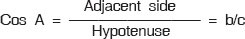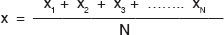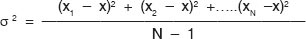MATTER AND ENERGY
Physics is a science dealing with nature. It is concerned with the study of two concepts, matter and energy, and how they interact with each other. Matter is one, which occupies space, and it is made up of molecules or atoms, e.g. gold, wood, water and air. Matter exists in solid, liquid, gas, liquid crystal, and plasma state. Matter can be converted from one form to another by physical or chemical means, e.g. melted ice converts from solid to liquid form by physical process and burning of wood into ash is a chemical process.
Energy is the ability to do work, it has several forms, and it can be converted from one form to another, e.g. human body converts chemical energy (food) into kinetic energy (work). Law of conservation of energy states that energy can neither be created nor destroyed, and the total energy in the universe is constant. This law holds good for all forms of energy.
In general, physicist studies the behavior of matter and energy under different physical conditions.
MEASUREMENT AND UNITS
To study, the matter and energy and their various properties, measurements of physical quantities, such as length, mass, and time are required. Physical quantity is measured accurately in terms of its own standard, e.g. distance is measured in meter, mass in kilogram, and time in second. Therefore, unit is a quantity adopted as a standard of measurement in terms of which similar quantities can be measured. The units which are independent of one another and having their own standard (base) are called fundamental units, e.g. kilogram, meter and second. The units, which are not having their own standard (base) and obtained 2from the fundamental units are called derived units, e.g. area-meter2, velocity-meter per second, and density-kilogram per meter3.
One meter is the distance traveled by light (Krypton-86) in 1/299,792,468 second. One kilogram is the mass of 1000 cm3 of water at 4°C. The second is measured by an atomic clock and is based on the vibration of atoms of cesium.
SI UNITS
In 1960, a new system of units called Systems International d'units (SI Units) was introduced. The SI system is superior to all other systems and more convenient in practice and is used throughout the world. There are 7 fundamental units and 2 supplementary units in the SI system as shown in the Table 1.1.
Conventions for SI Units
- When the unit is named after a scientist, it should not be written in a capital initial letter, e.g. newton, ampere. The symbol of the unit is expressed in capital letters, e.g. N for Newton.
- The symbol of all other units should be written with small letters, e.g. ‘m’ for meter.
- Only singular form of the unit is to be used, e.g. 500 meters is written as 500 m. No full stops or punctuation marks should be used at the end of the symbol.
- Space is to be left between the numerical and symbol, e.g. 20 s and not as 20s.
- In the temperature unit Kelvin no degree sign is used, e.g. 273 K and not as 273° K.
Prefixes
Though the SI units are a coherent system, they are found to be either too large or low in practice, e.g. the activity of an isotope for bone scan is expressed in billions of becquerel's. Hence, prefixes are used to overcome the above difficulty, as shown in Table 1.2. These prefixes are conveniently used to describe very large or small physical quantities. In radiation physics, giga becquerel (GBq), kilovolt (kV), centi gray (cGy), milli ampere (mA), and nanometer (nm) are commonly used.
DENSITY, MOLE, PRESSURE, AND GAS LAWS
DENSITY
The density of a body (ρ) is defined as the ratio of its mass (m) and volume (v) and its unit is kgm−3. The density of a body is same, if it is made up of identical material. If its composition is changed, its density will be vary.
ρ = m/v
The relative density or specific gravity of a substance is the ratio between its density with that of water.4
MOLE
The amount of matter in a body is expressed by the number of elementary particles (atoms or molecules) it contains and its unit is mole. One mole of matter contains 6.022 × 1023 elementary particles, and it is known as Avogadro's number.
PRESSURE
The total force acting on a liquid surface is called thrust. The pressure (p) is defined as the force (F) per unit area (A) and its unit is Nm−2 or pascal (Pa). The atmospheric pressure is about 1.01 × 105 Pa. The pressure is caused by the weight of material pressing on its surface. It may be also due to collisions of atoms or molecules of a gas within a container. The pressure of a liquid at rest is always perpendicular to the surface in contact with it. The pressure at a point within a liquid is directly proportional to the depth of the point from the free surface, density, and acceleration due gravity.
GAS LAWS
Boyle's law states that the volume (V) of a given mass of gas is inversely proportional to its pressure (P), at constant temperature. Charles's law states that volume of a given mass of gas, at constant pressure, is proportional to its temperature (T). The above two laws can be combined and stated as follows:
PV/T = constant
This is known as the perfect gas equation.
MECHANICS
VELOCITY AND ACCELERATION
Displacement (d) is defined as the shortest distance between the initial and final positions of a body. The velocity (v) of a moving body is the rate of change of displacement of the body in a particular direction and its unit is ms−1. The magnitude of velocity is called speed, which is a scalar quantity. Velocity is a measure of how fast the matter is moving or rate of change of its position with time. It is given by the relation;
v = d/t, where d is the displacement in t seconds.
Acceleration (a) is defined as the rate of change of velocity and its unit is ms−2. It is a measure of how quickly or slowly the velocity 5is changing. If the velocity is constant, the acceleration is zero. It is given by the relation
a = (vf – v0)/t
where, v0 is the initial velocity and vf is the final velocity, that undergone during the time interval t.
SCALAR AND VECTOR QUANTITIES
All physical quantities can be classified into two broad categories, namely, scalar and vector quantities. Quantities that have only magnitude and no direction are called scalar quantities, e.g. length, mass, time, etc. Quantities that have magnitude as well as direction are called vector quantities, e.g. displacement, velocity, force, etc.
A vector quantity is usually represented graphically by an arrow (→), whose length is proportional to the magnitude of the vector. In an equation, vector quantity is represented by bold letters, e.g. F = ma, where, force and acceleration are vectors and mass is a scalar quantity.
FORCE
Force is the influence that changes the state of rest or uniform motion of the body along a straight line. If a force F acts on a body of mass m, and produces an acceleration a, then F = m × a. Hence, the force acting on the body is equal to the product of mass of the body and the acceleration produced by the force on the body.
The SI unit of force is newton and it is denoted by the letter N. One newton is the force acting on a body of mass one kilogram producing an acceleration of one ms−2 in its direction.
WORK
If a force acts on a body and the point of application of the force moves, then work is said to be done by the force. If the force F moves a body through a distance s in its direction, then the work done by the force is given by W = F × s. The displacement does not always take place in the direction of force. If the direction of displacement s is inclined to F at an angle of θ, then the work done,
W = F cos θ × s,
where, F cos θ is the component of force. The SI unit of work is joule (J). One Joule is the amount of work done, when the point of application 6of force of one newton acting on a body, moves it through a distance of one meter in the direction of force.
POWER
The rate of doing work is called power. It is measured by the amount of work done in unit time. If W is the work done in time t, then power P = W/t.
The SI unit of power is joule per second (Js−1). It is also given by a special unit watt, which is equal to 1 joule per second. A larger unit of power is called kilowatt, which is equal to 1000 watt. The unit of electrical energy consumption is kilowatt-hour (kWh). One kilowatt-hour is the power consumed at the rate of 1000 watts for one hour. 1 kWh = 1000 × 60 × 60 = 36,00,000 watt per second = 36,00,000 joules. The older unit of power is horse power (HP), and 1 HP is equal to 746 watts.
ENERGY
The Energy of a body is its ability to do work. It is measured by the amount of work that it can perform. The SI unit of energy is joule. The electron volt (eV) is also used as unit of energy in radiation physics. There are many forms of energy, such as mechanical energy, heat energy, light energy, electrical energy, chemical energy, atomic energy, etc. There are two forms of mechanical energy, viz. potential energy and kinetic energy.
Potential Energy
The potential energy of a body is the energy it possesses by virtue of its position or state of strain, e.g. water stored up in a reservoir, a wound spring, compressed air, etc. For a body of mass ‘m’ remaining at rest at a height h above the ground, the potential energy is equal to the work done in raising the body from the ground to that height.
The work done | = force × displacement = mg × h |
Potential energy | = mgh joule, where ‘g’ is the acceleration due to gravity. |
Worked Example 1.1
A patient of weight 50 kg on a wheel chair has to be lifted onto a examination couch, which is 25 cm higher than wheel chair. Calculate the work done to carry out the above task (g = 9.81 ms−2).7
W = Force × distance
= mg × distance
= 50 × 9.81 × 0.25
= 120 J
The work done in lifting the patient on to the couch needs 120 J energy, which will increase the potential energy of the patient.
Kinetic Energy
The kinetic energy of a body is the energy possessed by the body by virtue of its motion. Let a body of mass m moves with a velocity v, then,
Kinetic energy = (1/2) mv2 joule
Worked Example 1.2
A film cassette of mass 2 kg is kept in a shelf at a height of 1.5 m, possess a potential energy of 25 J. If the cassette falls on to the floor, what will be its speed?
Kinetic energy | = ½ × 2 × v2 |
25 | = ½ × 2 × v2 |
v | = 5 ms−1 |
The cassette may fell on the floor with a speed of 5 ms−1.
MOMENTUM
The momentum (P) of a moving body is the product of mass (m) and velocity (v) and it is given the relation:
P = mv
The momentum is a vector quantity and its direction is the same as its velocity, the unit is kg-ms−1.
TEMPERATURE AND HEAT
Matter is made up of atoms or molecules. These atoms and molecules are in regular movement in solids and random movement in liquids and gases. They possess potential energy as well as kinetic energy. The total energy of the molecules in the system is called as internal energy of the system. The kinetic energy is responsible for the hotness and coldness of the body.
Temperature is the measure of hotness and coldness of the body. When a body is heated, its molecules are in vigorous movement, and 8therefore have high energy, and the body is said to be in high temperature. When a body is cooled lower and lower, its kinetic energy decreases, and the body is said to be in lower temperature. Change of temperature may alter the electrical resistance, conductivity, viscosity and rate of chemical reaction of the substance, e.g. change of body temperature alter metabolism. Temperature is measured in degrees with the help of thermometers. There are three scales of temperature, namely, (i) Celsius scale, (ii) Kelvin scale, and (iii) Fahrenheit scale.
Celsius Scale
In this scale, the temperature of the melting of ice is zero (0°C) and temperature of the boiling water is 100°C. The range between melting point and boiling point is divided into 100 intervals called degrees.
Kelvin Scale
In the Kelvin scale or absolute scale of temperature, 0 degree is named as absolute zero and it is denoted as 0 K. The absolute zero is the temperature at which the molecules will have zero speed. In this scale, the temperature of melting ice is 273.15 K and the temperature of boiling water is 373.15 K. The range between the two is divided into 100 intervals. One interval is the same in both centigrade and Kelvin scale of temperature. The 0 K temperatures is equal to −273°C in Celsius scale. At 0 K, the atomic particles are at rest and hence, it is called absolute zero. It means that the body do not have internal energy at absolute zero.
Fahrenheit Scale
In this scale, the melting ice is at 32°F and boiling water is at 212°F. The entire range is divided into 180 degrees. The body temperature is about 98.4°F equal to 37°C or 310 K. The relation between Celsius and Fahrenheit scale is given by
C/100 = (F – 32) ÷ 180 or 1.8 C = F – 32, or C = (F – 32) ÷ 1.8
Worked Example 1.3
Convert 86° F into degrees of celsius
Here | F = 86 C = (F – 32) ÷ 1.8 = (86–32) ÷ 1.8 = 54 ÷ 1.8 = 30°C. |
HEAT
Heat is a form of internal energy, which can be transferred from one part of the body to another. If a hot body and a cold body are placed 9in close contact, the hot body will transfer some of its heat energy to the cold body until the temperature of the two become equal. The difference in temperature creates temperature gradient. There are three methods of heat transfer, namely, conduction, convection and radiation.
Conduction
It is the process in which heat energy is transferred by collisions between neighboring atoms, without the visible motion of the particles. Conduction takes place in solids, liquids and gases. Let us consider a rod of length L and area A and temperature θ1 and θ2 of at their ends. The rate of flow of heat (dQ/dt) is directly proportional to cross-sectional area (A), temperature gradient (θ1– θ2)/L and thermal conductivity (k) of the material. The thermal conductivity of a material is its inherent ability to conduct thermal energy and it is expressed in Wm−1K−1. The relation for thermal conductivity is given by
dQ/dt = kA (θ1 – θ2)/L
The thermal conductivity of various materials are listed in Table 1.3. Metals in general are good conductors of heat, e.g. silver, copper, etc. Nonmetals are bad conductors of heat, e.g. glass, rubber, wood, etc.
Convection
It is the process in which heat energy is transferred by the actual motion of the particles of the body. Heat in liquid causes the fluid to expand and making it less dense and starts rising. The cold, dense fluid molecules move to their place from other area. Convection takes place in liquids and gases, e.g. trade winds, land and sea breezes.
|
Convection current in air remove heat from X-ray tube housing to the atmosphere. Oil and then water circulation remove heat from large X-ray systems like CT scan. Convection forms the basis for domestic heating system and air-conditioning. Convection may be caused by natural or forced circulation
Radiation
It is the process by which heat energy is transmitted from one place to another without the aid of any material medium. When a body has internal energy, its atoms and molecules vibrate and emits electromagnetic radiation, which can transport energy across a vacuum, e.g. heat reaches the earth from the sun. A black body and matt surface will radiate and absorb energy efficiently, while white and glossy surface will not. Stefan's law states that the rate of heat energy emission (dQ/dt) is directly proportional to the area of the emitting surface (A) and the fourth power of its temperature (T)
dQ/dt ∝ σAT4
where, σ is the Stefan–Boltzmann constant = 5.670 × 10−8 W m−2K−4
The SI unit of heat is joule. However, the special unit calorie is still in use. One calorie is the amount of heat which will raise the temperature of one gram of water by one degree Celsius. 1 calorie = 4.2 joules.
HEAT CAPACITY
The heat capacity of a material is the heat required to raise its temperature by 1 K. It is independent of material size or shape and expressed in JK−1. The heat required to raise temperature of a 1 kg material by 1K is called specific heat capacity, and it is expressed in Jkg−1K−1.
ATOMIC STRUCTURE
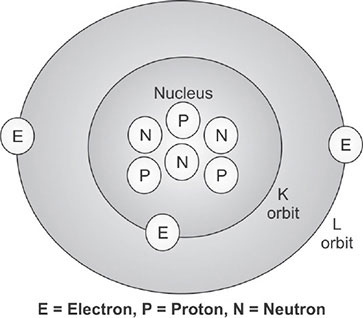
All matter is composed of elements and compounds. Elements are the simplest chemical entity, which cannot be broken further, e.g. hydrogen, carbon. Two or three elements form a compound, e.g. water. The smallest particle of an element is the atom, which forms the fundamental unit of matter. The atoms are very small and its diameter is of the order of 10−10 m. Every atom posses a central core called a nucleus, which is positively charged. The diameter of the nucleus is of the order of 10−14 m (Fig. 1.1).11
The nucleus consists of two particles called protons and neutrons and collectively known as nucleons. The protons are positively charged and the neutron has no charge. The space around the nucleus consists of another important particle, called electron. The electrons are negatively charged particle, and they circulate around the nucleus at varying distances, similar to planets rotation around the sun. The number of electrons in an atom is equal to the number of protons and hence, atom is said to be neutral.
There are two types of forces exist in the nucleus. The electrostatic repulsive force, exist between particles of similar charge. The strong forces (attractive) resulting from the exchange of pions among all nucleons, hold the nucleus together. These two forces act in opposite directions. The nucleus has energy level and the lowest energy state is called the ground state. Nuclei with energy excess of the ground state are said to be in an excited state. Excited states that exists > 10−12 s are referred to as meta stable or isomeric states.
ATOMIC NUMBER AND MASS NUMBER
In 1913, HGJ Mosley stated that the atomic number of an atom is the number of protons in the nucleus. It is also equal to the number of electrons of the atom, which is represented by Z. The mass number of an atom is the total number of protons and neutrons in the nucleus and it is denoted by A. An element (X) is symbolically described as ZXA. The subscript gives the atomic number Z while superscript gives the mass number A. Some of the important elements, their symbol, atomic number and mass number are given in Table 1.4.12
|
EFFECTIVE ATOMIC NUMBER
The effective atomic number (Zeff) is meant for a compound or mixture, which has more than one element. Zeff is the atomic number of an element with which photons interact the same way as with the given composite material. Mayneord has defined the effective atomic number as follows:
Zeff = (a1Z12.94 + a2Z22.94 + …….anZn2.94) 1/2.94
where, a1, a2…… an are the fractional contribution of each element to the total number of electrons in the mixture. The density and effective atomic number of few compounds are given in Table 1.5.
ISOTOPES
The atoms composed of nuclei with the same number of protons but different number of neutrons is called isotopes. In other words, isotopes have the same atomic numbers and different mass numbers, e.g. hydrogen have 3 isotopes, namely:
1H1 have 1 proton (Hydrogen),
1H2 have 1 proton and 1 neutron (Deuterium)
1H3 have 1 proton and 2 neutrons (Tritium).
Isotopes of an element have the same chemical properties but have different physical properties. Isotopes capable of performing radioactivity are called radio-isotopes and their nucleus is said to be unstable.13
|
Nuclides having the same mass numbers but different number of protons are called isobars. Nuclides having same number of neutrons but different number of protons are called isotones. An isomer is the excited state of a nucleus, and it will have same number of proton and neutron.
ELECTRON SHELLS
In 1921, Burry and Bohr independently gave a scheme for the arrangement of electrons in an atom. According to this scheme, the orbits in the atom are named as shells and denoted as K, L, M, N, etc., from the nucleus. The following are the rules of their scheme: The maximum number of electrons in each shell can be obtained from the formula 2n2 where n = 1, 2, 3, 4, etc. In the case of K shell, n = 1, the number of electrons in the K shell = 2 × 12 = 2. In the case of L shell, n = 2, the number of electrons in the L shell = 2 × 22 = 8 and so on. Each shell is provided with subshells, which are denoted as s, p, d, f, etc. The K shell (n = 1) has one subshell, namely, 1s. The L-shell (n = 2), has two subshells, namely, 2s and 2p and so on. One electron in the s subshell of K shell is denoted as 1s1, while 2 electrons in the same subshell is denoted as 1s2.
The outermost orbit is called valence shell, which is responsible for chemical, thermal, optical and electrical properties of the element. No valence shell has more than 8 electrons, e.g. metals have one, two or three valence electrons. The elements are arranged in the periodic table based on the similarities of chemical properties of different elements. As we go across the periodic table the atomic number of the atom increases. The number of electron also increases in the same step.14
QUANTUM NUMBER
The energy level of an electron or position in an atom is described by quantum numbers as follows:
- The principle quantum number (n) defines the main energy level or shell of an orbiting electron. For K shell, n = 1; for L shell, n = 2 and so on.
- The azimuthal quantum number (l) describes the angular momentum of the orbiting electrons. It can have values 0, 1, 2, 3…. n-1, e.g. M shell principal quantum number is 3 and its azimuthal quantum numbers are 3 − 1 = 2, which are 0, 1 or 2.
- The magnetic quantum number (m) describes the spatial orientation of the plane of the orbiting electron and it can have values from − l to + l. When l = 1, m can have −1, 0, + 1 values.
- The spin quantum number (s) describes direction of spin of the electron and it can value + 1/2 (spin up) or −1/2 (spin down).
IONIZATION
Removal of one or more electrons from a neutral atom is called ionization. After ionization, the remainder of the atom is left with positive charge and is known as positive ion. The positive atom and the removed electrons form one ion pair.
BINDING ENERGY
The binding energy of an electron in an atom is the energy required to remove the electron completely from the atom against the attractive force of the positive nucleus. The magnitude of the binding energy depends on the atomic number and the shell from which the electron is being removed. It is greater for elements of higher atomic number and greatest for the K shell (inner most shell).
Binding energies are negative because they represent amounts of energy that must be supplied to remove electrons from atoms. Electron shells are often described in terms of the binding energy of electrons occupying the shells, e.g. the binding energy of hydrogen K shell is − 13.5 eV and − 3.4 eV for L shell. The K-shell binding energies of various elements are given in Table 1.6.
EXCITATION
In an atom, if energy is supplied, the electrons can be moved from the inner orbit to the outer orbit. Now, the atom will have more energy than its normal state.15
|
It is said to be in an excited state and the process is known as excitation. For example, to move an electron from K to L shell of the hydrogen atom, the energy required is (–3.4 eV) – (–13.5 eV) = 10.1 eV.
ELECTRON VOLT
The electron volt (eV) is the unit of energy in radiation physics, where it deals with microscopic objects. One electron volt is the kinetic energy imparted to an electron accelerated across a potential difference of one volt. In practice, we use kiloelectron volt (keV) and million electron volt (MeV) and
1 eV = 1.6 × 10−19 J = 1.6 × 10−12 erg = 4.4 × 10−26 kWh
The electron volt describes potential as well as kinetic energy. The binding energy of an electron in an atom is a form of potential energy and it is expressed in keV.
ELECTROMAGNETIC RADIATION
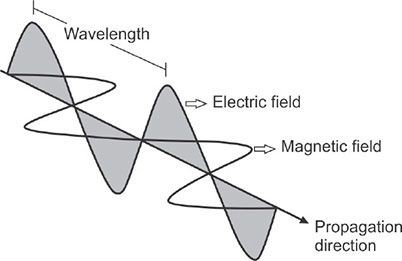
An electric charge is surrounded by an electric field and if the charge moves, a magnetic field is produced. When the charge undergoes an acceleration or deceleration, the magnetic and the electric fields of the charge will vary. The combined variation of the electric and magnetic fields results in loss of energy. The charge radiates this energy in a form known as electromagnetic radiation. The electromagnetic radiation moves in the form of sinusoidal waves (Fig. 1.2). The nature of the electromagnetic radiation (X-rays, ultraviolet, etc.) depens on the way in which the electric charges are disturbed. Electromagnetic radiations 16are transverse waves that transfer energy away from the electric charge. Electromagnetic radiations may be absorbed or scattered in a medium, resulting in loss of energy.
WAVE CHARACTERISTICS
The electromagnetic wave have wavelength (λ), frequency (ν), and velocity (c). The distance between two consecutive positive peaks is known as wavelength. The number of cycles of the wave which pass a fixed point per second is known as the frequency of the wave. The velocity of the wave is the distance traveled per second by the wave. The relation between wavelength, frequency, and velocity of the electromagnetic wave is
c = νλ
All electromagnetic waves, travel at the same velocity in a given medium and its velocity in vacuum is about 2.998 × 108 ms−1. The wavelength of X-rays and gamma rays are in nanometers (nm).
PARTICLE CHARACTERISTICS
Though electromagnetic radiations have the properties of waves, they also behave like particle during interaction with matter. The actual amount of energy (E) carried by a photon is given the equation E = hν, where, h is the Planck's constant = 6.63 × 10−3 4 J. Substituting the value of ν = c/λ in the above equation, the energy
E (keV) = hc/λ = 1.24/λ
where, λ is in nanometer (nm). It is seen that the energy of the photon is inversely proportional to its wavelength and as the wavelength decreases, the energy increases.17
MASS ENERGY EQUIVALENCE
Einstein's theory of relativity states that mass and energy are equivalent and are interchangeable. In any reaction, the sum of the mass and energy must be conserved. Einstein showed that the speed of some nuclear processes approach the speed of light. At these speeds, mass and energy are equivalent.
E = mc2
where, E represents the energy equivalent to mass ‘m’ at rest and ‘c’ is the speed of light in a vacuum. For example, the energy equivalent of an electron of mass 9.109 × 10−31 kg is
E = 9.109 × 10−31 kg × (2.998 × 108 m/s)2
= 0.511 MeV
ELECTROMAGNETIC SPECTRUM
Electromagnetic spectrum includes radiowaves, microwaves, infrared, visible light, ultraviolet, X-rays, gamma rays and cosmic rays (Fig.1.3). All of them travel at a velocity ‘c’ in a vacuum. The wavelength and photon energy of the whole range of electromagnetic radiation are summarized in Table 1.7.
IONIZING RADIATION AND NON-IONIZING RADIATION
Ionization is a process of removal of electron from neutral atom. The radiation which does ionization in a medium, by removal of electron is called ionizing radiation, e.g. UV, X-rays, and gamma rays have sufficient energy to do ionization. As a result, ionized atoms and molecules or ion-pairs are produced. This forms the basis for biological effects of radiation. Radiation that do not have sufficient energy to produce ionization are called non-ionizing radiation, e.g. visible light, infrared, radiowaves, and TV broadcasts, etc.
FLUORESCENCE
When electromagnetic radiation falls on a phosphor, visible or ultraviolet light is emitted from the phosphor and it is called as luminescence. The electromagnetic radiation raises the valence electrons to the conduction band, which return to the valence band to fill up the holes. As electron falls through the luminescence centers, they emit the surplus energy in the form of flashes of light, called luminescence. If the luminescence is instantaneous, within 10−8 s, it is called fluorescence.18
|
The energy of light emitted depends on the difference in energy across the luminescence centers. It is always less than the energy which originally stimulated the fluorescence, e.g. a phosphor exposed to ultraviolet may emits visible light. Fluorescent phosphors, such as thallium activated sodium iodide (NaI:Tl, gamma camera), terbium activated gadolinium oxysulfide (intensifying screen) and sodium activated cesium iodide (image intensifier) are used in diagnostic radiology.
If the emission of light is delayed beyond 10−8 s, it is called phosphorescence. When the valence electrons are stimulated, they get trapped in the conduction band. They acquire energy from the atom (internal energy) and return to the valence band by emitting luminescence. It is a random process, which takes time to accomplish. The emission of light decays exponentially with a time constant, that depends upon the temperature of the phosphor.19
INVERSE SQUARE LAW
The intensity of electromagnetic radiation is inversely proportional to the square of the distance from its source. Let us consider a point source ‘s’, emitting radiation at constant rate. The radiation spread over the inner surface of an imaginary sphere of radius d with surface area 4πd2. Then the radiation intensity at a point ‘d’ is given by the relation
I ∝ 1/d2
The inverse square law is based on the following assumptions:
- The source of radiation is a point source.
- The radiation travels in straight lines.
- The radiation is emitted equally in all directions.
- The energy is radiated at a constant rate.
- No radiation energy is lost on its way from the source to the point of measurement.
Let 100 mR be the radiation exposure at 1 m for a point source (Fig. 1.4). The radiation exposure at 2 m is found to be 25 mR, by inverse square law. Hence, if distance is doubled, the radiation is reduced by a factor of 4. Keeping higher distance always reduce radiation exposure.
RADIOLOGICAL MATHEMATICS
LOGARITHMS
The logarithm of a decimal number is the exponent to which the base must be raised to produce the number. For example, the logarithm of 1000 to base 10 is 3, because 1000 is 10 to the power 3: 1000 = 103 = 10 × 10 × 10. More generally, if x = by, then y is the logarithm of x to base b, and is written as logb(x), so log10(1000) = 3. There are three types of logarithms, namely, common logarithm (log10), natural logarithm (loge), and binary logarithms (log2), where ‘e’ = 2.71828, e.g. log102 =0.301, the base 10 must raised to power of 0.301, 100.301= 2. 20Similarly, loge 2 = 0.693, the base ‘e’ must be raised to power of 0.693, e0.693 = 2.
The measurements of optical density and sound intensity are expressed in logarithm to base 10. Radioactive decay, and X-ray attenuation uses logarithm of base ‘e’, which is denoted by lne (natural logarithm). Logarithmic scales reduce wide-ranging quantities to smaller scopes. Logarithm is useful to describe many radiation events such as X-ray absorption, radioactive decay, etc.
GRAPHS
Graph gives the relationship between physical quantities, plotted as series of points or lines with reference to the set of axis. A Cartesian graph has two axis, namely, ‘x’ axis called abscissa and ‘y’ axis called ordinate. The x axis contains independent variable (time, distance) and the ‘y’ axis contains dependent variable (velocity, exposure).
If a physical quantity ‘y’ varies with ‘x’ in a proportional way, then a linear plot can be drawn. It is straight line graph obeying the equation
y = mx + c
where, m is the slope of the line and c is the intersection with the y axis.
Logarithmic functions such as ex and e-x can also be plotted as curve, where a rapid increase or rapid decrease may be seen. A semi-log graph is a way of visualizing such data that are changing with an exponential relationship. One axis is plotted on a logarithmic scale and the other in linear scale. On a semi-log graph the spacing of the scale on the y-axis is proportional to the logarithm of the number, not the number itself. It is equivalent to converting the Y values to their log, and plotting the data on linear (lin-lin) scales. The term log-lin is used to describe a semi-log plot with a logarithmic scale on the y-axis, and a linear scale on the x-axis (Fig.1.5).
This kind of plot is useful when one of the variables being plotted covers a large range of values and the other has only a restricted range. The advantage being that it can bring out features in the data that would not easily be seen if both variables had been plotted linearly. Semi-log plot requires only few measurements of the exponential function.
TRIGONOMETRY
Trigonometry is a mathematics which deals with triangles and the relation between angle and sides (Fig.1.6).21
If one angle of a triangle is 90 degrees and the other angle is known, then the third angle can be obtained easily. Since the sum of the angles is 180 degrees, the two acute angles therefore added up to 90 degrees, they are said to be complementary angles. The shape of a triangle is determined by the angles. Once the angles are known, the ratios of the sides can be determined, regardless of the overall size of the triangle. If the length of one of the sides is known, the other two can be determined. These ratios are given by the following trigonometric functions of the known angle A, where a, b and c refer to the lengths of the sides in the accompanying figure:
Sine function (sin), defined as the ratio of the opposite side to the hypotenuse.
Tangent function (tan), defined as the ratio of the opposite side to the adjacent leg.
The hypotenuse is the side opposite to the 90 degrees angle in a right triangle; it is the longest side of the triangle, and one of the two sides adjacent to angle A. The adjacent leg is the other side that is adjacent to angle A. The opposite side is the side that is opposite to angle A. The terms perpendicular and base are sometimes used for the opposite and adjacent sides, respectively.
STATISTICS
Source of errors: There are three types of errors in measurements, namely, systemic error, random error and blunder. Systemic error occurs when measurements differ from the correct values in a systemic fashion. Random error is caused by random fluctuations in the measurement process itself. The processes by which radiation is emitted and by which radiation interacts with matter are random in nature. Therefore, all radiation measurements are subject to random error. The counting statistics helps us to judge the validity of measurements.
Accuracy and precision: If a measurement is close to the correct value, it is said to be accurate. If measurements are reproducible, they are said to be precise. Precision does not imply accuracy. If a set of measurements differ from the correct value in a systematic fashion, the data are said to be biased.
Mean, median and standard deviation
The mean is the arithmetic average of a group of data. The mean (x) of a set of measurements is defined as
where, N is the number of measurements. The median is measure of the central tendency and is the value that separates the data in half and defines the 50%. It is the middle most measurement, if the number of measurements is odd. It is the average of the two middle 23most measurements, if the number measurements are even. For example, the median of the five measurements 5, 8, 9, 12 and 14 is 9.
The variance (σ2) and standard deviation (σ) are measures of the variability of a set of measurements. The standard deviation is used to describe the spread of a data set and is the square root of the average of the square of all the sample deviations. The variance is determined from a set of measurements as follows
where, N is the total number of measurements and x is the sample mean. The standard deviation is the square root of the variance,
s = √σ2
When samples are taken from a large population, there is uncertainty between the sample mean and the actual population mean. This is measured by the standard error, given by the relation
Standard error = σ/√N
The coefficient of variation (CV) is a measure of spread within the samples, given in percentage. It is given by the relation
CV = (σ / x)100
where, σ / x is the fractional error in the measurements.