INTRODUCTION
Our planet is about 4.5 billion years old. Life has been around since the Archean era, some 3.8 billion years ago. The great dinosaurs ruled in the Triassic, Jurassic, and Cretaceous Periods from 248 to 65 million years. Our furry mammalian kin crept in during the demise of the dinosaurs and came to prominence in the Paleocene epoch. Some 60 million years ago, our own siblings, members of the order Primates (the “Firsts”) crept quietly along their little branches or out of their nocturnal caves as mammals came to prominence. From these relatives a host of lemurs, monkeys, apes, and eventually our ilk, came to the fore. Depending on how you define us, our own species, Homo sapiens, arrived maybe a few hundred thousand years ago. We are, literally, a blink in the timeline of our planet's history.
Since we, Homo sapiens, arrived, however, we have become the unquestioned masters of this world. Yes, it was tricky at first, as we possessed neither the great strength of mammoths nor the speed of gazelles. We lacked distinctive dentition, such as massive canines, that could frighten away competitors. We had no claws or even a quill or two. We could not swim very well. We could not climb very well. Neither our vision nor our hearing was particularly impressive. Physically, by mammalian standards, we just “were not all that”, as our kids would say today.
We did accrue, however, something very special along the path of our evolution: a remarkable ability that no other animal on our planet either now or in our long history has had. We have a unique voice – and the ability to use that voice to produce articulate speech. Together, this duo has enabled the panoply of our multilinguistic abilities and has given us our unique, species-specific, language. With these, we have dominated our planet.
WHAT ARE VOICE, SPEECH, AND LANGUAGE?
These are topics for treatises by philosophes, linguists, and others of deep thought. They clearly overlap in meaning, and one can argue until the next phase of our evolution regarding how such terms may or may not apply to different species. Do bees have language? Can a dolphin speak? Does a parrot have cognitive abilities? What is a cat “thinking” when it looks at the moon? It is, however, necessary to have general, working, definitions for otolaryngologists so we are on the same page. Such is offered below.
Voice, sometimes called “vocalization”, usually refers to sounds that are produced at the laryngeal vocal folds, mostly through air from the lungs. In humans, vocalizations comprise the fundamental components of speech (vide infra), but not all vocal sounds are part of the speech spectrum. Indeed, we utter many involuntary sounds—from coughs to an infant's babble—that are generated at the vocal folds but not morphed into articulated sounds of speech. “Phonation” is the term that describes the production of the voice via vocal fold modifications. The essence of voice is, thus, its tie to vocal fold anatomy, physiology, and neuromuscular control. Of course, “voice” has also come to have a variety of societal, literary, and even philosophical meanings that go far beyond the confines of the larynx.14
Speech is more complex than voice to define. For humans, we can consider speech as the verbal–vocal communication system regularly used by all living people. By this definition nonhuman species would thus not have speech. As many species arguably have advanced vocal systems, they are often referred to as having “speech”, e.g. “ape-speech” or “the speech of prehumans”. Accordingly, what we produce is often given the modifier, “human”, and our speech indicated as “human speech” to distinguish what we do from other vocal utterances. For our purposes here, speech will be defined as what we do, and other species vocal communication considered and referred to as just that, vocal communication. Whales and apes may call to complain (please leave a vocal message if you can).
Returning to our species, speech is the product of both the central (brain) and peripheral nervous systems and the aerodigestive tract (ADT), particularly that component of it known as the “vocal tract”. Of particular interest to the otolaryngologist is the supralaryngeal component of the vocal tract, the region that is responsible for the modification of the fundamental sounds (voice) produced at the vocal folds. As will be emphasized below, it is important to always keep in mind that there is not an anatomically or physiologically separate “vocal tract” that functions solely for this purpose, rather the components are inherent parts of a complex, multifunctional ADT that evolved for primary purposes other than speech (a point often overlooked by our linguistic colleagues).
Lastly, there is language, the most complex concept of them all.2,3 To define language in a few words would do it injustice, but as a working definition for us we can view it as the global expression of human communication either spoken, written, or gestural, consisting of words in a structured, ordered, and conventional manner. Inherent components of human language include brain (mental) functions of cognition, grammar, and syntax, among others. The crucial peripheral components of the input systems (i.e. auditory, visual) and output (voice, speech) are, obviously, inextricable elements.
THE ANATOMY OF VOICE AND SPEECH PRODUCTION: PART OF A LARGER SYSTEM WITH A LARGER ROLE
What is often lost in discussions of voice or speech—or the conceptualizations regarding how these abilities arose in humans or our relatives – is that the anatomical mechanisms for sound generation and/or modification did not evolve for the purposes of making or modifying sounds. While the larynx – the central element in sound generation – is better known to those outside the medical world by its pseudonym, “voice box”, it emphatically did not come on the scene so that we could chat about the weather. That majestic conglomeration of cartilages, membranes, nerves, blood vessels, and an assortment of internal plica came about for three primary biophysiological functions: (1) regulation of air to and from the lungs, (2) protection of the airway, and (3) maintenance of intrathoracic and intra-abdominal pressure. That is the “Big Three”, and there are NO more important baseline functions for our body. We are air-breathing mammals and control, regulation, and protection of our airway is paramount.4,5
THE LARYNX: THE HOLY TEMPLE OF OUR BODY
The larynx is the guardian of our lungs and thus has evolved as a special and protected structure. Although we have ascended the evolutionary ladder, the primary function of our larynx remains true to its origins: it is still essentially a valve, regulating and guarding the airway. As mammals morphed from their amphibian and reptilian ancestors their larynx also accrued added importance in new activities such as effectuating intra-abdominal pressure control during the transition from egg laying to birthing, and control of intrathoracic stabilization as movement of the upper limb required rib stabilization during climbing. Although the larynx of diverse mammalian species shares many homologous components, the specifics of structure for larynges of species that inhabit often vastly differing environments have been modified extensively during the course of evolution.5–8
The anatomical mechanisms for these controls and protections are the internal membranes, their folds, and controlling intrinsic muscles. While a detailed histology of the folds is beyond the scope of this essay, the basic composition of the structure has been divided by Hirano9 into five layers: squamous epithelium, superficial lamina propria (SLP), intermediate lamina propria (ILP), deep lamina propria (DLP), and vocalis muscle. These layers essentially function in mechanically “decoupled” groupings of the layers to form: the “Cover” or mucosa (epithelium and SLP); the “Transition area”, the vocal ligament itself (ILP and DLP); and the “Body” of the fold produced by the vocalis muscle.
Although comparative and descriptive histology of the larynx, in general, and vocal folds, in particular, have5 been done, many aspects still remain unclear, particularly as concerns the direct relationship to functions.6 Indeed, the relatively new field of “Voice Science”, and how specific structures/layers of the folds directly relate to normal or abnormal function, and medical or surgical treatment is in its nascent stages. Furthermore, what remains largely unstudied and unknown is how the different compartments of the vocal folds morphed and changed over the millennia to accommodate the evolutionary forces compelling their protective and pressure control roles.
While the initial roles of the larynx and its internal membranes of mammals, in general, and primates (prosimians, monkeys, apes, humans, and all of their ancestors), in particular, were sphincteric and protective, phonatory importance may well have played a part, though to what extent is unclear. Certainly, as we came to a hominid (direct human ancestors) grade of evolution, phonation/vocal communication, and even singing (as an important part of social communication) would have begun to take on increasing importance. Such importance would have had a selective advantage in evolutionary terms, working to select for vocal fold histology in ways conducive to sound production, distinct, and with possibly greater ranges than our closest relatives. Some studies are already pointing to this. For example, only humans seem to possess a deep layer of the lamina propria, which may account for species-level differences between humans and our nonhuman primate relatives.10 Along similar lines, it has been shown that the human thyroarytenoid muscle (the muscle within the body of the vocal folds; the most medial fibers are often named the “vocalis”) may uniquely exhibit slow tonic muscle fibers with rare contraction properties, such as contractions that are prolonged, stable, precisely controlled, and fatigue resistant. Such properties have been suggested to be a unique specialization (called an “autapomorphy”, or uniquely derived characteristic, in anthropological terms) that may underlie our unique “voice” and help effectuate speech.11
It should be noted that while our field is collectively trying to identify that which is special or comparatively distinct about the anatomy/histology of human vocal folds, there remains much we do not know about developmental changes in this regard. Indeed, we humans go through a remarkable developmental scenario, particularly in the changes to the aerodigestive region (vide infra). Corresponding, and perhaps cotemporaneous, developmental changes may well occur in the internal laryngeal environment. Indeed, recent studies have shown that significant histological differences may exist in vocal fold development, for example, the deep layer of the lamina propria is not present at birth and not fully developed until the age of 11 or 12 years.10,12 While the functional and clinical implications of such observations are not yet fully clear, these may well have major implications for laryngeal functions, ranging from protection to voice production and speech attainment.
SETTING THE STAGE: THE AERODIGESTIVE TRACT AMONG MAMMALS
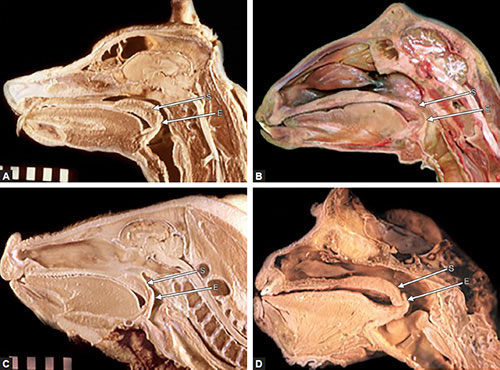
While mammals exhibit great variation in body plan, the general template for their throat regions is remarkably similar.13,14 Most mammals are characterized by having an epiglottis that can make contact with, or overlap, the soft palate during both normal respiration and deglutition (Figs. 1.1A to D). This is accomplished in most species by a larynx positioned, at all stages of postnatal development, relatively “high” in the neck when related to the basicranium and/or cervical vertebrae. Its position, measured from the cranial aspect of the epiglottis to the caudal border of the cricoid cartilage, corresponds to the level of the basiocciput or first cervical vertebra (C1) to the third or fourth cervical vertebrae (C3 or C4) in most terrestrial mammals. Concomitantly, the hyoid bone and associated suprahyoid and infrahyoid muscles (i.e. muscles largely responsible for raising or lowering the larynx) are also relatively high. The tongue at rest lies almost entirely within the oral cavity, with no portion of it forming part of the anterior pharyngeal wall. Because of this high position of the larynx, the supralaryngeal region of the pharynx is noticeably small; the pharynx has little or no oral portion and significantly reduced nasal and laryngeal segments. Inferiorly, the striated muscle fibers of the pharynx blend with the longitudinal striated fibers of the esophagus to form a continuous functional unit.
The high position of the larynx enables the epiglottis to pass upward behind the soft palate and “lock” the larynx directly into the nasopharynx. This configuration provides a direct air channel from the external nares through the nasal cavities, nasopharynx, larynx, and trachea to the lungs. Liquids, and in some species even chewed or solid material, can pass on either side of the interlocked larynx and nasopharynx by way of the isthmus faucium, through the piriform sinuses to the esophagus, following the so-called “lateral food channels.”6
Figs. 1.1A to D: Midsagittal sections of the head and neck regions of: (A) adult dog, Canis familiaris; (B) adult goat, Capra aegagrus; (C) juvenile hog (pig), Sus scrofa; (D) adult spider monkey, Ateles paniscus. (E: Epiglottis; S: Soft palate).
This anatomic configuration permits the patency of the laryngeal airway while streams of liquid or semisolid food are transmitted around each side of the larynx during swallowing. Two largely separate pathways are created: a respiratory tract from the nose to the lungs and a digestive tract from the oral cavity to the esophagus. This arrangement confers on mammals the ability to use these two pathways simultaneously, including enabling: (1) nursing young to suckle while breathing, (2) ruminants to breathe while regurgitating cud, (3) carnivores to breathe while their mouth is clamped tightly closed around the neck of their prey, and (4) a variety of animals to breathe when the mouth is used as a tool (e.g. beavers gnawing trees, felines, or rodents grasping and carrying young). In addition, as many mammals are macrosmatic (i.e. largely dependent on olfaction for communication with their environment), the two-tube system is particularly valuable as this arrangement allows, for example, grazing or drinking herbivores to simultaneously detect the scent of a predator.
While the larynx is consistently high in most mammals, its exact position and the extent of its placement in the nasopharynx can vary considerably among species. Studies of cetaceans (i.e. whales, dolphins, porpoises), for example, have shown that the larynx in some species is positioned so high (rostral) that it is no longer in the neck but rather lies largely within the head.15 In many odontocetes (toothed whales), the larynx, from the tip of the epiglottis to the lower border of the cricoid cartilage, corresponds to the level of the presphenoidal synchondrosis to the caudal border of the basiocciput. The larynx thus usually never reaches as far caudally as the compressed cervical vertebrae characteristic of these7 mammals. The epiglottis and corniculate cartilages form the anterior portion of an elongated larynx, which is encircled by a strong palatopharyngeal sphincter muscle (homologous to the soft palate and palatopharyngeal arch in humans). This sphincter grips the top of the larynx and keeps its aditus intranarial, thus effectively sealing the respiratory tract from the digestive route. Although exhibiting the basic mammalian pattern of a high larynx, odontocetes appear to have exaggerated it by placing the larynx even higher (or more rostral) than their terrestrial relatives. This extrahigh position ensures these mammals of a larynx fitted snugly into the nasopharynx and indeed may make it habitually intranarial, that is, it is not usually retracted from its position behind the soft palate. This arrangement may allow them to swallow whole fish while communicating with each other or while echolocating to orient themselves in their environment.
Although cetaceans demonstrate an example of larynges that have both migrated cranially and elongated their rostral cartilages, some terrestrial species have larynges that have expanded their caudal components (e.g. thyroid cartilage) so that they appear to extend considerably into the neck. For example, some male artiodactyls (red and fallow deer, Mongolian gazelle) exhibit particularly large larynges that seem to be located more caudally in the neck compared with other related species.16 However, although the larynges of these animals are elongated, they still retain roughly the same position opposite the cervical vertebrae as most other terrestrial mammals (extending from the basiocciput to C2–C3). Maintenance of this typical mammalian position is due to concomitant elongation of the cervical vertebrae. These animals also exhibit an elongated and elastic velum (red and fallow deer) and an elongated epiglottis (Mongolian gazelle) that appear to assist in epiglottic/palatal contact and, therefore, the maintenance of the “two-tube” system. Thus, while larynges differ considerably in position, the basic, ancestral two-tube configuration is essentially maintained. These animals have modified a basic plan; they have not changed it.
Postmortem dissections, and a range of imaging studies, including cineradiography, computed tomography, and magnetic resonance imaging, of our closest relatives, the nonhuman primates, show that their upper respiratory anatomy is also similar to the general mammalian pattern.4,17–19 As in other mammals, nonhuman primates exhibit a larynx positioned high in the neck, usually corresponding to the first to third cervical vertebrae. This position allows for epiglottic-soft palate apposition and the possibility of an intranarial larynx, thus providing for a direct airway from the nose to the lungs, whereas the alimentary tract passes around the larynx en route to the esophagus (Figs. 1.2A to C). Cineradiographic studies have confirmed that nonhuman primates exhibit mostly separate respiratory and digestive routes and the ability to breathe and swallow almost simultaneously.17,20 Because of this configuration, nonhuman primates, like other mammals, appear strongly, if not totally, dependent on nasal breathing. As occurs in many mammals, the connection between the epiglottis and the soft palate can be broken, as the larynx exhibits extensive mobility and can be transiently lowered. This can occur for a number of reasons, including some vocalizations, swallowing certain foods (e.g. a large bolus of meat), or due to disease.
Although this anatomic arrangement may enable almost simultaneous breathing and swallowing, it severely limits the array of sounds an animal can produce. The high position of the larynx means that only a small supralaryngeal portion of the pharynx exists. In turn, only a very reduced area is available to modify the initial sounds generated at the vocal folds. Due to this limitation, most mammals therefore depend primarily on altering the shape of the oral cavity and lips to modify laryngeal sounds. Although some animals can approximate some human speech sounds, they are anatomically incapable of producing the range of sounds necessary for human speech.21,22
THE DEVELOPMENT OF THE HUMAN AERODIGESTIVE TRACT: THE BASIS FOR OUR SPEECH ABILITIES
One of the most distinguishing features of humans is the anatomy and inherent functions of our ADT. Our specialized anatomy underlies our distinctive modes of breathing and swallowing, and our unique ability for speech. Indeed, the highly derived characteristics of the adult human ADT reflect both a distinctive developmental path as well as an evolutionary one.
The human ADT, particularly the larynx and its positional relationships to contiguous structures, undergoes dramatic changes during development.
The major morphologic events in embryonic development (0–8 weeks) of the human larynx have been well documented and new data from homeobox genes are shedding further light on the mechanisms of early spatial establishment in the head and neck.23,248
Figs. 1.2A to C: Midsagittal sections and images of the human head and neck of: (A) 24-week fetus; (B) newborn infant; (C) magnetic resonance imaging of an adult. Note the apposition of the epiglottis and soft palate in A and B, and the descent of the larynx away from the soft palate in C. (E: Epiglottis; S: Soft palate).
The proper development of the larynx during the embryonic period is obviously crucial to its later normal function, with miscues during this phase of development resulting in a range of serious congenital, often life-incompatible, anomalies. Although aspects of laryngeal development during the embryonic period have been well studied, changes during the fetal period (eight weeks to birth) have not been as extensively explored. To elucidate the latter, studies by our laboratory and others have investigated fetal laryngeal development both through postmortem study employing precise means of age determination and ultrasonography of fetuses in utero.25–27 These studies have shown that the fetal period is a time of extensive laryngeal growth and of significant changes in the positional relationships of the larynx. The second trimester (13–26 weeks), in particular, is an active period for laryngeal development. By week 15, earlier than was previously reported, the epiglottis is already present, indicating that the epiglottic primordium may appear earlier in development than classically believed. Throughout this period, the larynx is found high in the neck, generally corresponding, from the epiglottic tip to the inferior border of the cricoid, to the level of the basioccipital bone to the third cervical vertebra. By week 21, the epiglottis is found to be almost in apposition to the uvula of the soft palate. Between weeks 23 and 25, the epiglottis and soft palate overlap for the first time, providing the anatomical “interlocking” of the larynx into the nasopharynx characteristic of mammals previously described.
The attainment of larynx–nasopharynx interlocking is a significant maturational horizon in the development of the aerodigestive region. Establishment of this anatomic relationship allows the creation of essentially separate respiratory and digestive routes that will function as such in the newborn infant (vide infra). Our ultrasound investigations have shown upper respiratory activity patterns that strongly suggest an operational two-tube system is beginning to function prenatally, in which the larynx remains highly positioned and intranarial during fetal swallowing movements. A critical time in the9 development of the entire upper respiratory region may take place during the period between weeks 23 and 25. Not only does the larynx attain a position that places it intranarially, but contiguous portions of the skull base—the de facto roof of the upper respiratory tract (vide infra)— also appear to be undergoing remodeling at this time. As portions of the cranial base are intimately related to the larynx and its contiguous musculature, cranial shape development and laryngeal/ADT positions may also be linked, although the precise extent and relationships are still unclear. What may be beginning during this period is a remodeling and refinement of the positional anatomy of the entire aerodigestive region—soft tissue and cartilaginous structures, such as the larynx, and bony skeletal parameters, such as the skull base—to provide the anatomical framework for the newborn's upper respiratory and digestive tract. It should be noted that while these laryngeal and basicranial modifications are occurring in the upper respiratory region, concomitant changes are also occurring in the lower respiratory tract. For example, the period from weeks 23 to 25 corresponds to the maturation of the pulmonary glandular epithelium. This alveolar epithelium is responsible for the production of fetal lung surfactant, a substance that is essential for independent respiratory function. The contemporaneous development of the fetal larynx to permit soft palate–epiglottic overlap with increasing levels of lung surfactant suggests that the time frame for the maturation of the upper and lower respiratory tracts are closely related. Normal fetal maturation of the larynx may be an essential factor in determining the beginnings of respiratory independence and overall fetal viability.
The morphologic pattern of high laryngeal position established during fetal life continues past the perinatal period and into infancy. Indeed, the newborn/young infant period may more accurately be seen as an extension of the pattern established during the late second and early third fetal trimesters rather than as a distinct entity. Postmortem dissections and imaging studies have shown that the positional relationships in the aerodigestive region of human newborns and young infants closely resemble the basic primate and mammalian pattern. In newborns and infants until approximately 1½ to 2 years of age, the larynx remains high in the neck.4,14,28–32 Its position corresponds to the level of the basiocciput/C1, extends to the superior border of C4 in newborns, and descends slightly to the level between C2 and C5 by approximately 2 years of age. The tongue at rest can be found entirely within the oral cavity, with no portion of it forming the upper anterior wall of the pharynx.
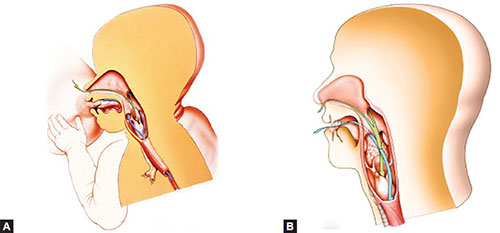
Largely separate respiratory and digestive pathways, similar to those described in most other terrestrial mammals, are effectuated by the high laryngeal position in newborns and young infants (Figs. 1.3A and B).
Figs. 1.3A and B: (A) The ADT of a newborn human during suckling and (B) the aerodigestive region in an adult human. Green arrows = Respiratory route, Blue arrows = Digestive route. Note that the high laryngeal position in the infant effectuates largely distinct pathways, whereas the lowered position of the larynx and tongue in the adult mandates the crossing of pathways.
This arrangement prevents the mixing of ingested food and inhaled air, thereby enabling the baby to breathe and swallow liquids almost simultaneously in a manner similar to that of monkeys. Thus the baby can breathe through the nose with only minimal, if any, cessations as liquid flows from the oral cavity around the larynx into the esophagus. Because of this high laryngeal position, newborns are essentially, if not obligatorily, nose breathers. As with nonhuman primates, the connection between the epiglottis and the soft palate is usually constant but may be interrupted during the swallowing of a particularly large or dense bolus of food or liquid, during vocalization or crying, or because of disease as noted above.
Although the high position of the larynx in a human newborn or young infant effectuates the dual-pathway system, it severely limits the array of sounds babies produce. Many studies have shown that the high position of the larynx greatly restricts the supralaryngeal portion of the pharynx/tongue available to modify the initial, or fundamental, sounds produced at the vocal folds. Thus, an individual with a larynx situated high in the neck, as is found in a newborn human or monkey, would have a more restricted range of vocalizations available than would individuals with larynges and tongues placed lower in the neck. Indeed, linguistic analyses have identified the quantal vowels [i], [u], and [a] as sounds that human infants or nonhuman primates cannot produce. As these vowels are the limiting articulations of a vowel triangle that is language universal, their absence considerably restricts speech capabilities.
Although the larynx remains high in the neck until around the second year, functional changes, such as the first occasional instances of oral respiration, have been noted to occur considerably earlier, indeed within the first six months of life.29 The period between four and six months, in particular, may represent a crucial stage in upper respiratory activity. At this time, neuromuscular control mechanisms of the larynx and extralaryngeal pharyngeal control are beginning to change even before true structural “descent” of the larynx has occurred. This changeover period may also indicate a time of potential respiratory instability because of the transition from one respiratory pattern to another.
The time and manifestation of both prenatal and postnatal maturation within the central nervous system relates directly to normal and abnormal upper respiratory and swallowing functions. For example, studies from our laboratory have suggested that there are crucial prenatal periods for the development of mammalian upper respiratory motor nuclei in the brainstem and that insults in utero could affect postnatal functions.33,34 The combination of subsequent, postnatal central nervous system maturation and developmental changes in respiratory patterns may predispose the infant to several developmentally related problems. The sudden infant death syndrome, SIDS, for example, may be related to these first postnatal upper respiratory changes and to the subtle changes in laryngeal position or central and peripheral neuromotor control of the larynx.33,35 The precise time of the shifts that occur in breathing patterns, their relationship to laryngeal changes, and the neurophysiologic mechanisms that accompany them are obviously crucial questions that are still poorly understood and require more specific and detailed study.
The larynx of human infants may remain high in the neck until approximately 1½ to 2 years of age. Our studies and others have confirmed that around the second year, children begin to show positional rearrangements of the ADT that differ sharply from the condition in newborns and early infants. Around this period the larynx begins its permanent, structural descent into the neck. Although minor topographic changes in laryngeal position continue until puberty and beyond, the major qualitative change probably occurs between the second to third years of life. Although the exact timing of laryngeal descent has yet to be fully determined, imaging and postmortem observations indicate that by the third year the position of the larynx has been significantly lowered.
Although the internal nature of the larynx changes relatively little after the third year (except for normal maturational changes at puberty), positional changes relative to contiguous upper respiratory structures are considerable. The tongue no longer lies entirely within the oral cavity at rest, as in the newborn infant or nonhuman primate. The posterior portion of the tongue, from the foramen cecum caudally, has descended into the neck and now forms the upper anterior wall of the pharynx. The larynx is now situated considerably lower in the neck. For example, in a seven-year-old child, the larynx—from the tip of the epiglottis to the inferior border of the cricoid cartilage—corresponds to the level between the upper border of C3 and the lower border of C5. In the adult, the larynx has further descended, lying between the lower border of C3/upper part of C4 to the upper border of C7. Concomitantly, the hyoid bone and its associated suprahyoid and infrahyoid muscles are relatively lower in the neck.11
Due to the descent of the larynx, tongue, and hyoid apparatus in children after the second to third year, the epiglottis can no longer approximate the soft palate, even during maximal laryngeal elevation. As the larynx cannot lock into the nasopharynx, there no longer exists the possibility of a continuous, tube-like airway from the external nares to the lungs. The lower position of the larynx alters dramatically the way humans, after the early years of life, breathe and swallow. The loss of the ability of the epiglottis to make contact with the soft palate means that the possibility of having two separate pathways, one for air and one for liquid—the basic ancient terrestrial template—no longer exists. Unlike this general mammalian pattern, the respiratory and digestive tracts now cross each other in the area of the pharynx. This low position of the larynx also results in a large supralaryngeal portion of the pharynx. A permanently enlarged oropharynx is now present even during maximal laryngeal elevation. These changes have pronounced effects upon our respiratory behavior. For example, we are habitual nose breathers but, unlike newborn infants, adults have a greater ability for oral respiration and do so more frequently. Loss of the two-pathway system has now made it imperative that the respiratory tract be sealed off during swallowing.
The permanent intersection of the respiratory and digestive pathways has created a de novo “aerodigestive” tract, a first of its kind in mammals. This has created many problems, which, if not unique to us, are certainly accentuated in our kind. A major problem is that a bolus of food can easily become lodged in the laryngeal aditus leading to the common occurrence of food “going down the wrong pipe”; however, if the bolus is large or not expelled rapidly enough one may choke to death. This event is often referred to as a “cafe coronary”, because it frequently occurs in restaurants and may be mistaken for a heart attack. Similarly, another disadvantage of the crossed pathways is the relative ease with which vomitus can be aspirated into the larynx and trachea and passed to the lungs.
Not only has a permanently lowered larynx proven to be a danger in the ingestion of material, but it may serve as the anatomic basis allowing for gastroesophageal reflux of stomach contents to enter the pharyngeal or oral cavities.36,37 The human ADT has clearly not been evolutionarily selected for the purpose of efficiently dealing with constant, retrograde emissions into the supraesophagus. Indeed, our ADT appears to be particularly poorly designed to handle any esophageal or gastroesophageal reflux. This is most clearly demonstrated by two features: (1) our uniquely low laryngeal position and (2) the relatively unprotected posterior larynx. Low laryngeal position, by creating a permanent oropharynx, has by definition created a greatly expanded supraesophageal region. It should also be noted that as the larynx has migrated caudally, so too has the location of the cricopharyngeal sphincter (i.e. the upper esophageal sphincter) at the caudal-most extent of the pharynx. Thus by definition, adult humans have a relatively shorter esophagus and relatively longer supraesophagus than most other mammals. At the very least, then, the highly acidic contents of human gastroesophageal reflux have gained access to a proportionally greater surface area of pharyngeal mucosa with which they may interact.
Laryngeal descent in humans has altered considerably the way we breathe and swallow. From a comparative perspective, we have lost the basic mammalian ability to breathe and swallow simultaneously or almost simultaneously. We have also accrued a number of most unwanted guests, including the relative ease of lodging material in the airway or refluxing contents to the supraesophagus and other portals. A litany of other diseases and/or incoordination clinicopathologies, ranging from otitis media and frequent rhinosinusitis38 to SIDS and aspects of obstructive sleep apnea39 may also be a price we pay for peripheral and central neuromuscular rearrangements that accompany ADT modifications. The lowered position of our larynx has, however, provided one major positive aspect: a greatly expanded supralaryngeal portion of the pharynx. This enlarged supralaryngeal pharynx has “liberated” the tongue from the oral cavity confinement, now having its posterior portion form the movable anterior aspect of this enlarged chamber. This configuration allows for enhanced oral tidal respiration, which may have occurred in temperate environments for our early ancestors. In addition, pharyngeal/lingual modification of sounds produced at the vocal folds is considerably greater than that possible for newborns, early infants, or any nonhuman mammal. In essence, it is the unique marked descent of the larynx and the resultant expansion of the pharynx and liberation of the posterior tongue that gives us the anatomic ability to produce fully articulate speech.
WHEN DID WE GAIN OUR VOICE AND OUR ABILITY TO SPEAK?
When, how, and why the unquestionably unique ADT that we exhibit today came about during the course of our evolution is an ongoing topic of often-heated (and vocal)12 debate. The reason for the intensity, of course, is that it speaks to the centrality of what makes us “human” and what separates us from both other living animals and from our own nonhuman ancestors.
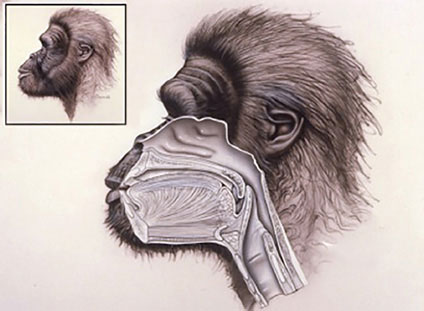
While fossilized throats do not remain, some researchers, our lab included, have developed methods to reconstruct the structure of the region through the use of remnant portions of the cranial base, the de facto “roof” of the ADT, as a guide.40–43 Reconstruction of the ADT of fossil human ancestors – ranging in age from our earliest direct relatives known as australopiths (who can be traced to over four million years before the present) to early members of our own genus, Homo (appearing over a million and a half years ago), to our own species, Homo sapiens (arriving perhaps 200,000 to 300,000 years before the present)—have enabled us to trace the changes in the region through our history. For example, our reconstructions have suggested that the earliest hominids likely exhibited an ADT largely similar to those of the extant apes, with the larynx positioned high in the throat and the epiglottis able to contact the soft palate during normal tidal respiration. These early ancestors likely breathed and swallowed essentially as do our living monkey and ape relatives, being essentially nasal breathers with a modified two-tube system. The high position of their larynx would, by necessity, limit the supralaryngeal area of the pharynx and the freedom of the tongue to modify sounds as extensively as modern adult humans can. This suggests that they were restricted in the types of sounds that they could make, probably being incapable of producing a number of the universal vowel sounds found in human speech patterns.4,41,42 What the internal anatomy/microanatomy of their vocal folds was exactly like, and whether changes toward the human condition were occurring in the distribution, structure or innervation patterns of the intrinsic musculature, remains unknown. If the global reconstructions given above for their ADT are correct and they were indeed very similar to living apes, then it is reasonable to hypothesize that their vocal fold anatomy would also not be very different from those of the living apes and monkeys. They may well, for example, have lacked a deep layer of the lamina propria, again a feature suggested to be found only in living humans and not in other primates (Fig. 1.4).
If our earliest relatives were still “ape-like” in their ADT/vocal tract component, when did things change en route to us? This is a key question of our evolution and while definitive answers in evolution are rare, clues are beginning to appear. Our fossil data—and by extension reconstructions—suggest that the region was starting to change with the first members of our own genus, Homo, some million—plus years before the present on the plains of Africa.
Fig. 1.4: Reconstruction of the head and neck anatomy of Australopithecus africanus, an early human ancestor, during quiet nasal respiration (based upon the fossil Sts 5 from Sterkfontein, South Africa; for discussion see text and reference 42). As with living monkeys and apes, we hypothesize that the earliest hominids would exhibit a highly positioned, intranarial larynx during nasal breathing, as well as during ingestion of some foods. The high larynx would also have limited the supralaryngeal area and thus the ability to modify laryngeal sounds compared to living humans.
It was at this time that the ancient, two-tube system in place for millennia was evolving into something different. The basicranium was changing, indicating that the larynx was changing in position, likely becoming disengaged from the nasopharynx. Such a shift would have radically altered the way our ancestors breathed (e.g. increasing oral respiration possibilities) and how they swallowed. Indeed, as we have previously hypothesized,4 the reason behind the advent of such changes may have been linked to increased need for oxygen due to lifestyle demands such as running on the African plains. In addition, such ancestors would also no longer have had the ability to breathe and swallow almost simultaneously. A new paradigm would have come in place. Such a shift would have required not only obvious anatomical uncoupling and rearrangements, but also a “rewiring” of central and peripheral neural processing. As with developmental shifts noted previously, change has its costs, and many of our early ancestors likely paid the ultimate price. Scenarios such as increased choking and other upper respiratory and upper digestive maladies probably evolved along with our laryngeal shifts.
While breathing and swallowing abilities were changing, so too were vocal and speech capabilities. On the one hand, the brain size in our early Homo ancestors was13 increasing over that found in earlier australopith-level hominids. Concomitant with that were indications that the internal complexity of the brain was changing as well. Together with the ADT changes noted above, the stage was set for vocal tract and intrinsic laryngeal change.
A watershed level of change was beginning with the advent of a new genus, one more advanced linguistically and cognitively than any that had preceded it. If, indeed, such changes were now becoming widespread, it is likely that the vocal component was taking on an ever-increasing communication role. Accordingly, microanatomical changes in vocal fold anatomy and function were also beginning to occur, as would have been alterations in intrinsic laryngeal muscle fiber type. While en route to the modern condition, our early Homo ancestors were probably not yet there. It is difficult, yet intriguing, to envision relatives who had an anatomical/physiological substrate different from either ourselves or our ape-like, earliest ancestors. The early members of our genus were remarkable in their own way, neither ape, nor australopith, nor us, humans, as we are today.
While changes may have begun with early members of our genus, it was not until the appearance of early members our own species, Homo sapiens, some 200,000 to 300,000 years before the present (or even much later depending on who one sees as worthy of H. sapiens designation) that our reconstructions indicate an ADT similar to ours appeared.42 These early H. sapiens, probably functioned much as we do today as regards their breathing, swallowing, and communicative parameters. Certainly, their extensive vocal tract component would have allowed for the production of the sounds of extant speech. We can also hypothesize that their internal laryngeal milieu was very similar to ours, but exactly when the type of muscle fibers, or presence of a lamina propria such as those shown today, actually appeared remains to be determined. Could there have been stages of change even within the timeframe of our own species? Could some of the answers to the late appearance of advances in the archeological record that point to highly advanced cognitive abilities44 be reflective of subtle, yet seminal, changes in our voice or speech capabilities? Clearly, as we uncover more of the secrets buried within our unique structures for voice and speech, so too will be able to uncover their deeply entwined role with the story of how we came to be.
REFERENCES
- Abitbol J. The Odyssey of the voice. San Diego, CA: Plural Publishing; 2006.
- Lieberman P. The biology of language. Cambridge, MA: Harvard University Press; 1984.
- Chompsky N. Reflections on language. New York: Pantheon Press; 1976.
- Laitman JT, Reidenberg JS. Evolution of the human larynx: nature's great experiment. In: Fried M, Ferlito A (eds), The larynx, 3rd edn. San Diego, CA: Plural; 2009:19–38.
- Laitman JT, Reidenberg JS. The evolution and development of human swallowing: the most important function we least appreciate. Otol Clin N Am. 2013;46(6):923–36.
- Harrison DFN. The anatomy and physiology of the mammalian larynx. Cambridge, UK: Cambridge University Press; 1995.
- Reidenberg JS, Laitman JT. Morphophysiology of the larynx. In: Van De Water T, Staecker H (eds), Basic science review for otolaryngology. New York, NY: Thieme; 2005:505–15.
- Kirchner JA. The vertebrate larynx: adaptations and aberrations. Laryngoscope. 1993;103:1197–201.
- Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatrica Logopaedica. 1974;26:89–94.
- Benninger MS. The human voice: evolution and performance. Music Med. 2010;2(2):104–8.
- Han Y, Wang J, Fischman DA, et al. Slow tonic muscle fibers in the thyroarytenoid muscles of human vocal folds: a possible specialization for speech. Anat Rec. 1999;256(2): 146–57.
- Hartnick CJ, Rebhar R, Prasad V. Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope. 2005;115:4–15.
- Laitman JT, Reidenberg JS. Specializations of the human upper respiratory and upper digestive systems as seen through comparative and developmental anatomy. Dysphagia. 1993;8:318–25.
- Laitman JT, Reidenberg JS. Comparative and developmental anatomy of human laryngeal position. In: Bailey B (ed), Head and neck surgery – otolaryngology, 2nd edn, vol. 1. Philadelphia, PA: Lippincott Company; 1998:45–52.
- Reidenberg JS, Laitman JT. The position of the larynx in Odontoceti (toothed whales). Anat Rec. 1987;218:98–106.
- Frey R, Gebler A. The highly specialized vocal tract of the male Mongolian gazelle (Procapra gutturosa Pallas, 1777- Mammalia, Bovidae). J Anat. 2003;203(5):451–71.
- Laitman JT, Crelin ES, Conlogue GJ. The function of the epiglottis in monkey and man. Yale J Biol Med. 1977;50: 43–9.
- Laitman JT, Crelin ES. Tantalum markers as an aid in identifying the upper respiratory structures of experimental animals. Lab Anim Sci. 1980;30(2):245–8.
- Flugel C, Rohen JW. The craniofacial proportions and laryngeal position in monkeys and man of different ages. (A morphometric study based on CT scans and radiographs.) Mech Ageing Develop. 1991;61:65–83.
- German RZ, Crompton AW. Integration of swallowing and respiration in infant macaques (Macaca fascicularis). Am J Phys Anthropol. 1993;(Suppl 16):94.
- Lieberman P, Laitman JT, Reidenberg JS, et al. The anatomy, physiology, acoustics and perception of speech: essential elements in analysis of the evolution of human speech. J Hum Evol. 1992;23:447–67.
- Som PM, Smoker WR, Reidenberg JS, et al. Embryology and anatomy of the neck. In: Som PM, Curtin HD (eds), Head and neck imaging, 5th edn., New York, NY: Mosby; 2011:2117–64
- Laitman JT, Noden DM, Van De Water TR, Formation of the larynx: from homeobox genes to critical periods. In: Rubin JS, Sataloff RT, Korovin GS, Gould WJ (eds), Diagnosis and treatment of voice disorders, 4th edn., San Diego, CA: Plural Publishing; 2014.
- Magriples U, Laitman JT. Developmental change in the position of the fetal human larynx. Am J Phys Anthropol. 1987;72:463–72.
- Wolfson VP, Laitman JT. Ultrasound investigation of fetal human upper respiratory anatomy. Anat Rec. 1990;227: 363–72.
- Isaacson G, Birnholz JC. Human fetal upper respiratory tract function as revealed by ultrasonography. Ann Otol Rhinol Laryngol. 1991;100:743–7.
- Laitman JT, Crelin ES. Developmental change in the upper respiratory system of human infants. Perinatol Neonatol. 1980;4:15–22.
- Sasaki CT, Levine PA, Laitman JT, et al. Postnatal descent of the epiglottis in man: a preliminary report. Arch Otolaryngol. 1977;103:169–71.
- Westhorpe RN. The position of the larynx in children and its relationship to the ease of intubation. Anaesth Intensive Care. 1987;15:384–8.
- Schwartz D, Keller M. Maturational descent of the epiglottis. Arch Otolaryngol Head Neck Surg. 1997;123:627–8.
- Vorperian HK, Kent RD, Linstrom MJ, et al. Development of vocal tract length during early childhood: a magnetic resonance imaging study. J Acoust Soc Am. 2005;117: 338–50.
- Friedland DR, Eden AR, Laitman JT. Naturally occurring motoneuron cell death in rat upper respiratory tract motor nuclei: a histological, Fast-DiI and immunocytochemical study in the nucleus ambiguus. J Neurobiol. 1995;26: 563–78.
- Friedland DR, Eden AR, Laitman JT. Naturally occurring motoneuron cell death in rat upper respiratory tract motor nuclei: a histological, fast-DiI and immunocytochemical study in the hypoglossal nucleus. J Neurobiol. 1995;27: 520–34.
- Tonkin SL, Gunn TR, Bennet L, et al. A review of the anatomy of the upper airway in early infancy and its possible relevance to SIDS. Early Hum Dev. 2002;66:107–21.
- Laitman JT, Reidenberg JS. The human aerodigestive tract and gastroesophageal reflux: an evolutionary perspective. Am J Med. 1998;103:3–11.
- Lipan M, Reidenberg JS, Laitman JT. The anatomy of reflux: a growing health problem affecting structures of the head and neck. Anat Rec. B: the new anatomist. 2006;289B: 261–70.
- Bluestone CD, Pagano AS, Swarts JD, et al. Consequences of evolution: is rhinosinusitis, like otitis media, a unique disease of humans? Otol Head Neck Surg. 2012;147: 986–91.
- Davidson T. The great leap forward: the anatomic basis for the acquisition of speech and obstructive sleep apnea. Sleep Med. 2003;4:185–94.
- Laitman JT, Heimbuch RC, Crelin ES. Developmental change in a basicranial line and its relationship to the upper respiratory system in living primates. Am J Anat. 1978; 152:467–83.
- Laitman JT, Heimbuch RH, Crelin ES. The basicranium of fossil hominids as an indicator of their upper respiratory systems. Am J Phys Anthropol. 1979;51:15–34.
- Laitman JT, Heimbuch RC. The basicranium of Plio-Pleistocene hominids as an indicator of their upper respiratory systems. Am J Phys Anthropol. 1982;59:323–44.
- Reidenberg JS, Laitman JT. Effect of basicranial flexion on larynx and hyoid position in rats: an experimental study of skull and soft tissue interactions. Anat Rec. 1991;220: 557–69.
- Tattersall I. The human odyssey: four million years of human evolution. New York: Prentice Hall; 1993.