broadly classified as either A or B type lamin depending on their amino acid sequence, behavior at mitosis and tissue specific patterns. Each lamin molecule consists of an N-terminal head, long “coiled-coil” α helical rod and globular C-terminal tail domain.27 The central “coiled-coil” rod domains of two lamins interact to form lamin dimers and the lamin dimers are arranged with each other in an anti-parallel manner. The INM contains a number of integral proteins such as lamin B receptor (LBR), lamina associated polypeptide (LAP), MAN 1, emerin, nurim and ring finger binding protein (RFBP).28
Functions
- Acts as a physical barrier between the cytoplasm and nucleus.
- Various integral proteins such as LBR, LAP, RFBP help in chromatin remodeling and gene expression. LAP and lamin A/C bind with Rb-protein that further recruits histone deacetylases (HDAC), DNA methyl transferases (DNMT 1), histone methyl transferases (HMTase), and heterochromatin protein 1 (HP1). The action of these enzymes changes the higher-order conformation of chromatin and ultimately causes gene silencing by inhibiting transcriptional activation of E2F.29,30
Nuclear Pore
At certain positions, the ONM and INM fuse with each other and therefore, make the hole on the nuclear membrane. The nuclear pore is the direct communication site between the nucleus and cytoplasm. The diameter of each nuclear pore is about 100 nm. The number of nuclear pore varies from few hundreds to thousands depending on the metabolic activity of the cell. The nuclear pore complex (NPC) is the gateway of the nucleus across the double membrane nuclear envelope (Fig. 1.17).31The NPC selectively exchanges the macromolecules between the nucleus and cytoplasm.30
Nuclear pore complex consists of a cytoplasmic ring, a nuclear ring, and a distal ring connected by nuclear basket (Fig. 1.17).32
Function
The main function of the nuclear pore is the facilitation of the cytoplasmic to nuclear traffic and vice versa.
Nuclear Matrix
The nuclear matrix is the internal skeleton of the nucleus and consists of an RNA network, protein complexes, peripheral nuclear lamin, and residual nucleoli.
The composition of the nuclear matrix is dynamic and varies with nuclear activities. The nuclear matrix protein (NMP) is tissue specific and participates in many vital cell functions, such as gene transcription and translation.
Nuclear Chromatin
Chromatin Structure
Chromatin represents the uncoiled chromosome of the interphase nucleus (Box 1.14). It is composed of DNA, histone, and nonhistone proteins.33
In the interphase nucleus, the individual chromosomes occupies specific position of the nucleus which is referred to as chromosomal territories and the chromosomes are separated by channels called interchromosomal domains. Chromatin can be classified as heterochromatin and euchromatin. The heterochromatin is the condensed portion of chromatin where genes are usually inactive. Heterochromatin usually is found on the nuclear membrane (Fig. 1.2) and can be further divided into facultative and constitutive. In case of facultative heterochromatin, the genes are inactive in certain cell types in certain stages of development. The constitutive heterochromatin consists of chromosome structural components such as telomeres and centromeres. Heterochromatin and euchromatin are seen only in ordinary light microscopy of fixed preparation of cells.
Nucleosome—Basic Unit of Chromatin
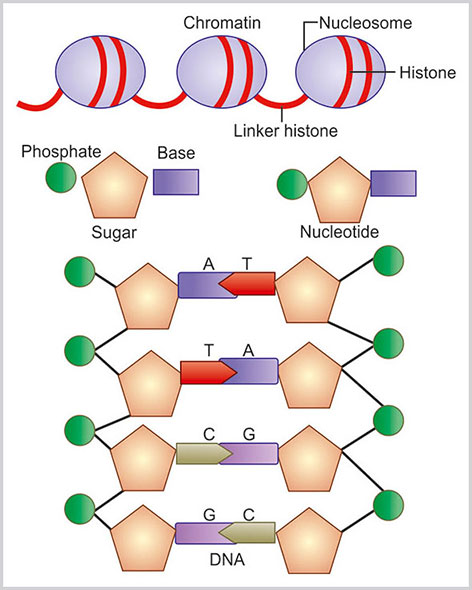
Nucleosome is the basic unit of chromatin. Each nucleosome consists of approximately 146 bp of DNA. This DNA encircles in two turns around a central octameric protein core containing two copies each of histone H2A, H2B, H3 and H4 (Fig. 1.19). The central core histone proteins are arranged as a (H3-H4)2 tetramer and two H2A-H2B dimers located on either side of the tetramer.
Fig. 1.19:
Nuclear chromatin and DNA structure. Double helix DNA structure is made of sugar, phosphate back bone and four bases—adenine (A), guanine (G), cytosine (C) and thymine (T)
Histone H1 is known as the linker histone. This linker histone binds to the DNA joining nucleosomes together and to core histone. The strings of linked nucleosomes are helically twisted into a 10 nm fiber, which, in turn, is folded into a 30 nm fiber and forms the higher order organization of chromatin. The thread of chromatin fiber makes a bend or loop and the DNA at the base of the loop is attached to the NMP. This is known as matrix attachment region (MAR). MAR takes essential role in gene expression.34
Nucleolus
Nucleolus is the subnuclear round to oval small structure within the nucleus and about 1 micron in diameter (Box 1.15). It is not a membrane-bound structure. Nucleolus is usually situated in the center of the nucleus; however, the position of the nucleolus may vary. The number of nucleolus may vary from 1 to 3. The size of the nucleolus depends upon the requirement of ribosome and protein synthesis. So, it is expected that metabolically active cell with higher amount of protein synthesis will have larger nucleoli. Nucleolus is easily detectable by light microscope. In hematoxylin and eosin stained histological section, the nucleoli are stained as deep eosinophilic round structures. In May Grunwald Giemsa stained cytology smears, the nucleoli are stained as light-blue colored structures.
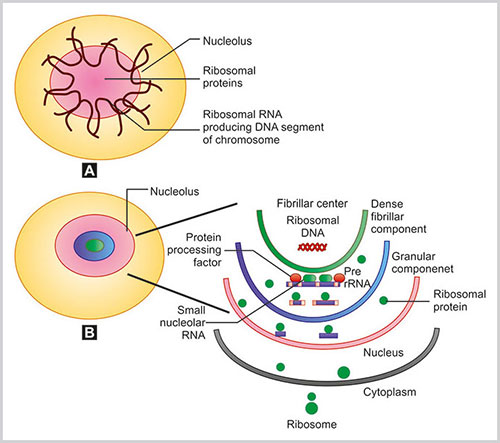
The nucleoli are formed at the end of the mitosis around the tandemly repeated clusters of ribosomal DNA (rDNA) genes (Fig. 1.20A). These specific genetic loci of the origin of nucleoli are known as nucleolar organizing regions (NORs). The nucleolar organizer loci are seen in homologues chromosomes of 13, 14, 15, 21 and 22. Therefore at the end of mitosis, tiny 10 nucleoli appear to from the NOR of the five pair of chromosomes (total 10 chromosomes). These small nucleoli conglomerate to form a single larger nucleolus. The nucleolus contains protein and rRNA. The protein and rRNA are surrounded by chromosomal DNA of the nucleolus.
Structure
At the electron microscopic level, the nucleolus exhibits three major subregions35(Fig. 1.20B).
Figs 1.20A and B:
A) Organization of nucleoli. Five pairs of chromosomal parts that produce ribosomal DNA with ribosomal proteins make nucleoli; B) Nucleolar structure and functions are highlighted
- Fibrillar center (FC): It looks like variable sized round structure with very low electron opacity.
- Dense fibrillar component (DFC): It is located on the outer rim of FC and is composed of densely packed fibrils.
- Granular components (GC): This is the outermost region and is composed of granules.
These different regions of nucleoli probably indicate the stages of RNA transcription and ribosomal assembly.
Function
The nucleolus is the site of rRNA transcription, processing, and ribosomal assembly.
- Ribosomal RNA transcription: Transcriptionally active rRNA genes are located in the FCs and DFCs. The transcription of rRNA genes occurs by RNA polymerase I enzyme. The primary transcript of the ribosomal DNA is the large 45S pre rRNA. This contains the 18S, 5.8S and 28S rRNAs. Subsequently, the preribosomal RNA transcripts are processed in the DFC with the help of small nucleolar RNA and other protein processing factors. A series of cleavages occur during the processing of preribosomal RNA to mature rRNA. In addition, considerable amount of methylation of the bases and ribose residues also happens. The various proteins in the NORs are selectively stained by the silver staining method and these proteins are labeled as AgNOR proteins.36
- Ribosomal synthesis (Fig. 1.20B): Outside the nucleus, the genes of the ribosomal proteins are transcribed and from the cytoplasm, these proteins are transported to the nucleolus. With the help of rRNA these ribosomal proteins are assembled within the nucleolus to form preribosome.
The preribosome is transported back to the cytoplasm for final maturation.
DEOXYRIBONUCLEIC ACID
Deoxyribonucleic acid, the nucleic acid, carries the vital genetic information of the cell (Box 1.16). The portion of DNA that carries the genetic information is known as gene. Within the nucleus, DNA is coiled and supercoiled to make a thread-like structure known as chromosome. During cell division, the chromosomes are easily visible as distinct entity by light microscope. However, in the interphase, the chromosomes remain partly condensed and partly extended. Therefore, they are not visible as a distinct entity. There are 23 pairs of chromosomes, out of which 22 pairs are autosomes and one pair is sex chromosome. Sex chromosomes in the male are X and Y chromosomes, and in the female are X and X chromosomes.
Structure of Deoxyribonucleic Acid
Deoxyribonucleic acid is made of double helical strands containing a sugar phosphate backbone and bases attached with the sugar molecule.37 Each strand of DNA is made up of alternate sugar and phosphate molecules. The sugar molecule is a pentose sugar and it is attached with the phosphate by 3rd and 5th carbon atom, alternatively. The nucleobase is attached with each sugar molecule and then links with the other base of the opposite strand by a weak hydrogen bond. There are four base pairs: adenine (A), cytosine (C), guanine (G) and thymine (T). Adenine and guanine are purine bases. Cytosine and thymine are pyrimidine bases. Adenine only joins with thymine and cytosine only joins with Guanine. This is known as complementary base pairing. There is another pyrimidine base known as uracil (U) that is found in RNA (Fig. 1.19).
Nucleotides
It is the basic structural unit of DNA. It consists of a pentose sugar, phosphate and nucleobase (adenine, cytosine, guanine or thymine). Nucleobase and sugar molecule form a nucleoside.
Gene
Gene is the specific portion of DNA with particular arrangement of nucleobases that carry the genetic information for making a particular protein. It is the sequence of A, T, C and G that determine the genetic information. Triplet of three consecutive bases of DNA is known as codon and each codon is specific for a particular amino acid. This specifies the sequence of amino acids, and subsequently, the protein formation. Only certain parts of the DNA are involved in carrying the genetic information, and the “in between part” of the DNA is commonly known as noncoding DNA or junk DNA. The exact biological function of the noncoding DNA is not known. However, evidences suggest that noncoding DNA interacts with micro-RNA and thereby controls transcriptional and translation of protein coding sequences.38
DNA Replication
Deoxyribonucleic acid replication is a semi-conservative process by which genetic inheritance is maintained. When a cell divides, DNA replication process happens. Here each strand of DNA serves as a template and an identical complementary daughter DNA strand is formed. Each of the newly formed daughter cell contains DNA make up of one original strand and one freshly made strand. Therefore, the DNA double helix replication is a semi-conservative process. This replication process is well controlled and free of mistakes because of stringent proof reading and error checking mechanisms (Fig. 1.21).
Deoxyribonucleic acid replication process needs following enzymes:
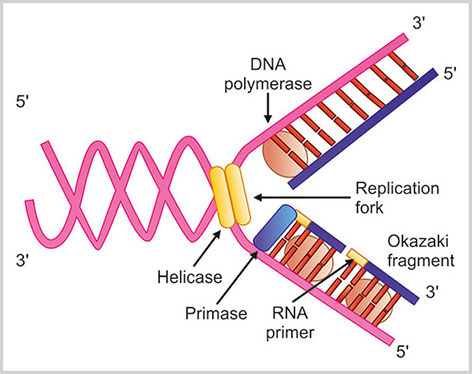
- DNA polymerases: These are the major enzymes in DNA replication process.Fig. 1.21:DNA replication steps are highlighted in this schematic diagram. Helicase enzyme breaks the double stranded DNA and a replication fork is formed. With the help of DNA polymerase enzyme DNA strand is made. In the leading strand DNA is synthesized in continuous manner at the direction of replication fork whereas in lagging strand DNA is synthesized in opposite direction as small segments. These small segments are known as Okazaki fragmentsThese enzymes help in the polymerization of deoxyribonucleotides into the DNA strand. The DNA polymerase enzyme reads the intact template DNA strand to make the complementary DNA strand. On the basis of sequence homology and structural similarities, DNA polymerases are classified into five major families:39 A, B, C, X and Y. The three major varieties of eukaryotic DNA polymerases α, δ and ε belong to family B. The mitochondrial DNA polymerase γ belongs to family A. There are two important and fundamental properties of DNA polymerases:
- They only can add free nucleotides in the 5’ to 3’ direction.
- They need a preformed primer strand which is attached to the template DNA by hydrogen bonding. DNA polymerase adds a deoxyribonucleoside 5’ triphosphate to the 3’ OH group of the primers strand.
DNA replication is the coordinated activities of various enzymatic processes. The basic mechanisms of DNA replication process is evolutionary preserved. DNA replication events are initiated in many hundreds of points in chromosomes. This initiation point of the segment of DNA is known as origin. The protein complex that acts on the origin as initiator of the DNA replication is known as origin recognition complex (ORC). The human ORC is site nonspecific. However, it is suggested that in somatic differentiated cells human ORC binds to genomic DNA with certain specificity.40 DNA is replicated in S phase of the cell cycle. Total time of replication of DNA is usually fixed.
For the purpose of description, the replication process can be divided into series of steps:
- At first two strands of DNA are separated at a particular point known as origin.Here, the initiator protein along with other associated protein forms a prereplication complex that separates the two strands of DNA. Therefore, a fork-like structure is formed known as replication fork. Helicases enzymes break the hydrogen bonds in between the bases and the unwound DNA strands are stabilized by single stranded DNA binding proteins.
- The binding of RNA primase in the initiation point of the 3’ to 5’ parent chain: there is extension of the RNA primers by DNA polymerase that binds to the DNA nucleotides of the 3’ to 5’ strand due to the hydrogen bonds between the bases.
- DNA polymerase adds the matching loose nucleotide: DNA polymerase can act only from 5’ to 3’ direction. Therefore, DNA replication is different in two strands of DNA. Original 5’ to 3’ strand of DNA replication starts from 3’ end and proceeds to the direction of the breakage of the replication fork. The strand of DNA here is known as leading strand. In leading strand DNA is synthesized in continuous manner. In other strand of DNA, known as lagging strand, the process of DNA synthesis is in discontinuous manner, opposite to the direction of the replication fork. This occurs in the multiple areas of the DNA strand. Therefore, the multiple small pieces of DNA are synthesized that are known as Okazaki fragments.
- Joining of intact lagging strand: The RNA strands are removed by the action of RNase enzyme and DNA Pol I exonuclease. The lagging strands are joined by DNA ligase.
Ultimately, the DNA replication is terminated. Each double helix DNA contains one old template strand and one newly synthesized fresh strand.
DNA Transcription and Protein Synthesis
The principal key factor of protein synthesis remains in the DNA sequence of the nucleus. At first, the portion of DNA template is copied into the messenger RNA (mRNA). This mRNA is processed within the nucleus, and finally comes out from the nucleus to the cytoplasm through nuclear pore. Within the cytoplasm, on the small subunit of ribosome attached with RER, the mRNA is decoded and protein is synthesized with the help of t-RNA (Box 1.18).
The two major steps of the protein synthesis are:
- Transcription: It is the process of making mRNA (messenger RNA) from DNA.
- Translation: The process of synthesizing protein from the mRNA code is known as translation.
Transcription
In this process, mRNA is formed from the particular sequence of nucleotides of DNA carrying the genetic information to make a particular protein. Therefore, in this step, the information of DNA is transferred to the corresponding mRNA. The basic information of DNA remains same, so the process is known as transcription (Fig. 1.22).RNA is essentially same as DNA in structure except in certain points:
- This is a liner polymer of nucleotides
- The sugar moiety is ribose
- RNA contains the base uracil (U) instead of thymine (T)
There are three important steps of transcription: initiation, elongation, and termination.
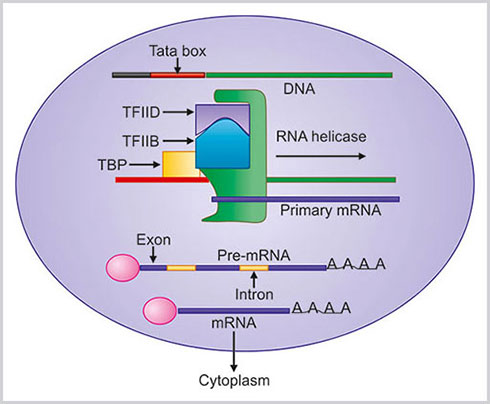
- Initiation: Initiation of the mRNA synthesis needs a DNA chain, transcription factors and RNA polymerase II. At first TATA binding protein (TBP) binds with TATA box of DNA. TBP is a part of general transcription factor called TFIID. The binding of TFIID promotes the binding of another protein known as TFIIB. This complex helps in binding RNA polymerase, to DNA. After the recruitment of RNA polymerase another two transcription factors, TFIIE and TFIIH binds, with this complex and initiation complex is completed.
- Elongation: The helicases enzyme of TFIIH unwind DNA, and the RNA polymerase starts synthesis of mRNA from the DNA strand.RNA polymerase now moves from the promoter and elongation phase starts. The DNA strand is read from 3’ to 5’ direction and subsequently RNA strand is made from 5’ to 3’ direction.
- Termination: When the RNA polymerase reaches to the terminal codon of DNA, the mRNA synthesis is stopped.
Translation
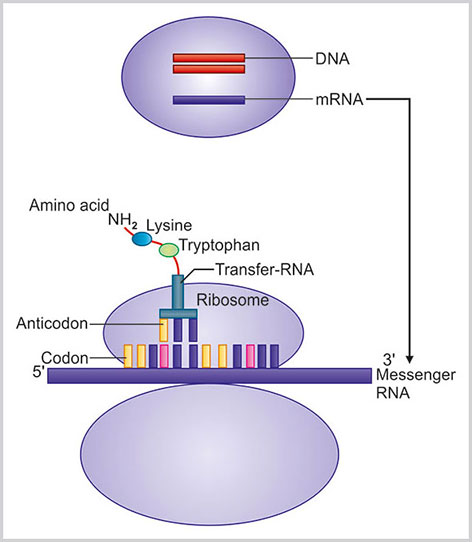
In translation phase, information of mRNA is decoded and the protein is synthesized. The newly formed mRNA passes through the nuclear pore and binds properly with the small unit of ribosome. Ribosome contains many proteins and rRNA. There are four nucleotides (adenine, guanine, cytosine and uracil) in RNA and each group of three consecutive nucleotides of mRNA is called a codon (Box 1.19). Each codon indicates one specific amino acid. More than one codon may also specify a particular amino acid. Now for each codon of m-RNA there is a specific transfer RNA (tRNA) carrying the complementary codon nucleotide sequence called anticodon. Each such tRNA molecule is linked with a particular amino acid. The recognition and attachment of the specific amino acid with this tRNA is dependent on enzymes known as aminoacyl-tRNA synthetase.
At first, mRNA is attached with the surface of ribosome. Then specific tRNA is attached to the start codon of the mRNA carrying a particular amino acid. Ribosome moves from 5’ to 3’ direction of mRNA and then a new tRNA with another particular amino acid is attached next to the previous one. The former tRNA is released from the mRNA. This process continues till the end of the tRNA meets the stop codon and ribosome stops translation. The complete protein is synthesized and comes to the cytoplasm (Fig. 1.23).
Fig. 1.23:
Translation of mRNA to protein synthesis in ribosomal surface is highlighted. Information of mRNA is decoded and tRNA with complementary anticodon brings a specific amino acid to form a protein
REFERENCES
- Singer SJ and Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(4023):720–31.
- Jacobson K, Sheets ED, Simson R. Revisiting the fluid mosaic model of membranes. Science. 1995;268:1441–2.
- Luttrell LM. Transmembrane signaling by G protein-coupled receptors. Methods Mol Biol. 2006;332:3–49.
- Tuteja N. Signaling through G protein coupled receptors. Plant Signal Behav. 2009;4(10):942–7.
- Macara IG. Parsing the polarity code. Nature Rev Mol Cell Biol. 2004;5:220–31.
- Januschke J, Gonzalez C. Drosophila asymmetric division, polarity and cancer. Oncogene. 2008;27:6994–7002.
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–25.
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38.
- Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83.
- Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin. 2008;40:643–50.
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–9.
- Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death and Differentiation. 2011;18:1470–7.
- Kemler R, Ozawa M. Uvomorulin-catenin complex: cytoplasmic anchorage of a Ca2+ dependent cell adhesion molecule. Bio Essays. 1989;11:88–91.
- Hazan RB, Kang L, Whooley BP, et al. N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun. 1997;4(6):399–411.
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4(3):181–91.
- Ellgaard L, Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol. 2001;13(4):431–7.
- Wilson C, Venditti R, Rega LR, et al. The Golgi apparatus: an organelle with multiple complex functions. Biochem J. 2011;433(1):1–9.
- Nilsson T, Au CE, Bergeron JJ. Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett. 2009;583:3764–9.
- Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–9.
- Missiaen L, Dode L, Vanoevelen J, et al. Calcium in the Golgi apparatus. Cell Calcium. 2007;41:405–16.
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–60.
- Brighton Carl T, Hunt Robert M. Mitochondrial calcium and its role in calcification. Clinical Orthopaedics and Related Research. 1974;100(100):406–16.
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–908.
- Jurgensmeier JM, Xie Z, Deve raux Q, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002.
- Fuchs E. Intermediate filaments: Structure, Dynamics, Function, and Disease. Annu Rev Biochem. 1994;63:345–82.
- Steinert PM, Chou YH, Prahlad V, et al. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999;274:9881–90.
- Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly, and interactions. J Struct Biol. 1998;122:42–66.
- Markiewicz E, Dechat T, Foisner R, et al. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell. 2002;13:4401–13.
- Dey P. Nuclear margin irregularity and cancer: a review. Anal Quant Cytol Histol. 2009;31(5):345–52.
- Lim RYH, Aebi U, Fahrenkrog B. Towards reconciling structure and function in the nuclear pore complex. Histochem Cell Biol. 2008;129:105–116.
- Beck M, Lucic V, Forster F, et al. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature. 2007;449:611–5.
- Dey P. Chromatin remodeling, cancer and chemotherapy. Curr Med Chem. 2006;13(24):2909–19.
- Dey P. Chromatin pattern alteration in malignant cells: An Enigma. Diagn Cytopathol. 2005;32(1):25–30.
- Boisvert FM, Koningsbruggen SV, Navascues J, et al. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8;574–85.
- Sirri V, Roussel P, Hernandez-Verdun D. The AgNOR proteins: qualitative and quantitative changes during the cell cycle. Micron. 2000;31: 121–6.
- Watson JD, Crick FHC. A structure for deoxyribose nucleic acid. Nature. 1953;171(4356):737–8.
- Elgar G, Vavouri T. Tuning in to the signals: noncoding sequence conservation in vertebrate genomes. Trends Genet. 2008;24(7):344–52.
- Ohmori H, Friedberg EC, Fuchs RP, et al. The Y-family of DNA polymerases. Mol Cell. 2001;8(1):7–8.
- Vashee S, Cvetic C, Lu W, et al. Sequence-independent DNA binding and replication initiation by the human origin recognition complex. Genes Dev. 2003;17:1894–908.
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–74.