ANATOMY OF THE OLFACTORY SYSTEM
Introduction
It is estimated that approximately 1–2% of the population suffers from disorders of taste and smell.1 There are several categories of olfactory dysfunction including anosmia, hyposmia and dysosmia. Anosmia is the total loss of ability to detect odorants while hyposmia is the decreased ability to detect such odors. Dysosmia has two types, referred to as phantosmia and parosmia. Phantosmia is characterized as the perception of an odorant being present when it is not, whereas p arosmia is the perception of an altered sense of smell. Patients with dysosmia tend to perceive malodorous smells such as rotten fumes and gas leaks.
Olfactory Anatomy
Nasal Passage
Olfaction is determined primarily by the ability of odorants to reach the olfactory epithelium at the roof of the nasal cavity. This action occurs through inhalation into the nasal cavity and through retronasal flow from the nasopharynx and oropharynx. Based on a large scale model study, there is evidence that 50% of the total airflow goes through the middle turbinate but only 15% of airflow reaches the olfactory region.2
Olfactory Mucus
Once the odorants have traversed the nasal passageway, they must interact with the olfactory mucus which covers the olfactory epithelium. Odorants are classified based on their solubility, but this is not the only variable in reaching the olfactory receptors. The composition of the mucus also determines the transit time to the receptors, and adrenergic, cholinergic and peptidergic agents are known to affect the thickness of the mucus layer.2
Olfactory Epithelium
The olfactory epithelium is located approximately 7 cm into the nasal cavity, where it spans around 1 cm2 on each side of midline. It is found in the cribriform plate, upper septum, and the medial portion of the middle and superior turbinates. On nasal endoscopy, it appears as thick pale mucosa adjacent to pink respiratory epithelium. The olfactory epithelium consists of the olfactory mucosa and lamina propria, which is separated by a basement membrane. The olfactory mucosa contains olfactory receptor neurons (ORNs), sustentacular cells, basal cells, microvillar cells, and the Bowman's gland ducts. The lamina propria contains the Bowman's gland, bundles of olfactory axons, and blood vessels. The ORN is a bipolar neuron which has a club shaped end that projects peripherally and contains immotile cilia. The olfactory receptors which are found on these cilia are the gateway to olfactory transmission. Single nonmyelinated axons project toward the olfactory bulb after they have come together to form myelinated fascicles and are referred to as filae olfactoria. These filae olfactoria travel through the foramina of the cribriform plate to reach the olfactory bulb. Sustentacular cells form a tight barrier around the distal dendrite of the ORN and are thought to aid in the removal of odorants after perception and to prevent toxin exposure. The basal cells of the olfactory epithelium include the horizontal and globose cells, which are thought to be the stem cells of the olfactory system. Within the lamina propria, the Bowman's glands produce mucus which travels through ducts in the olfactory mucosa and are secreted onto the olfactory epithelium.
Olfactory Bulb
The olfactory bulb is located at the base of the frontal cortex in the anterior cranial fossa. The bulb consists of multiple layers including the glomerular layer, olfactory nerve layer, external and internal plexiform layers, mitral and granule cell layers. Olfactory nerve bundles from the ORN from glomeruli that subsequently synapse with second cell neurons (mitral and tufted cell) and intrinsic neurons. From the olfactory bulb, synapses are transmitted to the olfactory cortex. The olfactory cortex has extrinsic connections to other areas of the brain including the lateral hypothalamus and the hippocampus, which may explain why smell often evokes strong memories.3
Olfactory Physiology
The process of olfaction occurs when inhalation of an odorant reaches the olfactory cleft and goes through the olfactory mucus. 3From here the odorant must attach to specific olfactory receptors on the cilia of the ORN. This elicits a G protein second messenger pathway which upregulates cyclic adenosine monophosphate (cAMP) leading to depolarization of the cell and firing of action potentials to the olfactory bulb. A decrease in the odor perception is due to adaptation. It is believed that increased intracellular Ca+2 ions block cAMP, and therefore plays a vital role in adaptation.4,5
Differential Diagnosis
The most common causes of olfactory disorders are post-upper respiratory infection (URI), head trauma, nasal and sinus disease, toxins, congenital disorders, and idiopathic processes. These etiologies can be separated into conductive and sensorineural disorders.
Conductive Disorders
Conductive disorders include septal deviation, nasal polyps, sinonasal tumors, nasal and sinus disease, and prior surgery.
Depending on the significance of the septal deviation there may be some obstruction of nasal airflow. Several studies have reported traumatic nasal deformity as a cause for olfactory loss, but none have used objective olfactory testing to confirm this hypothesis. A few studies have looked at the impact of surgery on olfactory disorders and there has not been a meaningful improvement documented in these.6 Many authors would agree that a severe septal deflection may cause olfactory loss but deformity to this degree is rare.
Nasal polyps may cause obstruction in airflow leading to anosmia or hyposmia. Sinonasal tumors such as inverting papilloma, adenomas, squamous cell carcinomas, and esthesioneuroblastomas may also cause nasal obstruction and hyposmia. However, these lesions commonly cause unilateral olfactory loss and therefore may not be the presenting symptom.
Chronic rhinosinusitis and edema of the nasal mucosa can cause obstruction of airflow to the olfactory cleft. In addition to obstruction, a study by Kern in 2004 showed evidence of apoptosis of the olfactory epithelium due to chronic sinusitis and persistent swelling.7
Prior surgeries including total laryngectomy and tracheostomy divert airflow away from the nasal cavity, and therefore lead to a decreased sense of smell.
Sensorineural Disorders
Typically olfactory loss due to a URI recovers within a few days of illness; however, a small subset of these patients never recovers normal olfactory function. The incidence of this is much higher in women and the elderly population. Thirty-two to sixty-seven percent of patients regain a significant amount of function, which can take anywhere from a couple of weeks to several years after the illness has resolved.8 Unfortunately, less than 10% of those patients who experience persistent olfactory loss after a URI will return to absolutely normal function.
The incidence of olfactory loss after trauma is between 5% and 10%. Head trauma is more likely to result in total anosmia when compared to URI etiology. The loss of olfaction in head trauma is likely due to shearing effect of the axons by the cribriform plate.9 Blunt trauma to the forehead or occiput is more likely to lead to anosmia, with occipital trauma being five times more likely to result in complete loss.10
Congenital disorders account for about 3% of anosmia patients. This is typically an isolated finding seen in the preteen and adolescent age group. The pathology of congenital disorders shows a degeneration or atrophy of the olfactory epithelium or bulb. The most common congenital disorder is Kallmann syndrome. This syndrome is characterized as an autosomal dominant or X-linked syndrome with agenesis of the olfactory bulb and hypogonadotropic hypogonadism.
Aging has long been known to affect olfactory function. As we age, functional olfactory epithelium is replaced with respiratory epithelium. By age 65, 20% of the patient population has olfactory dysfunction, and this increases to 50% by age 80. Alzheimer's and Parkinson's disease have been associated with olfactory dysfunction. In fact, recent studies have shown that olfactory loss is an early sign for both disease processes, even when the patient is otherwise asymptomatic.
Other causes of sensorineural disorders include toxins, medications and HIV. Tobacco smoke is associated with hyposmia in active smokers; however, once smoking cessation occurs, olfactory status returns to baseline. Well debated in the literature, zinc gluconate has been associated with loss of smell after URI. Additionally, HIV has been associated with olfactory loss which does not correlate with disease progression.
Workup
The most important part of the workup is a detailed medical history and physical examination. Key portions of the history that should 5be elicited include timing of onset, severity, and symptoms around the time of onset, including URI or trauma. A thorough review of symptoms and past medical history is also warranted. A history of hypothyroidism, neurodegenerative disorder in the patient or their family, and the use of certain medications may endorse a more systemic cause for the disorder. Physical examination should focus on the nasal endoscopy, cranial nerve (CN) examination, and oral cavity examination. Typically, an identification test should be performed in the office to objectively evaluate the severity of the olfactory loss. The University of Pennsylvania Smell Identification Test (UPSIT) is frequently used in clinical situations. This is a 40 question scratch and sniff identification test with a score greater than five, but less than 20, representing anosmia. Other testing such as blood work and imaging may also be warranted. If the patient has an anatomic deformity or obstruction, a history of sinonasal disease, or a diagnosis that is not clear based on history and physical, then a CT scan of the sinuses is appropriate. Images in the coronal section are most helpful as they identify sinus disease in the ethmoid region, tumors, and bony deformities leading to obstruction. A magnetic resonance imaging is useful for viewing soft tissue and potential olfactory bulb abnormalities. Patients who come in with a complaint of taste dysfunction along with a decreased sense of smell should be asked about their ability to taste sweet, sour, bitter and salty. If the patient is able to decipher these flavors, it is unlikely that they have a problem with taste and their symptoms are most likely secondary to an olfactory dysfunction.
Management
The management of olfactory disorders depends primarily on the cause. Medical treatments including nasal and oral steroids are used to treat nasal and sinus disease such as chronic rhinosinusitis, nasal polyps and nasal edema. Antihistamines and antibiotics can also be used in those with allergic rhinitis or chronic or acute sinusitis, respectively. Oral steroids have been quite effective in improving olfactory function when narrowed airflow passages are due to edema and inflammation. This medication is typically tried for 1–2 weeks but cannot be used on a long-term basis because of the potential side effects of persistent use. Surgical options are also a possibility for patients with chronic rhinosinusitis with or without nasal polyps. Surgical correction of a severely deformed nasal septum may improve airflow but has not been found to make a significant difference in olfactory function. Patients with previous nasal surgery including rhinoplasty, resection of intranasal lesions, and 6skull base tumors may have scarring that limits nasal airflow, in which case surgery may lead to improvement in olfaction. Unfortunately, a significant number of etiologies do not have a proven therapeutic option. Reports of vitamin and mineral supplementation have shown inconsistent results. Vitamin A and zinc are the most studied supplements for olfaction; however, to date, there is no conclusive evidence that either treatment has an impact on smell.11 Additionally, this improvement is difficult to prove given the possibility of spontaneous recovery of olfaction. A randomized, double blind, crossover study by Henkin et al. showed that zinc is no more effective than placebo in improving olfactory function.12
The most important job of the physician is to educate their patients about the condition and give them steps to improve their safety and quality of life. This includes adding color, texture, and spice to the food they consume and counseling them on the importance of functioning smoke detectors and natural gas detectors in the home.13 Patients are instructed to be vigilant about checking the expiration dates on foods and looking at the appearance of food before cooking. For those patients with dysosmia, this can be significantly disruptive to their lives. Some literature would even suggest that it is even more limiting than those patients who suffer from anosmia or hyposmia. These patients may benefit from the use of gabapentin and clonazepam which has been prescribed for off label use.
Conclusion
Olfactory dysfunction can have a significant impact on patient quality of life. Many patients suffer with weight loss, depression, and social isolation because they can no longer perceive food, drink, and environmental stimulus in the manner they once did. This condition is especially concerning for patients working in industries where taste and smell are vital to their productivity and safety. There are multiple etiologies for this relatively common disorder; therefore, a detailed history and physical including nasal endoscopy is warranted. Smell identification test along with imaging studies will help narrow the differential diagnosis and provide the best management strategy for this often difficult to treat condition.
SINONASAL PHYSIOLOGY
The Nose
The first of many protective features of the sinonasal cavity are the vibrissae, which are the hairs located just within the nasal meatus. 7They help to filter large, aerosolized particles from the inspired air. The next tier of sinonasal defense is the nasal valves, which regulate inspired airflow. The external nasal valve is defined by the angle between the medial and lateral crus of the lower lateral cartilage, the columella, and the nasal sill. The internal nasal valve, which is the narrowest portion of the upper airway, is defined by the angle between the caudal edge of the upper lateral cartilage, the nasal septum, and the anterior face of the inferior turbinate. The nasal valves are dynamic and can increase and decrease the amount of nasal airflow to ensure that air is not inspired faster than it can be warmed, humidified and cleaned.
The Turbinates
Inspired air then reaches the turbinates of the nasal cavity. The superior and middle turbinates are formed from the ethmoid bone, while the inferior turbinate is an independent osseous structure. Most inspired air passes between the inferior and middle turbinates. The turbinates increase the total surface area of the nasal cavity, thus significantly contributing to warming and humidifying inspired air. The shape of the turbinates allows air to move posteriorly toward the nasopharynx while changing the airflow from a laminar to a transitional pattern.
The Nasal Cycle
In a normal nasal airway, most people experience asymmetric airflow through the nose, with one nasal passage being more patent to airflow and having increased secretions from serous and mucus glands, whereas the other nasal passage is more congested with reduced secretions. This nasal cycle is regulated by neural control and vasomotor input that results in alternating engorgement and constriction of the venous sinusoids within the erectile mucosa of the nasal passage. The nasal cycle alternates every 2–7 hours on average.
The Sneeze Reflex
The sneeze reflex of the upper airway is analogous to the cough reflex of the lower airway. It protects the upper airway by removing irritants from the nasal cavity. The olfactory nerve (CN I) conveys special olfactory senses while the trigeminal nerve (CN V) conveys thermal, noxious and mechanical stimuli from the nasal cavity. When the offending agent is present in the nose, afferent fibers of CN V are activated. The efferent parasympathetic fibers 8then stimulate the nasal mucosa to increase secretions. At the same time, the phrenic nerve stimulates the diaphragm to active inspiration. The anterior abdominal wall muscles then contract, generating a powerful exhalation (sometimes up to 100 miles per hour) during a brief Valsalva maneuver, forcing the pressure exhalation through the nasal cavity.14
The Mucosa and Mucociliary Clearance
When airborne pathogens pass through the first lines of sinonasal defense, they become trapped in the mucus layer (made from goblet cells) of the sinonasal mucosa. Mucociliary clearance removes both healthy secretions and pathogens from the sinonasal airway and is the principal mode of defense of the respiratory system, in particular the paranasal sinuses.
The anterior margin of the nasal vestibule is composed of stratified squamous epithelium. Around the area of the nasal valves, the epithelium transitions to pseudostratified columnar ciliated epithelium, which is found throughout the rest of the nasal cavity (except for the olfactory epithelium).
Mucociliary clearance in the maxillary sinus flows in a superomedial direction against gravity, with the help of propulsion of cilia, toward the natural ostium in the superior medial wall. The anterior ethmoid cells direct their mucus toward their individual ostia and then into the middle meatus. The posterior ethmoid cells, on the other hand, direct their mucus toward the superior meatus and eventually into the sphenoethmoidal recess. The sphenoid sinus also drains into the sphenoethmoidal recess. The mucus in the medial portion of the frontal sinus is carried superiorly (away from the natural ostium) and then laterally along the roof of the sinus. The mucus along the floor and inferior portions of the sinus is carried medially toward the natural ostium, where it then drains into the frontal recess and into the ethmoid infundibulum.
The mucociliary flow from the maxillary, anterior ethmoid, and frontal sinuses is carried to the ostiomeatal complex and then to the posterior nasopharynx. The mucociliary flow from the posterior ethmoid and sphenoid sinuses travels posterior toward the posterior nasopharynx. From here, swallowing directs the mucus into the gastrointestinal tract.
EMBRYOLOGY OF THE NOSE AND PARANASAL SINUSES
The embryo develops separate nasal cavities around the fourth to eighth week of gestation as the frontonasal and maxillary processes join. The ethmoturbinals develop from the lateral nasal wall at 9the eighth to tenth week of gestation. The first ethmoturbinal has an ascending portion which becomes the agger nasi cell and a descending portion which becomes the uncinate process. The second ethmoturbinal forms the middle turbinate. The space between the first and second ethmoturbinal becomes the middle meatus. The third ethmoturbinal forms the superior turbinate. The fourth and fifth ethmoturbinals form the supreme turbinate (when present). The maxilloturbinal (which is not part of the ethmoid bone) forms the inferior turbinate.
The nasal septum arises from the posterior midline growth of the frontonasal process and midline extensions of the mesoderm from the maxillary processes. The descending septum merges with the fused primary and secondary palatal shelves to create two distinct nasal cavities.
The maxillary sinus develops during the tenth week of gestation from the invagination of the middle meatus. Also during the same time, the uncinate process and the ethmoidal bulla form a narrow groove known as the hiatus semilunaris. The anterior ethmoidal cells appear as invaginations from the upper middle meatus and the posterior ethmoidal cells from invaginations of the floor of the superior meatus, both during the fourteenth week of gestation. Development of the sphenoid sinus begins in the third to fourth month of fetal life with pneumatization beginning around age one and completing growth by age twelve. The frontal sinus is typically not present at birth, becomes present by age four, and continues to aerate and grow until adulthood (Fig. 1).
The ethmoid sinuses are the first set of sinuses to fully develop, followed by the maxillary, sphenoid and frontal sinuses (in that order).15
ANATOMY OF THE NOSE AND PARANASAL SINUSES
Inferior Turbinate
The inferior turbinate comes off the lateral nasal wall. It is composed of a central bony skeleton covered by a mucosal layer. It articulates with the perpendicular plate of the palatine bone and the nasal surface of the maxilla. Its function is to help regulate nasal airflow and humidification.
Nasal Septum
The nasal septum separates the right and left nasal cavities and provides structural support for the nose.10
Fig. 1: Postnatal development of maxillary and frontal sinus.
From Rhinology: In: David Kennedy, Peter Hwang (Eds). Diseases of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012.
It is made of cartilage and bone, which are covered by mucoperichondrium and mucoperiosteum, respectively. The majority of the anterior septum is composed of the quadrangular cartilage. The membranous septum connects the quadrangular cartilage to the columella. The perpendicular plate of the ethmoid bone forms the bony upper one-third and the vomer forms the bony posterior and inferior portion. Finally, the nasal, frontal, maxilla, and palatine bones contribute to the periphery of the septum (Fig. 2).
Ethmoid Sinus
The ethmoid sinuses are formed from five lamellae: (1) uncinate process, (2) ethmoid bulla, (3) basal lamella of the middle turbinate, (4) lamella of the superior turbinate and (5) supreme turbinate. The basal lamella divides the anterior and posterior ethmoid cells.
Middle Turbinate
The anterior attachment of the middle turbinate is adjacent to the crista ethmoidalis of the maxilla. The posterior end is attached to the crista ethmoidalis of the perpendicular process of the palatine bone.11
Superiorly and medially, it attaches to the lateral aspect of the cribriform plate (dividing it into a medial and lateral lamella). The middle third turns laterally across the skull base to the lamina papyracea, where it then turns inferiorly. The vertical portion of the middle turbinate basal lamella is this portion that runs in a coronal plane and attaches to the skull base superiorly and the lamina papyracea laterally (and separates the anterior and posterior ethmoid sinuses). The posterior segment then becomes horizontal. The middle turbinate lies in three planes, thus providing for its stability (Fig. 3).16
Uncinate Process
The uncinate process is a thin, bony structure that attaches to the perpendicular process of the palatine bone and the ethmoid process of the inferior turbinate. Superiorly, it has many possible areas of attachment, including the lamina papyracea and the skull base (Fig. 4). Due to these variations, the frontal sinus outflow tract may drain directly into the superior aspect of the ethmoid infundibulum (less commonly) or into the middle meatus without a direct connection to the superior aspect of the ethmoid infundibulum (more commonly).1712
Fig. 3: Sagittal section through the nose and paranasal sinuses. The basal lamella of the middle turbinate divides the anterior and posterior ethmoid air cells.
Figs. 4A to C: Variants of attachment of the uncinate process. (A) Most commonly, the superior attachment of uncinate process is laterally onto the lamina papyracea. (B) It may attach to the skull base centrally. (C) It may attach to the skull base medially.
Agger Nasi
The agger nasi refers to the remnant of the ascending portion of the first ethmoturbinal. It is the anterior most pneumatized ethmoid air cell and lies immediately anterior and superior to the insertion of the middle turbinate. It lies anterior and inferior to the frontal sinus and forms the anterior border of the frontal sinus outflow tract.13
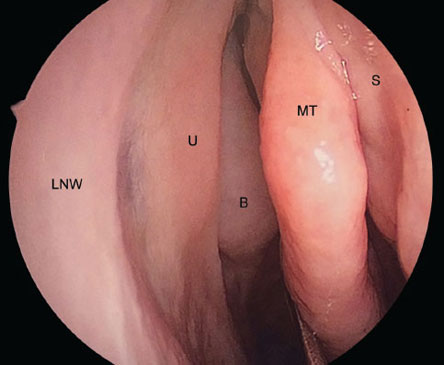
Fig. 5: Endoscopic view of right nasal cavity. (LNW: lateral nasal wall; U: uncinate process; B: ethmoid bulla; MT: middle turbinate; S: nasal septum.
From David Kennedy, Peter Hwang (Eds). Rhinology: Diseases of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012).
Ethmoid Bulla
It refers to the largest anterior ethmoid air cell. It forms from pneumatization of the bulla lamella. If it reaches the ethmoid roof, it can form the posterior wall of the frontal recess; however, if it fails to reach the skull base, it results in the formation of a space called the suprabullar recess. It is present medial to the lamina papyracea, posterior to the uncinate process, anterior to the vertical basal lamella of the middle turbinate, and posteroinferior to the frontal recess (Fig. 5).
Suprabullar and Retrobullar Recess (Sinus Lateralis)
The suprabullar recess is the superior space between the ethmoid bulla and the skull base. The retrobullar recess is the posterior space between the ethmoid bulla and the basal lamella of the middle turbinate. The suprabullar recess may extend into the retrobullar recess if the posterior wall of the ethmoid bulla is not in contact with the basal lamella of the middle turbinate. This space is bordered superiorly by the ethmoid roof, laterally by the lamina papyracea, inferiorly by the roof of the ethmoid bulla, and posteriorly by the basal lamella of the middle turbinate.14
Hiatus Semilunaris
It is a two-dimensional opening into the ethmoid infundibulum. It starts inferiorly from a plane between the free posterior margin of the uncinate process and the anterior face of the ethmoid bulla and extends superiorly to the space between the ethmoid bulla and the middle turbinate. The superior hiatus semilunaris is the opening to the retrobullar recess.
Infundibulum
The infundibulum is a funnel-shaped, three-dimensional structure. It is bordered medially by the uncinate process, laterally by the lamina papyracea, anteriorly/superiorly by the frontal process of the maxilla, superiorly/laterally by the lacrimal bone, posteriorly by the ethmoid bulla, and inferiorly by the natural ostium of the maxillary sinus (at its posterior and inferior one-third). It opens into the middle meatus through the inferior hiatus semilunaris (Fig. 6).15
Ostiomeatal Complex
This refers to the conglomerate of structures and sinuses that surround and drain into the middle meatus. It includes the anterior ethmoid, maxillary and frontal sinuses; the uncinate process; and the ethmoid infundibulum (Fig. 7).
Haller Cell
A Haller is also referred to as an infraorbital ethmoid cell as it is present in the floor of the bony orbit, which constitutes the roof of the maxillary sinus. It can cause narrowing of the ethmoid infundibulum or the maxillary sinus ostium. It most commonly pneumatizes from the anterior ethmoid, and less commonly, the posterior ethmoid sinuses (Figs. 8 and 9).
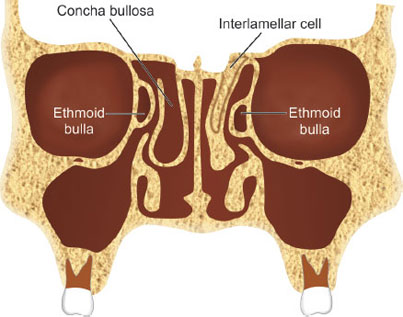
Concha Bullosa
The term concha bullosa is typically used to refer pneumatization of the middle turbinate, although it can apply to the superior turbinate as well (Fig. 10).16
The pneumatization typically originates from the frontal recess or the agger nasi. A concha bullosa is a normal anatomic variant; however, it may cause obstruction of the ostiomeatal complex and predispose a patient to sinusitis, in which case surgery may be appropriate. A concha bullosa can sometimes contain a mucocele or a mucopyocele.
Ethmoid Sinus Roof
The attachment of the middle turbinate to the skull base divides the cribriform plate into a medial and lateral lamella. The fovea ethmoidalis, which is the roof of the ethmoid sinus, is formed by the orbital plate of the frontal bone, laterally, and the lateral lamella of the cribriform plate, medially. The lateral aspect of the ethmoid roof is about 0.5 mm thick, whereas the lateral lamella has a thickness of only 0.2 mm. The thinnest area of the ethmoid roof is present along a groove in the lateral lamella at the site of the anterior ethmoid artery (AEA) (0.05 mm thick) and is the most common site for iatrogenic cerebrospinal fluid (CSF) leak during sinus surgery.
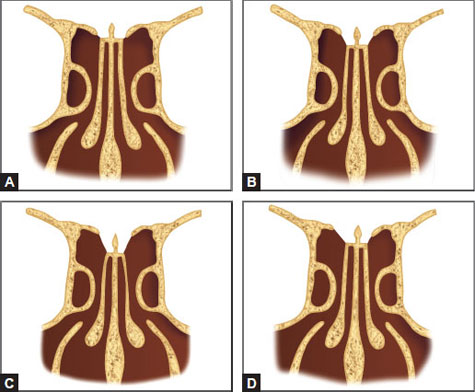
Keros described three configurations of the depth of the olfactory fossa, each of which depends on the length of the lateral lamella of the cribriform plate (Figs. 11A to D). As Keros type increases, there is lesser contribution from the thick frontal bone, with more of the ethmoid roof being formed by the thin lateral lamella. Thus, as Keros type increases, there is an increased risk of CSF leak.18
Figs. 11A to D: Keros classification. (A) Keros type type 1:1-3 mm; (B) Keros type 2:4-7 mm; (C) Keros type 3:8-16 mm (D) Asymmetric skull base.
Keros type 1: The olfactory fossa is 1–3 mm deep, the lateral lamella is short, and the fovea ethmoidalis is at almost the same plane as the cribriform plate.
Keros type 2: The olfactory fossa is 4–7 mm deep with a longer lateral lamella.
Keros type 3: The olfactory fossa is 8–16 mm deep, and the fovea ethmoidalis lies significantly above the level of the cribriform plate; this configuration leads to the highest risk of injury to the lateral lamella of the cribriform plate due to its steep angle and its thin, overlying bone.
The AEA is an important landmark within the ethmoid skull base. It runs in an anteromedial direction from the orbit to enter the skull base in the lateral lamella of the cribriform plate. The AEA then typically runs along the skull base, although, in well-pneumatized ethmoid sinuses, it may be found 1–3 mm below the roof of the skull base in a mesentery.
Posterior Ethmoid Sinus
The boundaries of the posterior ethmoid sinus are the basal lamella of the middle turbinate anteriorly, the anterior face of the sphenoid 19posteriorly, the lamina papyracea laterally, the superior/supreme turbinate medially, and the fovea ethmoidalis superiorly. The posterior ethmoid sinus drains into the superior meatus.
Superior and Supreme Turbinates
The superior and (if present) supreme turbinates are attached to the lateral nasal wall and the anterior skull base. The superior nasal meatus and supreme nasal meatus lie underneath their respective turbinates. The sphenoethmoidal recess is the space between the superior turbinate laterally, the roof of the nose superiorly, and the nasal septum medially. Its posterior border is the anterior face of the sphenoid sinus.
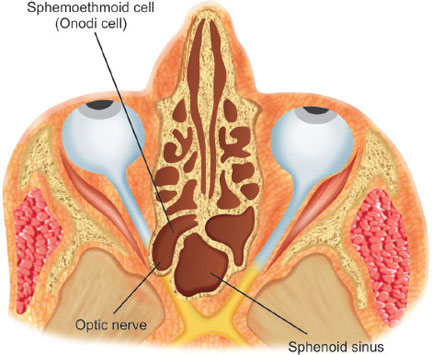
Onodi Cell
Also known as a sphenoethmoidal cell, an Onodi cell is a posterior ethmoid cell that is pneumatized far laterally and superiorly to the sphenoid sinus (Fig. 12). The sphenoid sinus lies medially and inferiorly to the most posterior cell of the posterior ethmoid complex. The optic nerve and carotid artery often are found in the lateral wall of the Onodi cell (as opposed to the lateral wall of the sphenoid sinus) and can, sometimes, be exposed.
Maxillary Sinus
Nasal Fontanelles
These are areas of the lateral nasal wall where no bone exists and are found above the insertion of the inferior turbinate. The middle meatus and maxillary sinus are separated only by a layer of periosteum, which can cause the fontanelles to be sites of accessory ostia to the maxillary sinus. The anterior fontanelle is anterior and inferior to the uncinate process while the posterior fontanelle lies posterior and superior.
The natural ostium of the maxillary sinus lies within the posterior one-third of the ethmoid infundibulum. The boundaries of a normal maxillary sinus are as follows: the alveolar portion of the maxilla inferiorly, the zygoma laterally, the floor of the orbit superiorly, the pterygopalatine fossa and infratemporal fossa posteriorly, and the inferior turbinate/uncinate process medially.18
Frontal Sinus
The frontal recess is the most anterior and superior part of the anterior ethmoid complex and communicates with the frontal sinus. The anterior and superior part of the middle turbinate forms the medial wall of the frontal recess while the lamina papyracea forms the lateral wall. It is bounded by the agger nasi cell anteriorly. The recess takes the shape of an inverted funnel in the sagittal section. The frontal recess has significant variation and can be obstructed by a well-pneumatized agger nasi cell or a large ethmoid bulla.
The frontal sinus aerates into the frontal bone. The anterior table (4–12 mm) is thicker than the posterior table (0.1–4.8 mm). The right and left frontal sinuses are typically separated by a thin bony partition called the frontal intersinus septum. Sometimes, an air cell may extend laterally over the superior orbital rim, giving the appearance of a septation in the lateral wall of the frontal sinus. This cell is called a supraorbital ethmoid cell since it is pneumatized from an ethmoid air cell of the orbital plate of the frontal bone. On endoscopic view, the ostium of a supraorbital ethmoid cell is located posterior and lateral to the frontal sinus ostium.
Four types of frontal cells have been described (Fig. 13):
Type 1: A single anterior ethmoid air cell located superior to the agger nasi cell, which does not pneumatize into the frontal sinus.
Fig. 13: Coronal representation of frontal sinus and recess anatomy.
(M: Maxillary sinus; F: Frontal sinus; AN: Agger nasi cell; 1–4: type 1–4 frontal cells; SOE: Supraorbital ethmoid cell; I: Frontal intersinus septal cell).
From David Kennedy, Peter Hwang (Eds). Rhinology: Diseases of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012.
Type 3: Single large anterior ethmoid cell superior to the agger nasi cell, which extends into the frontal sinus and has a connection to the frontal recess.
Type 4: An anterior ethmoid cell that appears to be completely contained within the frontal sinus.
Sphenoid Sinus
The sphenoid sinus is the most posterior and medially located paranasal sinus and drains into the sphenoethmoidal recess. The natural ostium of the sphenoid sinus is located on the face of the sphenoid sinus itself and is located 7 cm back (at a 30° angle) from the nasal spine in an adult.22
Fig. 14: Sphenoid sinus anatomy. The drawing is in an oblique plane, with the left side of the drawing demonstrating structures present more anteriorly, whereas the right side of the drawing shows more posterior structures.
From David Kennedy, Peter Hwang (Eds). Rhinology: Diseases of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012.
The ostium is located 1–1.5 cm above the superior aspect of the posterior choana and lies between the nasal septum and posterior insertion of the superior turbinate.
The roof of the sphenoid sinus is known as the planum sphenoidale. The sella turcica is the bony covering over the pituitary gland and is located in the posterior, superior aspect of the sphenoid sinus. The posterior wall of the sphenoid sinus meets the planum sphenoidale at the tuberculum sellae. The clivus lies just inferior to the sella turcica and is made of thick bone that forms the posterior inferior wall of the sphenoid sinus. The sphenoid rostrum forms the anterior face and floor of the sinus and articulates anteriorly with the vomer of the nasal septum. A sphenoid intersinus septum divides the right and left sphenoid sinuses. It is common for the right and left sphenoid sinuses to develop asymmetrically, and the intersinus septum can be deviated unilaterally with insertion onto vital structures such as the optic nerve or the internal carotid artery.
There are numerous important structures that surround the sphenoid sinus (Fig. 14). The pituitary gland lies posterior and superior in the midline within the sella turcica, just below the optic chiasm. The optic nerve and internal carotid artery can be seen as 23bony impressions in the lateral wall of the sphenoid sinus (with the optic nerve lying superior to the internal carotid artery). The opticocarotid recess can be seen between the optic nerve and internal carotid artery impressions. The cavernous sinus is located lateral to the lateral wall of the sphenoid sinus with CNs III, IV, V1, V2, VI and the internal carotid artery within it. The vidian nerve is located inferolaterally.
REFERENCES
- NIDCD Fact Sheet: Smell Disorder, Publication No. 09-3231. July 2009. Website https://www.nidcd.nih.gov/staticresources/health/smelltaste/SmellDisorders.pdf.
- Scherer PW, Hahn II, Mozell MM. The biophysics of nasal airflow. Otolaryngol Clin North Am. 1989;22(2):265–78.
- Engen T. The Perception of Odors. Academic Press; New York: 1982.
- Hadley K, Orlandi RR, Fong KJ. Basic anatomy and physiology of olfaction and taste. Otolaryngol Clin North Am. 2004;37(6):1115–26.
- Leopold D, Holbrook E. Physiology of olfaction. Cummings Otolaryngology—Head and Neck Surgery, 5th edition. 2010.; 41.
- Bramely P. Long-term effects of facial injuries. Proc R Soc Med. 1972;65(10):916–8.
- Kern RC, Conley DB, Haines GK 3rd, et al. Pathology of the olfactory mucosa: implications for the treatment of olfactory dysfunction. Laryngoscope. 2004;114(2):279–85.
- O’Brien E, Gurrola J, Leopold D. Olfaction and taste. Rhinology: Disease of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012; 57-66.
- Costanzo RM, Miwa T. Posttraumatic olfactory loss. Adv Otorhinolaryngol. 2006;63:99–107.
- Reiter ER, DiNardo LJ, Costanzo RM. Effects of head injury on olfaction and taste. Otolaryngol Clin North Am. 2004;37(6):1167–84.
- Reden J, Lill K, Zahnert T, et al. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: A double-blind, placebo-controlled, randomized clinical trial. Laryngoscope. 2012;122(9):1906–9.
- Henkin RI, Aamodt R, Babcock A, et al. Treatment of abnormal chemoreception in human taste and smell. In: Norris DM (Ed). Perception of Behavioral Chemicals. Elsevier/North-Holland Biomedical Press; Amsterdam: 1981.
- Malaty J, Malaty IA. Smell and taste disorders in primary care. Am Fam Physician. 2013;88(12):852–9.
- Gudis DA, Woodworth BA, Cohen NA. Sinonasal physiology. In: David Kennedy, Peter Hwang (Eds). Rhinology: Diseases of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012.
- Wise SK, Orlandi RR, DelGaudio JM. Sinonasal development and anatomy. In: David Kennedy, Peter Hwang (Eds). Rhinology: Diseases of the Nose, Sinuses, and Skull Base. Thieme Medical Publishers; 2012.
- Walsh WE, Kern RC. Sinonasal anatomy, function, and evaluation. In: Byron J Bailey, Jonas T Johnson (Eds). Head & Neck Surgery—Otolaryngology, 4th edition. Lippincott Williams & Wilkins: Philadelphia, PA; 2006. p. 307.
- Stammberger HR, Kennedy DW. Paranasal sinuses: anatomic terminology and nomenclature. Annals of Otology, Rhinology & Laryngology: Supplement, 167. 1995. pp. 7-16.
- Lang J. Clinical Anatomy of the Nose, Nasal Cavity, and Paranasal Sinuses. Thieme Medical Publishers; 1989.