INTRODUCTION
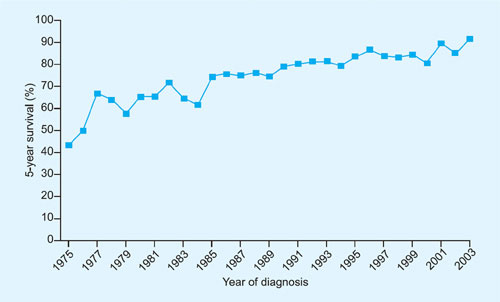
Treatment of acute lymphoblastic leukemia (ALL) represents one of the triumphs of modern oncologic chemotherapy. Once a uniformly fatal cancer, childhood ALL currently has around a 90% cure rate and majority of these children can be expected to lead long and productive lives (Figure 1-1).1 This remarkable success can be attributed to innovative drug design, rational combination of chemotherapeutic agents, astute clinical observations, and systematic performance of large clinical trials. These results become even more impressive when one considers the fact that most of this success was achieved before molecular pathogenesis of ALL was delineated using modern laboratory techniques.
Figure 1-1: Five-year relative survival of patients with acute lymphoblastic leukemia diagnosed before 20 years of age (1975–2003).
DEVELOPMENT OF MODERN CHEMOTHERAPEUTIC AGENTS
A brief history of the development of agents that form the backbone of modern chemotherapy for ALL follows.
Methotrexate
Observations in folic acid deficiency as well as “acceleration” of acute leukemia by folic acid conjugate therapy led Sidney Farber, a pathologist at Children's Hospital in Boston to postulate that folic acid antagonists would be effective in acute leukemia.2
A true pioneer in cancer chemotherapy, Yellapragada Subbarow, already an accomplished biochemist at Harvard University had moved to Lederle Laboratories (a division of American Cynamid Company) in Pearl River, New York after he was denied a permanent appointment at Harvard. At Lederle, Subbarow developed a method to synthesize folic acid following which he worked on various folic acid conjugates. He provided Drs Farber and Louis Diamond with adequate quantities of various folate antagonists including 4-aminopteroyl glutamic acid (aminopterin) for clinical testing.2,3 During the period from November 1947 to April 1948, these investigators treated 16 children with acute leukemia with various folate antagonists including aminopterin. Ten of these patients showed “clinical, hematologic, and pathological evidence of improvement” to aminopterin, a remarkable result at a time when no effective therapy existed and even temporary remissions were unheard of. Farber, Diamond, and colleagues published the details of five of the responders in a seminal publication in the New England Journal of Medicine on June 3, 1948.4 Probably unaware of the magnitude of their contribution to ALL chemotherapy, they were cautious in interpreting their results and made it clear in the paper that these remissions were “temporary” and did not represent a “cure”. It is a testament to Dr Farber's conviction in his ideas that he, a pathologist turned clinician persevered at a time when there was nihilism among clinicians about the value of treating pediatric leukemia as well as skepticism from his colleagues of the value of his findings. His work laid the foundation for specialized care for pediatric leukemia and led to the establishment of Children's Cancer Research Foundation which would later become the world renowned Dana Farber Cancer Institute.2
Eventually, the less toxic folate antagonist methotrexate (amethopterin, MTX) replaced aminopterin as a mainstay of ALL therapy. Subsequently, intrathecal administration of MTX was found to be effective in treating central nervous system (CNS) involvement of ALL.5,6
6-Mercaptopurine
Since around 1942, George Hitchings and co-workers at Wellcome Research Laboratories had been systematically studying the structure of purines and 3pyrimidines. Based on the hypothesis that malignant cells were different from normal cells in utilizing purines and pyrimidines for DNA synthesis, they synthesized various analogs of these compounds as potential chemotherapeutic agents. Research into antimetabolites had received a boost by the publication by Farber et al. on the activity of MTX. In collaboration with Hitchings’ group, investigators at Sloan Kettering Institute (now Memorial Sloan Kettering Cancer Center) in New York initiated testing of various purine and pyrimidine analogs in mouse models of leukemia as well as patients. Of the various compounds, 6-mercaptopurine (6-MP) was found to be the most active in a screening program using transplanted mouse tumors.7
In a landmark paper published in 1953, Burchenal et al. reported results of treating various cancers including acute leukemia with 6-MP. The majority of patients in this study were children with acute leukemia. These children had the best responses to 6-MP with 15 of 45 achieving “hematologic remissions” including some who were resistant to folic acid antagonists. These investigators were particularly surprised by the lack of non-hematologic toxicity especially when 6-MP was used to treat childhood acute leukemia.8 The pronounced antileukemic activity of 6-MP in ALL would soon be confirmed by other investigators (see below under Early Clinical Trials). It was also noted later that the antileukemic activity of different nucleoside analogs against acute leukemia was markedly different with agents like 6-azauracil showing no benefit.9 Hitchings, Elion, and Black were awarded the Nobel Prize in 1988 for “discoveries of important principles for drug treatment”.
Corticosteroids
The laboratory observations of Dougherty and White that increased adrenal cortical function in animals led to involution of lymphoid tissues10 as well as observation by Heilman and Kendall that lymphoid tumors in mice regressed after administration of adrenal steroids11 led Pearson and colleagues at Sloan Kettering Institute to investigate its activity in lymphoid tumors in man in the late 1940s. They observed regression of lymphadenopathy in patients with lymphoid tumors as well as clinical and hematologic remissions in patients with acute leukemia.12 Subsequently, in 1954, Fessas, Wintrobe, and colleagues published their experience with cortisone therapy in acute leukemia. Remarkably, among 22 children with ALL aged below 10 years, a complete remission was observed in 18 after cortisone therapy. These investigators made the definite conclusion that corticosteroid therapy only benefited acute leukemias of the lymphoid lineage and emphasized the importance of differentiating acute lymphoid from acute myeloid leukemia based on careful morphologic examination and cytochemical staining. They also recognized the need for objective criteria for treatment response and defined “complete remission,” principles that would be fundamental for later ALL studies.134
Vinca Alkaloids
The periwinkle plant had enjoyed a reputation as an oral hypoglycemic agent. While investigating its hypoglycemic properties, investigators at Lilly laboratories noticed survival prolongation in mice implanted with the ALL P-1534. Subsequently, various alkaloids active against this leukemia model including vinblastine and vincristine were characterized by Svoboda and colleagues at Lilly. Investigators at Lilly Clinic initially noticed responses to vincristine at low doses in lymphoid malignancies.14 Subsequently, larger doses were tested in childhood acute leukemia by Karon and colleagues at the National Cancer Institute with impressive results. In a report published in 1962, they reported complete remissions after vincristine therapy in at least 7 of 13 children who had previously failed 6-MP and MTX therapy.15 Unlike vincristine, the related vinca alkaloid vinblastine had minimal activity in acute leukemia.
EARLY CLINICAL TRIALS IN ALL
Once multiple agents with potent antileukemic activity against ALL were available, the need to conduct systematic clinical trials in a multicenter fashion was quickly recognized. These efforts were pioneered by the group of investigators at the Clinical Center of the National Cancer Institute (NCI) in Bethesda, Maryland led by Emil Frei III and Emil Freireich who conducted a series of clinical trials that would lay the foundation for modern clinical trials in oncology. Their earliest combination trial done in collaboration with Dr James Holland at Roswell Park Memorial Institute in Buffalo, New York and published in 1958 (Protocol 1) was a randomized study to test the effect of combining the two most active chemotherapeutic agents at that time, namely MTX and 6-MP. This study tested two dosing schedules of MTX (intermittent versus continuous) combined with 6-MP and concluded that these schedules were not significantly different.16
Soon other institutions would join this group which was named Acute Leukemia Cooperative Group B (ALGB), a precursor of the modern cooperative group Cancer and Leukemia Group B (CALGB).17,18 In one of their earliest trials (Protocol 2), this group conducted a 318 patient trial from 13 institutions and conclusively proved that treatment with 6-MP and MTX together produced higher rate of complete remission than either agent alone.19 The era of large cooperative group studies in acute leukemia had begun.
These early trials were limited in their use of modern statistical techniques. For the next trial (Protocol 3), however, the group with the assistance of statistician Edmund Gehan applied novel statistical techniques to the trial design.17 This was a trial comparing 6-MP to placebo maintenance in patients who had achieved a complete remission with corticosteroids alone. Patients in complete remission were randomly assigned to 6-MP or placebo in a double-blind fashion and patients on placebo 5could cross over to 6-MP if they had a relapse. The duration of remission between the arms was compared using a restricted sequential procedure where patients at each institution were paired and randomly assigned one or the other therapy followed by assessment of preference for 6-MP or placebo based on remission duration. The trial had to be stopped early when a clear preference for 6-MP maintenance was noticed. After enrolment of 21 patient pairs, the median duration of remission was 33 weeks for 6-MP versus 9 weeks for placebo.20 The statistical analytical techniques used in this study were truly novel at that time and in many ways this trial embodied much of the principles of a modern double-blind, randomized clinical trial.17
Drug screening in the 1950s was aided in large measure by the development of the carcinogen induced murine leukemia model L1210 by Lloyd Law at NCI which became the primary screening model for acute leukemia.21 Cyclophosphamide was found to be active in ALL by Donald Fernbach and colleagues.22 Subsequently, trials of multiagent combination chemotherapy would be conducted and were found to yield remissions of durations not achieved in ALL thus far. Notable in this regard are the NCI VAMP [vincristine, amethopterin (methotrexate), mercaptopurine, and prednisone) regimen and CALGB protocol 6313 (13th protocol of 1963) consisting of same agents as in VAMP plus cyclophosphamide and carmustine, both of which tested combination therapy with active agents available at that time.23,24 These regimens were formulated based on the fundamental principles of combination chemotherapy, i.e., combining active agents with different mechanisms of action and non-overlapping toxicity. Development of combination regimens was also helped by the studies of Howard Skipper, a mathematical biologist at Southern Research Institute in Birmingham, Alabama who formulated the “cell kill” hypothesis based on kinetics of the L1210 mouse model.23,25
Based on outcomes of the various clinical trials done thus far, Donald Pinkel at St. Jude Children's Research Hospital in Memphis, Tennessee introduced the concept of “Total Therapy” consisting of phases of induction, consolidation, CNS directed therapy, and maintenance, of all which form the cornerstones of modern ALL regimens. By around 1965, this therapy was yielding survivals of 5 years or more in a significant number of treated children and “cure” in ALL had become a reality.26,27 L-asparaginase was subsequently found to be active in ALL and added to pediatric ALL regimens (see chapter 8 for detailed history of its development). Over the next 4 decades or so, various cooperative groups including the Berlin-Frankfurt-Munster group formed in 1970 would obtain incremental improvements in survival by modifications of the concept of total therapy and achieve astounding success in the treatment of childhood ALL. It is important to note that the only major drug development during this period was the introduction of tyrosine kinase inhibitors which improved the outcome of Philadelphia chromosome positive ALL. The survival improvements in adult ALL have been very modest and painfully slow with 6long-term survival rates only around 50% at the present time. Various reasons have been attributed to this disparity between adults and children and are discussed in Chapter 7. Cooperative group trials also have made therapy better tolerated with less long-term side effects; the elimination of prophylactic CNS radiation is most notable in this regard.
IMMUNOTHERAPY FOR ALL
At a time when chemotherapy was making deep inroads into the therapy of ALL, Georges Mathé, initially at Institute Gustav Roussy and Hôpital Paul-Brousse in Villejuif, France took the unconventional approach of testing various forms of immunotherapy including adoptive immunotherapy for treatment of ALL in the late 1950s and 1960s. Mathé had observed antileukemic effect of allogeneic marrow grafted into mice with transplanted or spontaneous leukemia including virally induced leukemia. Another premise for trying immunotherapy was that some cases of childhood leukemia may have a viral origin and may be controlled by an immune response against viral antigens. This was based on Mathé's earlier observation in mice with leukemia induced by Charlotte Friend's virus that both the leukemia as well as the viral infection was controlled by grafting allogeneic bone marrow.28
E Donnall Thomas at University of Washington, Seattle who would later receive the Nobel prize for his pioneering work on bone marrow transplantation had done extensive studies in mice and dogs to define radiation doses for conditioning. In 1959, Thomas and colleagues performed syngeneic bone marrow transplants in two children with ALL who were conditioned with total body radiation. Although engraftment was achieved, both patients would succumb to disease relapse.29 Mathé is credited with successfully performing the first sibling bone marrow transplant for acute leukemia in April 1963. This patient, a 26-year-old physician with refractory ALL was conditioned with total body irradiation and infused with bone marrow from six donors including four siblings and both parents. Hematopoietic engraftment from one of the brothers was demonstrated and so was tolerance to that donor. This patient remained in remission until December 1964 when he died of complications from herpetic encephalitis.30 Although Mathé and colleagues had performed allogeneic bone marrow transplants in at least 21 ALL patients by 1965, there were very few long-term survivors with most patients succumbing to infections or graft-versus-host disease, the latter complication was well characterized in their reports and referred to as “secondary syndrome”.30,31 With knowledge of the genetics of human leukocyte antigens (HLAs) as well as refinements in HLA typing, developments in immunosuppression, and supportive care, allogeneic hematopoietic stem cell transplantation (HSCT) would become a widely used and effective therapy for ALL (see chapter 12).7
Mathé and colleagues also tested other forms of immunotherapy in ALL including Bacillus Calmette-Guérin (BCG), irradiated allogeneic lymphoblasts, and even infusion of leukocytes collected by leukapheresis from patients with chronic myeloid leukemia.28,32 In one controlled trial, they showed prolongation of remission duration by immunization with BCG or irradiated leukemic cells.28 These therapies were clearly innovative at that time. However, interest in immunotherapy other than hematopoietic stem cell transplantation waned due to the high efficacy of chemotherapy in childhood ALL, lack of adequate technology, as well as the belief that ALL was not amenable to immune mediated therapy, probably based on the lack of efficacy of donor lymphocyte infusions in inducing remissions in patients who had relapsed after allogeneic HSCT. This was generally perceived as evidence for lack of potent graft versus leukemia effect in ALL.
THE FUTURE
“Prediction is very difficult, especially about the future.”
—Niels Bohr
After decades of poor results with intensification of conventional chemotherapy in adult and encouraged by the success of tyrosine kinase inhibitors in Philadelphia chromosome-positive ALL, it was thought that agents targeting aberrant molecular pathways would provide the much awaited breakthroughs needed to improve results in adult ALL. Therefore, it came as tremendous surprise that two immune mediated therapies would hold the greatest promise for the future of ALL therapy, particularly in adults. These agents, namely, bispecific T-cell engaging (BiTE) antibodies and chimeric antigen receptor (CAR) modified T-cells targeting the CD19 antigen are highly active and have yielded unprecedented remission rates in relapsed ALL (discussed in detail in chapter 14). These agents have the potential to change the landscape of adult ALL therapy and salvage patients who have relapsed after chemotherapy or allogeneic hematopoietic stem cell transplantation.
Much progress needs to be made in adults to achieve results similar to childhood ALL. Paradoxically, in children, the current focus is to reduce or tailor therapy especially in good risk patients in order to minimize deleterious effects of therapy on a growing population of childhood ALL survivors. Assessment of minimal residual disease status and pharmacogenetic factors would be expected to allow such modifications of therapy (discussed in chapters 9 and 11, respectively).
The early history of ALL therapy is a remarkable story of successful collaboration between academia and industry as well as collaboration among clinical investigators to efficiently perform meaningful cooperative group clinical trials. Both of these aspects of clinical research have been the subject of much criticism recently. One can only hope that the current decade will see progress in adult ALL at a pace that is reminiscent of progress in childhood ALL therapy made in the 1950s.8
REFERENCES
- Diller L. Clinical practice. Adult primary care after childhood acute lymphoblastic leukemia. N Engl J Med. 2011;365:1417–24.
- Miller DR. A tribute to Sidney Farber—the father of modern chemotherapy. Br J Haematol. 2006;134:20–6.
- Hutchings BL, Mowat JH, Oleson JJ, et al. Pteroylaspartic acid, an antagonist for pteroyl glutamic acid. J Biol Chem. 1947;170:323–8.
- Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787–93.
- Whiteside JA, Philips FS, Dargeon HW, et al. Intrathecal amethopterin in neurological manifestations of leukemia. AMA Arch Intern Med. 1958;101:279–85.
- Hyman CB, Bogle JM, Brubaker CA, et al. Central nervous system involvement by leukemia in children. II. Therapy with intrathecal methotrexate. Blood. 1965;25:13–22.
- Clarke DA, Philips FS, Sternberg SS, et al. 6-mercaptopurine: effects on mouse sarcoma 180 and in normal animals. Cancer Res. 1953;13:593–604.
- Burchenal JH, Murphy ML, Ellison RR, et al. Clinical evaluation of a new antimetabolite, 6-mercaptopurine in the treatment of leukemia and allied diseases. Blood. 1953;8:965–99.
- Freireich EJ, Frei III E, Holland JF, et al. Evaluation of a new chemotherapeutic agent in patients with “Advanced Refractory” acute leukemia. Studies of 6-azauracil. Blood. 1960; 16:1268–78.
- Dougherty TF, White A. Effect of pituitary adrenotropic hormone on lymphoid tissue. Proc Soc Exp Biol Med. 1943;53:132–3.
- Hellman FR, Kendall EC. The influence of 11-dehydro-17-hydroxycorticosterone (compound E) on the growth of a malignant tumor in the mouse. Endocrinology. 1944;34:416–20.
- Pearson OH, Eliel LP, Rawson RW, et al. Adrenocorticotropic hormone- and cortisone-induced regression of lymphoid tumors in man: a preliminary report. Cancer. 1949;2:943–5.
- Fessas P, Wintrobe MM, Thompson RB, et al. Treatment of acute leukemia with cortisone and corticotropin. AMA Arch Intern Med. 1954;94:384–401.
- Johnson IS, Armstrong JG, Gorman M, et al. The vinca alkaloids: a new class of oncolytic agents. Cancer Res. 1963;23:1390–427.
- Karon MR, Freireich EJ, Frei III E. A preliminary report on vincristine sulfate-a new active agent for the treatment of acute leukemia. Pediatrics. 1962;30:791–6.
- Frei E III, Holland JF, Schneiderman MA, et al. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood. 1958;13:1126–48.
- Gehan EA, Freireich EJ. The 6-MP versus placebo clinical trial. Clin Trials. 2011;8:288–97.
- Keating P, Cambrosio A. From screening to clinical research: the cure of leukemia and the early development of the cooperative oncology groups, 1955–1966. Bull Hist Med. 2002;76:299–334.
- Frei E III, Freireich EJ, Gehan E, et al. Studies of sequential and combination antimetabolite therapy in acute leukemia: 6-mercaptopurine and methotrexate. Blood. 1961;18:431–54.
- Freireich EJ, Gehan E, Frei III E, et al. The effect of 6-mercaptopurine on the duration of steroid-induced remissions in acute leukemia: a model for evaluation of other potentially useful therapy. Blood. 1963;21:699–716.
- Law LW, Dunn TB, Boyle PJ, et al. Observations on the effect of a folic acid antagonist on transplantable lymphoid leukemias in mice. J Natl Cancer Inst. 1949;10:179–92.
- Fernbach DJ, Sutow WW, Thurman WG, et al. Clinical evaluation of cyclophosphamide. A new agent for the treatment of children with acute leukemia. JAMA. 1962;182;30–7.
- DeVita VT, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–53.
- Larson RA, Stone RM, Mayer RJ, et al. Fifty years of clinical research by the leukemia committee of the Cancer and Leukemia Group B. Clin Cancer Res. 2006;12:3556s–63s.
- Pinkel D. Five-year follow up of “total therapy” of childhood lymphocytic leukemia. JAMA. 1971;216:648–52.
- Pinkel D. Total Therapy of acute lymphoblastic leukemia. JAMA. 1972;222:1170.
- Mathé G, Amiel JL, Schwarzenberg L, et al. Active immunotherapy for acute lymphoblastic leukemia. Lancet. 1969;1:697–9.
- Thomas ED, Lochte HL, Cannon JH, et al. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–16.
- Mathé G, Amiel JL, Schwarzenberg L, et al. Successful allogenic bone marrow transplantation in man: chimerism, induced specific tolerance and possible antileukemia effects. Blood. 1965;25:179–96.
- Mathé G, Amiel JL, Schwarzenberg L, et al. Adoptive immunotherapy of acute leukemia: experimental and clinical results. Cancer Res. 1965;25:179–96.
- Schwarzenberg L, Mathé G, Schneider M, et al. Attempted adoptive immunotherapy of acute leukemia by leucocyte transfusions. Lancet. 1966;2:365–8.