INTRODUCTION
Human skin has been assigned an important task of discriminating from self to nonself and to protect ourselves from different harmful microorganisms. To carry out this task, human skin is endowed with a unique class of receptors, namely, Toll-like receptors (TLRs) which are constitutively present or expressed in various cutaneous cell components like keratinocytes, Langerhans cells, melanocytes, dendritic cells, mast cells and fibroblasts.1 TLRs play a role as a firstline of cutaneous host defense against microorganisms and a crucial initiator of innate (natural) immune responses.
Though the role of TLRs has been increasingly incriminated in various disciplines of medicine, the discussion of the topic will flow down in this chapter as (a) the general concept regarding TLRs at first and (b) the role of TLRs in relation to different dermatologic diseases in particular.
INNATE IMMUNITY: NO MORE ‘NONSPECIFIC’!
Although, in the past, innate immunity had been regarded as a nonspecific system (engulfing and destroying pathogens, triggering proinflammatory responses, helping present antigens) compared to adaptaive immune system (mediated by T cells and B cells having potential to recognize so called ‘immunologically processed novel antigens’), recent studies show that innate immune system has a high degree of target specificity by its ability to recognize pathogens by pathogen-associated molecular patterns (PAMPs), encompassing molecules from gram-positive and gram-negative bacteria, DNA and RNA viruses, fungi and protozoa.2–5 The mammalian receptors capable of recognizing PAMPs are called pattern recognition receptors (PRRs). The major pattern recognition receptors are Toll-like receptors (TLRs), Nod-like receptors (NLRs) and RIG-I-like receptors (RLRs).2
TOLL RECEPTORS AND TOLL-LIKE RECEPTORS
‘Toll’ is a transmembrane cellular receptor discovered in fruit fly named Drosophila melanogaster. Toll is a German slang, means ‘fantastic’. Hashimoto and colleagues cloned the Toll gene in 1988 in Drosophila and discovered that the gene encodes a novel type of transmembrane receptor.6 Activation of Toll pathway resulted in the production of antimicrobial peptides that are essential for Drosophila flies to combat fungal and bacterial pathogens. Soon after that, a number of structurally-related proteins were discovered in mammals and named Toll-like receptors.7 In 1997, Janeway and Medzhitov identified the first human homolog of Toll receptor of Drosophila, now known as TLR4.8 Till now fifteen TLRs are identified in different mammalian species, among them, eleven are found in human (named as TLR1—TLR11). The exact function of TLR10 in human is still unknown.
TLRs: STRUCTURE AND REPERTOIRE IN SKIN
TLRs are a group of glycoproteins and represent a family of type I transmembrane proteins located on cell surface, having an extracellular leucine-rich repeat domain, a transmembrane domain and intracellular cytoplasmic domain, similar to interleukin–1 (IL-1) receptor.9 Intracellular domain is also called Toll-interleukin-1 receptor (TIR) domain.
TLRs are present or expressed in almost all the cell types epidermis and dermis involved in innate defense system (keratinocytes, Langerhans cells, melanocytes, dermal dendritic cells, fibroblasts and mast cells being recently included), but the repertoire of TLRs varies considerably among these cells.10 The different TLRs and their level in skin resident cells are described in Table 1.1.
|
LIGANDS FOR TLRS
Revelation of structure of extracellular domain of several TLRs has provided structural insights indicating that several PAMPs act as ligands for TLRs. PAMPs recognized by TLRs are lipids, lipoproteins, and nucleic acids derived from bacteria, viruses, parasites and fungi.7, 20 TLRs are classified mainly into two subfamilies depending on their genetic tree. TLRs-1, 2, 4, 5 and 6 are able to identify mainly the bacterial cell wall components and classified as extracellular TLRs due to their expression on cell surface and their extracellular domain.10 TLRs 3, 7, 8 and 9 are located in the cytoplasm (on endosomes and lysosomes) and their action depends on the capacity of the microbial element to penetrate the host cell membrane.21 So these TLRs are known to interact mainly with viral nucleic acids. Though TLR-ligand binding is by and large specific, a TLR can recognize a diverse range of molecules and thus TLR-ligand interaction and recognition remains flexible. For an example, TLR4 not only recognizes lipopolysaccharides but also it interacts with fusion protein of respiratory syncytial virus, heat shock protein and fibronectin.1
TLR SIGNALING
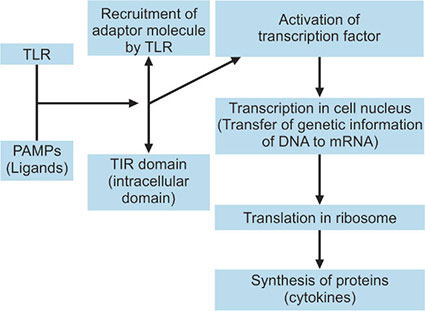
After ligand-TLR interaction, extracellular TLRs (1, 2, 4, 5 and 6) are internalized into phagosomes. TLR2 and TLR4 are also able to colocalize to phagosomes facilitating a very early contact with the immune system, especially for potentially dangerous microbial agents.22,23 Activated TLRs recruit different adaptor molecules which interact with the intracellular domain of the TLR (TIR domain).7 There are various adaptor molecules, namely MyD88 (myeloid differentiation factor-88), TIRAP (Toll-IL-1R domain-containing adaptor protein), TRIF (Toll-IL-1R domain-containing adaptor-inducing interferonβ) and TRAM (TRIF-related adaptor molecule) which activates different signaling pathways. MyD88, utilized by all TLRs except TLR3, activates in turn the transcription factor NFkB and mitogen-activated protein kinases (MAPKs).24 Transcription factors, by their ability to bind to the DNA molecule, regulate the gene activity by influencing the binding of RNA polymerases to the DNA molecule during the process of transcription. The next step is the genetic translation that leads to synthesis of proteins (inflammatory cytokines) (Flow chart 1.1).
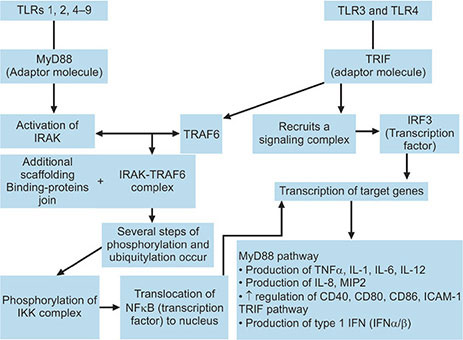
All TLR signaling are neither completely dependent on MyD88 nor it is required for recognition of all microbial ligands. TLR3 and TLR4 depend on a MyD88-independent pathway called TRIF pathway in which transcription factors, (interferon regulatory factor 3) IRF-3 and NFkB are involved producing interferon β (INF-β) and other inflammatory cytokines. TRAM and TIRAP act as sorting adaptors which help in recruiting other adaptors for TLRs, e.g. TRIF to TLR4 and MyD88 to TLR2 and TLR4.24, 254
The MyD88-dependent Pathway
Activation of MyD88 kicks off a signaling cascade which downstream the activation of IL-1R associated kinases (IRAK1, IRAK2, IRAK4 and IRAK-M).IRAK, in turn, interacts with tumor necrosis factor receptor-associated factor 6 (TRAF6). After joining of additional scaffolding and binding proteins to the IRAK-TRAF6 complex, several phosphorylation and ubiquitylation steps take place leading to the phosphorylation of the IKK complex (inhibitor of NFkB [IkB]-kinase complex). This results in degradation of IkB allowing NFkB translocation to the nucleus and induction of transcription of target genes. The IRAK-TRAF6 complex also stimulates the various members of MAPK family for induction of transcription of additional target genes. As a final outcome of this pathway, different cytokines like TNF-α, IL-1, IL-6 and IL-12, chemokines like IL-8 and MIP2 are produced. Upregulation of costimulatory molecules like CD40, CD80, CD86 and adhesion molecules such as ICAM-1 is also observed.25,26 Signaling of TLRs 1, 2, 4, 5, 6, 7, 8, 9 are MyD88-dependent. TLR2 and TLR4 also take help of the adaptor molecule TIRAP in MyD88-dependent pathway.
The TRIF-dependent Pathway
TLR 3 and TLR 4 utilize this pathway. In this pathway, transcription factors NFkB and interferon regulatory factor 3 (IRF3) are activated. TRIF recruits TRAF6 for NFkB activation similar to those of MyD88-dependent pathway and also recruits a signaling complex by which IRF3 is phosphorylated and tranlocated to nucleus. TRAF3 plays an important role in this pathway as a regulator. It promotes activation of IRF3 and IFNβ transcription and inhibits the MyD88-dependent pathway. Both Myd88-dependent pathway and TRIF-dependent pathway are summarized in Flow chart 1.2.5
OUTCOME OF TLR ACTIVATION
Activation of NFkB is considered as central signaling pathway. The final outcome of this pathway is the promotion of phagocytosis of the pathogenic microbes and inflammatory responses to phagosomes content. It also enhances expression of costimulatory molecules like CD80 and CD86, ensuing a second signal for full immune response.27, 28 So far, as intracellular TLRs activation is concerned, viral associated PAMPs are sensed and antiviral genes are induced and finally, type 1 INFs are produced. There is also enhanced presentation of viral antigens by major histocompatibility complex, ultimately leading to the activation of CD8 cells, which are considered as central weapon against virally-infected cells.29, 30
Apart from TLRs activation, production of antimicrobial peptides (AMPs) and their action is another important facet of innate immune system. TLR activation brings about the production of AMPs. Keratinocytes upregulate the expression of human β-defensin 2 (HBD-2) by stimulation through TLR2 or TLR4.31, 32 Interestingly, once the HBD-2 is induced, this AMP itself acts as a ligand for TLR4 and thereby, cyclically enhances the immune response.33
The recognition of different pathogenic microbes like bacteria, viruses and fungi by TLRs in the skin is essential for body defense. In case of fungal infections like Candida albicans, especially mucosal candidiasis, the regulation of TLR activation takes place through granulocytes.34 The most 6important distinctive feature of TLR activation in this regard is to create a pro-inflammatory milieu with the production of cytokines and chemokines through two different channels—TNFα and IL-12 for NFkB signaling TLR ligands and IFNα/β for IRF-3 signaling TLR ligands.35
Apoptosis (programmed cell death) can also be induced in certain tissues by TLR activation where caspase 8 and the TNF receptor superfamily, member 6 (FAS)-associated death domain protein are utilized. This apoptotic effect has been observed for mycobacterial, bacterial and mycoplasmal lipoproteins, and signals through TLR2 and TLR4.36, 37
Proinflammatory milieu having a balanced production of different cytokines and type 1 INFs plays an important key role in controlling tumor cell growth and autoimmune diseases. Negative regulation TLR-induced responses thus become extremely important to prevent tissue damage by suppressing inflammation and damaging immune responses in autoimmune diseases. Many negative regulator proteins have been identified to suppress the TLR signaling pathways, e.g splice variants for adaptor molecules and related proteins, ubiquitin ligases, deubiquitinases, transcriptional regulators and micro RNAs.24, 38–41
Various PAMPs as ligands, pathogens, induction of cytokines and final effector functions of TLRs are shown in Table 1.2.
TLRS IN DERMATOLOGY
The importance of TLRs does not restrict only to host defense to various microorganisms, its role in the pathophysiology of diseases has been also delineated in autoimmune diseases, diseases in central nervous system, lung, gastrointestinal tract, kidney, skin and malignancies.4,6,45,52,53 Significant advances in our understanding have also been noticed regarding the role of TLRs in skin inflammation and cutaneous malignancies. Another aspect now in research is the role of TLRs as therapeutic agents in the treatment of skin diseases. Research is also being carried out to develop agonists and antagonists of TLRs; inhibitor of TLRs signaling molecules as future drugs for a variety of therapeutic applications.
ROLE OF TLRS IN SKIN DISEASES
Adnexal Diseases
Acne Vulgaris
TLR2 has been implicated in the pathogenesis of this disease, as the expression of TLR2 at the site of inflammation of acne has been identified.54 The initiation and perpetuation of the inflammation by inducing cytokines production (IL-6, IL-8, IL-12) by Propionibacterium acnes (P. acnes) is dependent on TLR2. Expression of TLR2 has been found abundant on perifollicular and peribulbar macrophages.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The concentration of TLR2 expressing cells, particularly in the perifollicular region, shows a positive correlation with the severity of the acne lesions.55 The number TLR2–positive cells also increases with the long-term acne.4
The benefit of acne therapy with topical retinoids now can be explained by the role of TLR2, as retinoids has shown to decrease its expression.56,57 Nicotinamide has also been found to decrease IL-8 production through interaction with TLR2 on keratinocytes in a dose-dependent manner.58 Why and how topical corticosteroids induce and exacerbate acne vulgaris can be explained by the finding that shows boosted expression of TLR2 on keratinocytes after addition of glucocorticoids in human keratinocyte culture.59
Rosacea
Current concepts suggest a central role of TLR2 in the pathophysiology of rosacea. The keratinocytes of lesional skin of rosacea shows an altered TLR2 expression which, in turn, makes the individual susceptible towards innate immune stimuli.60–62 Activation of TLR2 leads to the expression of abnormally high level of cathelicidin, an AMP which upholds leukocyte trafficking through the induction of a chemokines CXCL8 and induce angiogenesis.63–65 The formation of pustules in rosacea is explained by the recruitment of neutrophils due to the crucial event of the induction of CXCL8 by the cathelicidin peptide LL-37 in keratinocytes.66 To add more to the mechanism of pustule formation, it has been found that IL-1 and TLR2-induced IL-6 and IL-23 induce differentiation of Th17 cells which act as an abundant source of IL-17, IL-21 and IL-22. IL-17/IL-22 provoke further induction of CXCL8 in keratinocyte to recruit neutrophil again to form pustules.6710
Apart from overexpression of cathelicidin, its processing enzyme kallikrein 5 (KLK5) is also overexpressed in keratinocytes and shows increased release, which is again based on TLR2 overexpression.62 Thus, increased TLR2 expression plays the central role, making the individual highly susceptible to innate immune stimuli, and explains the aberrant expression of cathelicidin and KLK 5 in rosacea.
The so called ‘clinical triggers’ of rosacea like ultraviolet (UV) radiation, heat, cold, stress, glucocorticoids, hormones, spicy food, and microbes have been found to generate or induce a variety of substances like reactive oxygen species (ROS), matrix metalloproteinases (MMPs), AMPs and above all TLR signaling.68 UV radiation-induced aggravation of rosacea now can be explained by MyD88-dependent TLR signaling as this adaptor molecule (MyD88) is found overexpressed in UV-irradiated human primary keratinocytes and chronically UV-exposed and photodamaged skin.69 The pathomechanism of rosacea is outlined in Flow chart 1.3.
Eczematous and Papulosquamous Diseases
Atopic Dematitis
The genetic polymorphisms of the TLRs and TLR signaling molecules have been identified in patients of Atopic dermatitis (AD). A strong association is found between TLR2 and symptoms of severe AD in some populations.70,71 There is high frequency (12%) of AD patients with known colonization of S. aureus infection where TLR single nucleotide polymorphism (SNP), especially TLR2 R753Q SNP has been identified.72 A severe phenotype of AD is detected with TLR2 R753Q SNP mutation compared to AD without this mutation. The enhanced skin inflammation in AD patients having TLR2 R753Q SNP is explained by increased production of proinflammatory cytokines IL-6 and IL-12 by monocytes, on stimulation of three different TLR2 ligands.73
Impairment of immunity in some cases of AD has also been attributed to the polymorphism of TLR9 gene. Recent study shows that impact on the functional aspect of antigen-presenting cells depends on TLR9 gene polymorphism.74 Polymorphism of Toll interactive protein (TOLLIP), an inhibitory adaptor molecule has been found associated with AD.75, 76
Allergic Contact Dermatitis
A specific autoreactive CD8 response against epidermis is found to take place, depending on TLRs 2, 4 and 9 signaling, when bacterial infections and hapten-self protein complex (complete allergen) leads to activation of dendritic cells in allergic contact dermatitis.20 Nickel-induced inflammatory response has been found to be dependent on direct activation of TLR4.77
TLR4 is also implicated in causing immunosuppression caused by UVB radiation. In a mice model, TLR4-endowed mice show significant suppression of contact hypersensitivity response than TLR4-deficient mice, when both strains of mice are subjected to prior UVB radiation (100 mJ/cm2) followed by sensitization with hapten dinitrofluorobenzene (DNFB).78 There is primarily generation of CD4+ CD25+ regulatory T cells which secrete large amount of IL-10 and TGF-β and cause suppression of immune response.
Psoriasis
Keratinocytes (KC) from psoriatic plaques show higher expression of TLRs 1, 2, 4, 5 and 9 than that of normal skin.1 Suprabasal KCs in psoriatic skin show upregulation TLR1 and TLR2 expression, whereas, basal KCs downregulate TLR5 expression, compared to normal skin.79 TLRs 2, 4 and also 3 upregulation induces NFkB nuclear translocation and there is eventual secretion of TNF α and IL-8 by KCs in psoriatic skin.80
The antimicrobial peptide LL-37, which is abundantly seen in psoriatic plaques but not in normal skin, also plays an important role in T cell activation and possibly promoting autoimmunity in psoriasis.20,55,81 It forms a complex with self-DNA and gains entry to the endosomal compartment of plasmacytoid dendritic cells (pDCs) and thereby activates TLR9 to produce INF-α.
Heat shock protein 60 (HSP60), a highly immunogenic protein, is greatly expressed in epidermal KCs in guttate and plaque psoriasis than KCs in normal skin.82 TLR2 and TLR4 have been reported to be the receptors of HSP60, but the exact mechanism of their interaction is yet to be elucidated.83, 84 It has been hypothesized that HSP60 triggers TLR2 and TLR4 resulting in development and/or exacerbation of psoriasis.82
TLR7 and TLR8 signaling has also been associated with psoriasis exacerbation. In a study, it has been demonstrated that imiquimod, a TLR7/TLR8 agonist, exacerbates a psoriatic lesion on application by local increase in IFN-α and 12increased recruitment of pDCs.85 Even why and how does HIV infection exacerbate psoriasis now can be explained by TLR7 signaling pathway where single-stranded mRNA from HIV acts as a naturally occurring ligand for TLR7.50, 86
The KC-derived ‘A domain’ of fibronectin, an endogenous ligand for TLR4 in APCs, has been identified at the basement membrane zone of the uninvolved skin in psoriasis patients compared to healthy control subject.87 This finding has enkindled a thought that libronectin-TLR4 signaling activates Langerhans cells to secrete TNF α and IL-12 and also promotes antigen presentation to pathogenic Th1 lymphocytes migrated to psoriatic plaques.86
Skin Infections
Staphylococcus Aureus Skin Infection
Immune response against S. aureus mainly depends on TLR2 induced MyD88-dependent pathway of cytokine productions and upregulation of AMPs, especially HBD-3, by keratinocytes.55 Binding of staphylococcal cell wall constituents, especially lipoteichoic acid, to TLR2 is partially responsible for the induction of HBD-3.88 Viable staphylococcus is more potent inducer of HBD-3 than cell wall component, suggesting that other factors, like staphylococcal secreted factors are involved.89 It has been documented that induction of HBD-3 by viable S. aureus is mainly NFkB dependent while S. aureus-secreted factors induced HBD-3 induction is TLR2 and NFkB independent, indicating involvement of two different signaling pathways.88
Interestingly, a mutually beneficial relationship between S. epidermidis and human keratinocyte inflammatory response has been documented.90 A small secretary molecule (<10 KDa) from S. epidermidis induces expression of HBDs in keratinocytes by TLR2 signaling.91 KCs incubated with S. epidermidis-conditioned media strongly upregulate AMPs production induced by S. aureus. Thus, it suggests that human KCs are sensitized by S. epidermidis towards pathogenic bacteria and the innate immune response is amplified.88
Herpes Simplex Virus (HSV) and Varicella-Zoster Virus (VZV) Infection
TLRs 2, 3 and 9 are implicated in the innate immunue response against HSV and VZV infections.1 TLR2 can recognize the viral envelope protein and lipopeptides of specific HSV glycoproteins. It also gets activated during VZV infection of monocytes.92,93 HSV infection, in a TLR3-deficient individual, can spread from keratinocytes to cranial nerves, increasing the susceptibility to HSV encephalitis.94 With the help of TLR9 expression, myeloid DCs and pDCs can sense HSV infection, as well as HSV DNA efficiently and initiate cytokine production accordingly.95
Candidiasis
One of the virulence factors of Candida albicans (C. albicans) is the phenomenon of phenotypic switching (yeast ↔ filament).96 It provides basis of 13activation of different pattern recognition receptor (PRRs) leading to different immune response. It is also important to remember that hyphal form is the main target for epithelial response and the yeast form is non-stimulatory.97 Among the several TLRs studied, it is accepted to date that TLR2 and TLR4 are the main TLRs involved in the signaling cascades induced by C. albicans. There are discrepencies in the results obtained in different studies using mice model so far as TLR2-mediated immune protection against disseminated candidiasis is concerned.98–100 These conflicting results are probably due to the use of different mouse model and candida strains. Similar conflicting results are found from different laboratories when the role of TLR4 for host defense against invasive candidiasis.101
Leprosy
It is now well-known that tuberculoid spectrum of the disease is characterized by Th1-dominated course with elaboration of Th1 cytokines and, in contrast, lepromatous spectrum comprises a T-cell anergic, Th2-dominated humoral course. Skin lesions of tuberculoid leprosy express a higher level of TLR2 and TLR1 compared to the skin lesion of lepromatous leprosy.102 The 19 kDa and 33 kDa lipoproteins of M. leprae act as ligand for TLR1/TLR2 heterodimer for activation of monocytes and dendritic cells.103
Single nucleotide polymorphism (SNP) of TLR2 gene has found to be associated with the susceptibility of developing lepromatous leprosy.104 SNP of TLR1 is also found associated with impaired mycobacterial signaling and protection from reversal reaction in leprosy.105 While analyzing three polymorphism in TLR2 gene (597C → T, 1350T → C and a microsatellite marker), a recent study shows both (597C → T) and a microsatellite marker polymorphism, induce susceptibility to reversal reaction.106
The nerve damage in leprosy can also be explained by TLR2-induced apoptotic genes. Schwann cells express TLR2 and its signalling, after binding with 19 kDa lipoprotein, induces apoptosis of the Schwann cells.107
Syphilis
The lipopeptide on the surface Treponema pallidum acts as a ligand for TLR2 on dendritic cells and the interaction is involved in mounting adaptive immunity through the cell activation and release of Th1 cytokines. Moreover, being T. pallidum a flagellated organism, its flagella (polymerized flagellin subunits) bind with TLR5 and lead to elaborate cytokines like TNF α.55 Flagellin also stimulates nitric oxide in macrophages involving TLR5 and TLR4 signaling pathways.108
Lyme Disease
The outer surface protein A lipoprotein (OspA) of Borrelia burgdorferi is the ligand for TLR2 and TLR6 to induce NFkB activation. Recent studies show that TLR2/TLR1 heterodimers are also necessary to identify OspA.1 TLR2-mediated 14signaling is involved in providing anti-bacterial action, inducing pathologic proinflammatory responses and stemming disease evolution.109 Like Treponema, Borrelia also has flagellin. Interestingly and quite opposingly to the antibacterial role, TLR2 activation has been shown to downregulate the expression of TLR5 in monocytes, making the host less responsive to Borrelia.110 Induction of MMPs by TLR2 stimulation also helps the migration of the organism within the cutaneous tissue to a large extent.111
Cutaneous Malignancies
Melanoma
Exposure to UV radiation is considered the main risk factor for developing this malignant tumor of melanocytes. UV radiation, other than its mutagenic effect, has the capacity to suppress immune system, both locally and systemically. Locally, high level of IL-10 secreted by regulatory T cells and systemically, elaboration of cytokines by KCs, notably IL-10 and TNFα, contribute to immunosuppression. Both IL-10 and TNFα render the host incapable to recall antigens even at the distant sites from UV exposure.9 Primary melanomas can also induce immunosuppression in the sentinel draining lymph node(SLN).112 Human melanocytes itself contribute also to the tumor progression in melanoma by inducing MMPs and other cytokines production through TLR4 signaling, acting on hyaluronic acid fragments.1
TLR7 immunomodulating pathways have been advocated as a basis of immunotherapy against melanoma. Imiquimod, aTLR7 agonist, has been found to enhance local and regional T cell numbers in both skin and SLN in a small pilot study involving treatment of primary melanoma.112 It is found also effective in both activating dendritic cells and producing tumor-specific cytotoxic T cells that arrest disease progression.113 Persistent TLR signaling may also be required to circumvent regulatory T cell induced immune tolerance or suppression.114 Thus, it shows, though not directly, that TLR7 plays a role not only in mounting protecting immune response against melanoma but also in arresting disease progression in melanoma.
Non-melanoma Skin Cancers
Among the non-melanoma skin cancers (NMSC), the two major types are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). In a recently published study aiming at determining the expression of selected innate immune genes in BCC and SCC arising in immunocompetent and immunosuppressed (organ transplant recipients) patients, it has been documented that SCC, but not BCC, showed significantly elevated mRNA expression of TLRs 1, 2, 3, 5, 6, 7, 8 TRIF and TRAF1. BCC shows increased IFN-γ. Human beta defensin 1 (HBD-1) and HBD-2, are significantly higher in SCC than BCC. SCC from 15immunosuppressed patients show only an increase in HBD-2 but not an increase of HBD-1 compared to SCC from immunocompetent patients.115 Thus, innate gene expression in SCC is distinct from BCC, and BCC shows lesser induction of innate immune genes.
TLR7 again and also TLR8 have been employed to activate the proinflammatory machinery against BCC and SCC. Imiquimod has been used successfully which helps to induce production of IFN-α and IL-2 mediated by TLR7.116–119
Autoimmune Diseases
Systemic Lupus Erythematosus
Defects in the induction of apoptosis, defects in the process in clearing of apoptotic cells and immunostimulatory cell debri, abnormal activated T cells and hyperactive B cell receptors are the compelling forces for the development of systemic lupus erythematosus (SLE). TLRs 7, 8 and 9 are implicated in the development in SLE.120–122
Circulating DNA-containing immune complexes (ICs) stimulate plasmacytoid DCs (pDCs) to produce INF-α through TLR9 activation. INF-α is thought to play a pathogenic role in SLE, as several studies observe the induction of SLE-like syndrome and anti-dsDNA antibodies in patients treated with INF-α.123–125 Interestingly, active SLE shows higher levels of IFN-α than in patients with inactive SLE. It has been also demonstrated that circulating pDCs are resistant to TLR9 signaling in patients with chronic SLE.126
TLR8 is also thought to play a potential role. Its ligand can induce production of Th1 cytokines.127,128 TLR8 mRNA is seen upregulated and TLR8 signaling is stimulated by ribonucleoprotein.
The role of TLR7 is incriminated due to the fact that apoptotic cells combined with sera from SLE patients and supernatants containing material released from dying cells augment INF-α response.129, 130
Sjogren's Syndrome
Like SLE, TLR8 mRNA upregulation and TLR8 signaling stimulated by ribonucleoprotein are also noticed in this autoimmune disease.
Major pathogenetic events related to different TLRs in skin diseases discussed here are shown in Table 1.3.
TLRS IN THE TREATMENT OF SKIN DISEASES
The basis of innate immune response initiation by TLRs, beneficial (e.g. in bacterial or viral infections) or harmful (autoimmune diseases) for the host, has created enormous interest in research to develop pharmalcologic molecules to manipulate innate immune response for the treatment of various TLR-related diseases including skin diseases. The concept of TLR agonists and antagonists explain whether intervention by a pharmacologic molecule augments the innate immune function of a TLR or TLR ligands (agonists), or structural similarity of the molecule with TLR blocks the damaging or harmful immune response on TLR signaling (antagonists).
TLR agonists are developed for the treatment of cancer, allergies and viral infections. These are also used as adjuvants for potent new vaccines to prevent or treat cancer and infectious diseases.131 The design of specific TLR agonists is made in such a way that it should have reduced toxicity, but increased potency to meet the stringent safety criteria required for prophylactic vaccines. APCs, mainly the dendritic cells, are the principal cellular targets, as they constitute the link between innate and subsequent adaptive immune responses. The main idea of employing certain TLR ligands to facilitate these vaccinations is two folds: (i) enhancement of CD8+ T cell responses to protein antigens necessitating cross-presentation of peptides generated from exogenous antigens and (ii) overcoming tolerance to self-antigens, probably necessary for generating responses to tumor-associated antigens having few differences from normal self-antigens.132 As our understanding grows regarding the role of inappropriate Toll-like receptor stimulation in inflammation and autoimmunity, significant efforts have begun to develop antagonists to Toll-like receptors as well.
TLR antagonists are structural analogs of agonists which probably bind to the receptor but are incapable in signaling. Other possible approaches include anti-TLR antibodies and small molecule antagonists selected from compound libraries. TLR antagonists appear quite promising for a number of inflammatory and autoimmune diseases.13119
Synthetic ligands (e.g. imiquimod) with varying degrees of similarity to natural ligands and, even more recently, endogenous (self) ligands (e.g. HSP60) have been described for most of the TLRs (Table 1.4).133–137 Therapeutic applications to date have used either synthetic versions of natural TLR ligands with optimized pharmacologic properties, or small molecule agonists derived from drug screening efforts.
Synthetic Ligands As Therapeutic Tool
Monophosphoryl Lipid A
Monophosphoryl lipid A, being identified as an active component of lipopolysaccharides for TLR4 signaling, is now used for specific allergen preparation in combination with allergen extracts and effectively introduced into immunotherapy for allergic diseases like allergic rhinitis.138 It is used as adjuvant in prophylactic vaccine against Hepatitis B infection.
Imiquimod and Resiquimod
Imiquimod and resiquimod, the members of the family of imidazoquinoline family and also TLR7 agonists, have potent antiviral antitumoral properties. Imiquimod has approved indications in treatment of genital warts, superficial basal cell carcinoma and actinic keratosis. It is interesting to note that keratinocytes do not express TLR7 or TLR8, but still they are the major target for anti-HPV therapy.
|
This incongruity is now can be explained by two recent discovery: (i) Keratinocytes upregulate TLR7 when virally (HPV) stimulated and139 (ii) a crucial role is played by the system of adenosine receptors besides TLRs in the recognition of imidazoquinolines in keratinocytes.140 This also explains how a good clinical effect by imidazoquinolines is seen in superficial epidermal cancers, in which transformed keratinocytes are also the targets, either directly or by the infiltration of primary immunocompetent cells. The several off-label indications for imiquimod include verruca vulgaris, HSV infection, Bowen's disease, squamous cell carcinoma, lentigo maligna, and Paget 's disease, Kaposi 's sarcoma, CTCL, keloid, vitiligo and alopecia areata.1 Imiquimod has been reported to clear plaques of mycosis fungoides which are otherwise shown resistance to psoralen combined with ultravoilet A (PUVA).141 Resiquimod, 100 times more potent than imiquimod, has been exploited mainly in the treatment of actinic keratosis and herpes genitalis caused by HSV2.142,143
Loxoribine and Bropirimine
Loxoribine, a guanosine ribonucleoside, and bropirimine, an aryl pyrimidinone class of antineoplastic compounds, both act as TLR7 agonists. They upregulate the activity of B cells, T cells, natural killer (NK) cells, macrophages and lymphokine-activated killer (LAK) cells.9 Both these immunomodulators are currently under clinical trials for treatment of carcinomas.
CpG ODN
Synthetic oligodeoxynucleotides (ODNs) which contains unmethylated cytosine-guanine motifs (CpG ODN) are identified as highly potent immune activators inducing both innate and adaptive immune responses through activation of TLR9. This TLR9 agonist is regarded as an effective adjuvant for vaccines and cancer immunotherapy because of its narrow expression profile among all TLRs.1 It has been demonstrated that CpG ODN can be used as anticancer vaccines against melanoma and other cancers. A response rate of 25% is noted in a preliminary clinical trial of CpG in mycosis fungoides and Sezary syndrome.141
SUMMARY
The discovery of TLRs is undoubtedly a major breakthrough in medical science. Our understanding of the role of TLRs in the pathophysiology of different skin diseases brings about an oneness among diverse etiologies. The several unanswered queries can now be explained in the light of TLRs’ contribution (e.g. how antimalarials work in SLE is now explained by the fact that chloroquine can inhibit TLR 7, 8, 9 at a very low concentration). Apart from their job in antimicrobial defense by initiating immune response, TLRs prove themselves as indispensible player in inflammatory skin diseases and 21cancers. Developments of TLR-based therapies against skin diseases and skin cancers have gained a lot of momentum in research nowadays. Imiquimod is just an example of the fruitful research in this direction. The various TLRs-based vaccinations, prophylactic or therapeutic, for viral skin infection (HSV), melanoma and other skin malignancies are recently under clinical trial. As TLR-signaling dysregulation is demonstrated, it makes us to start dreaming of an antiageing therapy by manipulating such versatile receptor like TLR.
REFERENCES
- Ermertcan AT, Öztürk F, Gündüz K. Toll-like receptors and skin. J Eur Acad Dermatol Venereol. 2011;25:997–1006.
- Ishii KJ, Akira S. Innate immunity. In Rich RR, Fleisher TA, Shearer WT, Schroeder HW Jr, Frew AJ, Weyand CM: (Eds). Clinical Immunology Principles and Practice, 3rd espn. Elsevier Ltd, Philadelphia. 2008: 39–51.
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20: 197.
- Chen K, Huang, J, Gong W et al. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7:1271–85.
- Liew FY, Xu, D, Brint, EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58.
- Ofodile ON. Disifin (Sodium tosylchloramine) and Toll-like receptors (TLRs): evolving importance in health and diseases. J Ind Microbiol Biotechnol. 2007; 34:751–62.
- Miller LS, Modlin, RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29:15–26.
- Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptative immunity. Nature. 1997; 338:394–397.
- Petry V, Gaspari, AA. Toll-like receptors and dermatology. Int J Dermatol. 2009; 48:558–70.
- Terhost D, Kalali, BN, Ollert, M, et al. The role of Toll-like receptors in host defences and their relevance to dermatologic diseases. Am J Clin dermatol. 2010;11:1–10.
- Mempel M, Voelcker, V, Kollisch, G, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121(6):1389–96.
- Kollisch G, Kalali, BN, Voelcker, V, et al. Various members of the toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114(4):531–41.
- Miller LS, Sorensen, OE, Liu, PT, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005;174 (10): 6137–43.
- Lebre MC, van der Aar AM, van Baarsen L, et al. Human keratinocytes express functional toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–41.
- Pivarcsi A, Bodai, L, Rethi, B, et al. Expression and function of toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15(6):721–30.
- Jin SH, Kang, HY. Activation of Toll-like receptors 1, 2, 4, 5, and 7 on human melanocytes modulate pigmentation. Ann Dermatol. 2010;22:486–89.
- Flacher V, Bouschbacher, M, Verronese, E, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177 (11): 7959–67.
- Kulka M, Alexopoulou, L, Flavell, RA, et al. Activation of mast cells by double-stranded RNA: evidence for activation through toll-like receptor 3. J Allergy Clin Immunol. 2004;114(1):174–82.
- Hari A, Flach, TL, Shi, Y, Mydlarski, PR. Toll-like receptors: role in dermatological disease. Mediators Inflapp. 2010;2010:437246. Epub 2010Aug22.
- Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol 2004;25(7):381–6.
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304 (5673): 1014–8.
- Underhill DM, Ozinsky, A, Hajjar, AM, et al. The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401 (6755): 811–5.
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–87.
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
- Tsuji S, Matsumoto, M, Takeuchi, O, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68 (12): 6883–90.
- Michelsen KS, Aicher, A, Mohaupt, M, et al. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS): peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276 (28): 25680–6.
- Heit A, Maurer, T, Hochrein, H, et al. Cutting edge: Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNAconjugated antigens but essential for cross-priming of CD8 T cells. J Immunol 2003;170 (6): 2802–5
- Zaks K, Jordan, M, Guth, A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176 (12): 7335–45.
- Becker MN, Diamond, G, Verghese, MW, et al. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275 (38): 29731–6.
- Hertz CJ, Wu, Q, Porter, EM, et al. Activation of toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171 (12): 6820–6.
- Biragyn A, Ruffini, PA, Leifer, CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298 (5595): 1025–9.
- Weindl G, Naglik, JR, Kaesler, S, et al. Human epithelial cells establish direct antifungal defense through TLR4-mediated signalling. J Clin Invest. 2007;117 (12): 3664–72.
- Dabbagh K, Lewis, DB. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr Opin Infect Dis. 2003;16(3):199–204.
- Lopez M, Sly, LM, Luu, Y, et al. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through toll-like receptor-2. J Immunol 2003; 170 (5): 2409–16.
- Palsson-McDermott EM, Doyle, SL, McGettrick AF, et al. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88- independent TLR4 pathway. Nat Immunol. 2009;10:579–86.
- Shi M, Deng, W, Bi, E, et al. TRIM30a negatively regulates TLR-mediated NF-jB activation by targeting TAB2 and TAB3 for degradation Nat Immunol. 2008;9: 369–77.
- Kayagaki N, Phung, Q, Chan, S, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318:1628–32.
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the micro RNA miR-21. Nat Immunol. 2010;11:141–7.
- Kurt-Jones EA, Popova, L, Kwinn, L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000; 1 (5): 398–401.
- Beg AA. Endogenous ligands of toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23 (11): 509–12.
- Okamura Y, Watari, M, Jerud, ES, et al. The extra domain A of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276 (13): 10229–33.
- Ospelt C, Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol. 2010; 42:495–505.
- So EY, Ouchi T. The application of Toll like receptors for cancer therapy. Int J Biol Sci. 2010;6:675–81.
- Alexopoulou L, Holt, AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413 (6857): 732–8.
- Hemmi H, Kaisho, T, Takeuchi, O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200.
- Heil F, Hemmi, H, Hochrein, H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303 (5663): 1526–9.
- Diebold SS, Kaisho, T, Hemmi, H, et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303 (5663): 1529–31.
- Hajjar AM, O'Mahony DS, Ozinsky A, et al. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166(1):15–9.
- Drexler SK, Foxwell, BM. The role of Toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:506–18.
- Montero Vega MT, de Andres Martin A. The significance of toll-like receptors in human diseases. Allergol Immunopathol. 2009;37:252–63.
- Kim J, Ochoa, MT, Krutzik, SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169(3):1535–41.
- Valins W, Amini, S, Berman, B. The expression of Toll-like receptors in dermatological diseases and the therapeutic effect of current and newer topical Toll-like receptor modulators. J Clin Aesthet Dermatol. 2010;3(9):20–9.
- Liu PT, Krutzik, SR, Kim, J, Modlin, RL. Cutting edge: all-trans retinoic down-regulates TLR2 expression and function. J Immunol. 2005;174(5):2467–70.
- Grange PA, Raingeud, J, Calvez, V, Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-jB and MAPK pathways. J Dermatol Sci. 2009;56:106–12.
- Shibata M, Katsuyama M, Onodera T, et al. Glucocorticoids enhance toll-like receptor 2 expression in human keratinocytes stimulated with propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol. 2009;129(2):375–82.
- Yamasaki K, Gallo, RL. The molecular pathology of rosacea. J Dermatol Sci. 2009; 55:77–81.
- Schauber J, Dorschner, RA, Coda, AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin Dependent mechanism. J Clin Invest. 2007;117:803–11.
- Yamasaki K, Kanada, K, Macleod, DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–97.
- De Y, Chen, Q, Schmidt, AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPR11) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80.
- Zhang J, Xu, X, Rao, NV, et al. Novel sulfated polysaccharides disrupt cathelicidins, inhibit RAGE and reduce cutaneous inflammation in a mouse model of rosacea. PLoS One. 2011;6:e16658.
- Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41:87–102.
- Gerber PA, Buhren, BA, Steinhoff, M, et al. Rosacea: the Cytokine and Chemokine Network. J Invest Dermatol. 2011;15:40–7.
- Lee Y, Kim, H, Kim S et al. Myeloid differentiation factor 88 regulates basal and UV-induced expressions of IL-6 and MMP-1 in human epidermal keratinocytes. J Invest Dermatol. 2009;129:460–7.
- Hasannejad H, Takahashi, R, Kimishima, M, et al. Selective impairment of Toll-like receptor 2 mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J Allergy Clin Immunol. 2007;120:69–75.
- Oh DY, Schumann, RR, Hamann, L, et al. Association of the toll-like receptor 2 A-16934T promoter polymorphism with severe atopic dermatitis. Allergy. 2009; 64:1608–15.
- Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, et al. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113:565–7.
- Niebuhr M, Langnickel, J, Draing, C, et al. Dysregulation of toll-like receptor-2 (TLR-2)-induced effects in monocytes from patients with atopic dermatitis: impact of the TLR-2 R753Q polymorphism. Allergy 2008;63:728–34.
- Novak N, Yu, CF, Bussmann, C, et al. Putative association of a TLR9 promoter polymorphism with atopic eczema. Allergy. 2007;62(7):766–72.
- Miller LS. Toll-like receptors in skin. Adv Dermatol. 2008;24:71–87.
- Lewis W, Simanvi, E, Li, H, et al. Regulation of ultraviolet radiation induced cutaneous photoimmunosuppression by Toll-like Receptor-4. Arch Biochem Biophys. 2011;508:171–7.
- Baker BS, Ovigne, JM, Powles, AV, et al. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic psoriasis. Br J Dermatol. 2003;148:670–9.
- Begon E, Michel, L, Flageul, B, et al. Expression, subcellular localization and cytokine modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol. 2007;17:497–506.
- Lande R, Gregorio, J, Facchinetti, V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9.
- Ohashi K, Burkart, V, Flohe, S, et al. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Tolll-like receptors-4 complex. J Immunol. 2000;164: 558.
- Zanin-Zhorov A, Nussbaum, G, Franitza, S, et al. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;127: 78.
- Gillet M, Conrad, C, Geiges, M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;104:1490–5.
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(Suppl. 2):S67–S80.
- Ting KM, Rothaupt, D, McCormick TS, et al. Overexpression of the oncofetal Fn variant containing the EDA splice-in segment in the dermal–epidermal junction of psoriatic uninvolved skin. J Invest Dermatol. 2000;114:706–11.
- Wanke I, Steffen, H, Christ, C, et al. Skin commensals amplify the innate immune response to pathogen by activation of distinct signalling pathways. J Invest Dermatol. 2011;131:382–90.
- Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human bet-defensin 3 in skin keratinocytes. Infect Immun. 2006: 74:6847–54.
- Gallo RL, Nakatsuji T. Microbial symbiosis with the Innate Immune Defense System of the Skin. J Invest Dermatol. 2011;131:1974–80.
- Li Y, Cogen, AL, Radek, KA, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–21.
- Sato A, Linehan, MM, Iwasaki, A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:17343–8.
- Wang JP, Kurt-Jones EA, Shin OS, et al. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79:12658–66.
- Zhang SY, Jouanguy, E, Ugolini, S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–7.
- Lund J, Sato, A, Akira, S, et al. Toll-like receptor 9-mediated recognition Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–20.
- Gauglitz GG, Callenberg, H, Weindl, G, et al. Host defence against Candida albicans and the role of Pattern recognition receptors. Acta Derm Venereol. 2012;92:291–8.
- Netea MG, Sutmuller, R, Hermann, C, Van der Graaf CA, Van der Meer JW, van Krieken JH, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004; 172:3712–8.
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–94.
- Villamon E, Gozalbo, D, Roig, P, O'Connor JE, Fradelizi D, Gil ML. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7.
- Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31:346–53.
- Krutzik SR, Modlin, RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol. 2004;16:35–41.
- McInturff JE, Modlin, RL, Kim, J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125(1):1–8.
- Kan T, Chae G. Detection of Toll-like receptor 2 (TLR2) mutation in lepromatous leprosy patients. FEMS Immunol Med Microbiol. 2001;31:53–8.
- Misch EA, Macdonald, M, Ranjit, C, et al. Human TLR1 deficiency is associated with impaired mycobacterial signalling and protection from leprosy reversal reaction. PLoS Negl Trop Dis. 2008;2 (5):e231.
- Bochud PY, Hawn, TR, Siddiqui, MR. Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J Infect Dis. 2008;197:253–61.
- Oliveira RB, Ochoa, MT, Sieling, PA, et al. Expression of toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect Immun. 2003;71(3):1427–33.
- Mizel SB, Honko, AN, Moors, MA, et al. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signalling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 2003;170:6217–23.
- Singh SK, Girschick, HJ. Toll-like receptors in Borrelia burgdorferi-induced inflammation. Clin Microbiol Infect. 2006;12:705–17.
- Cabral ES, Gelderblom, H, Hornung, RL, et al. Borrelia burgdorferi lipoprotein-mediated TLR2 stimulation causes the down-regulation of TLR5 in human monocytes. J Infect Dis. 2006;193(6):849–59.
- Behera AK, Hildebrand, E, Bronson, RT, et al. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin- 18-independent mice infected with Borrelia burgdorferi. Infect Immun. 2006;74(3):1462–70.
- Narayan R, Nguyen, H, Bentow, JJ, et al. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol. 2012;132:163–9.
- Steinmann A, Funk, JO, Schuler, G, Von den Driesch P. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43: 555–6.
- Yang Y, Huang, CT, Huang, X, et al. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–15.
- Muehleisen B, Jiang, SB, Gladsio, JA, et al. Distinct innate immune gene expression profile in Non-melanoma Skin Cancer of immunocompetent and immunosuppressed patients. PLoS One. 2012;7:e40754.
- van der Geer S, Ostertag JU, Krekels GA. Treatment of basal cell carcinomas in patients with nevoid basal cell carcinoma syndrome. J Eur Acad Dermatol Venereol. 2009;23:308–13.
- Kocabas E, Ermertcan, AT, Bilac, C, et al. Nonsyndromic multiple basal cell carcinomas successfully treated with imiquimod 5% cream. Cutan Ocul Toxicol. 2010;29:300–2.
- Micali G, Lacarrubba, F, Dinotta, F, et al. Treating skin cancer with topical cream. Exp Opin Pharmacother. 2010;11:1515–27.
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35.
- Krieg AM, Vollmer J. Toll-like receptors 7, 8 and 9: linking innate immunity to autoimmunity. Immunol Rev 2007; 220:251–69.
- Christensen SR, Shlomchik, MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007; 19:11–23.
- Ronnblom LE, Alm, GV, Oberg, KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med. 1990;115:178–83.
- Ronnblom LE, Alm, GV, Oberg, KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227:207–10.
- Ehrestein MR, McSweeney E, Sware M, et al. Appearance of anti-DNA antibodies in patients treated with interferon-alpha. Arthritis Rheum. 1993;36:279–80.
- Kwok SK, Lee, JY, Park, SH, et al. Dysfunctional interferon-alpha production by peripheral plasmacytoid dendritic cells upon Toll-like receptor-9 stimulation in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10:R29.
- Ghosh TK, Mickelson, DJ, Fink, J, et al. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines. I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57.
- Jurk M, Kritzler, A, Schulte, B, et al. Modulating responsiveness of human TLR7 and 8 to small molecule ligands with T-rich phosphorothiate oligodeoxynucleotides. Eur J Immunol 2006;36:1815–26.
- Bave U, Alm, GV, Ronnblom, L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-alpha inducer. J Immunol. 2000;165:3519–26.
- Lovgren T, Eloranta, ML, Bave, U, et al. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004; 50:1861–72.
- Kanzler H, Barrat, FJ, Hessel, EM, et al. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;5:552–9.
- Hodi FS, Dranoff G. Combinatorial cancer immunotherapy. Adv Immunol. 2006; 90:341–68.
- Krieg AM, et al. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev. Drug Discov. 2006;5:471–84.
- Tomai MA, Miller, RL, Lipson, KE, et al. Immune response modifiers: Imiquimod and future drugs for modulating the immune response. Drug Discov Today Ther Strateg [online]. 2006;3:342–52.
- Evans JT Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi. 529. Expert Rev. Vaccines. 2003;2:219–29.
- Marshak-Rothstein A, et al. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006;6:823–35.
- Mothes N, Heinzkill, M, Drachenberg, KJ, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33(9):1198–208.
- Kalali BN, Kollisch, G, Mages, J, et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol. 2008;181(4):2694–704.
- Schon MP, Schon, M, Klotz, KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126(6):1338–47.
- Hwang ST, Janik, JE, Jaffe, ES, et al. Mycosis fungoides and Sezary syndrome. Lancet. 2008;371 (9616): 945–57.
- Szeimies RM, Bichel, J, Ortonne, JP, et al. A phase II dose-ranging study of topical resiquimod to treat actinic keratosis. Br J Dermatol. 2008;159:205–10.
- Fife KH, Meng, TC, Ferris, DG, Liu, P. Effect of resiquimod 0.01% gel on lesion healing and viral shedding when applied to genital herpes lesions. Antimicrob Agents Chemother. 2008;52:477–82.
- Schmidt M, Raghavan B, Müller V, et al. Crucial role for human Tolllike receptor 4 in the development of contact allergy to nickel. Nat Immunol 2010;11:814–20.
- Seung NR, Park EJ, Kim CW, et al. Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor γδ in plaque and guttate psoriasis. J Cutan Pathol. 2007;34:903–11.