ORTHOPEDICS—HISTORY AND EVOLUTION
INTRODUCTION
It will be interesting to know that “orthopedics” was born as specialty for deformity correction in children. In 1741 Nicholas Andry (Fig. 1.1) coined the word “orthopaedics”, which was derived from Greek words for “correct” or “straight” (orthos) and “child” (paidion). Both “orthopaedics” and “orthopedics” are accepted spellings and in vogue worldwide. Until the end of 18th century “orthopedics” was limited to correction of deformity in children and fracture treatment was largely restricted to traction, splints and bandages.
In 19th century three landmark discoveries in surgical field which made surgeries safe, painless and enthusiastic were development of principles of antisepsis by Sir Joseph Lister (Fig. 1.2), the discovery of ether anesthesia in 1846 by William Morton (1819–1868) and the invention of X-rays by Wilhelm Konrad Roentgen (1845–1923). Discovery of X-rays revolutionized the way of making diagnosis in orthopedic cases. Another vital contribution which modernized the management of fractures was invention of plaster of Paris (POP) bandage by Antonius Mathysen in 1851. In 20th century World War I and II contributed a lot to development of core orthopedics. World wars produced countless number of patients requiring amputation, debridement, fracture management, tendon surgeries, etc. In fact many great orthopedic surgeons were military surgeons like Sir Robert Jones, Gerdhard Kuntscher, Antonius Mathysen to name few of them. In world war II, there were less numbers of amputations, fewer infections, less gangrene cases and better fixations of fractures because of the lessons learnt from World War I. Now the scope of orthopedics has extended beyond fracture fixation and deformity correction and many specialty branches have emerged like spine surgery, orthopedic oncology, pediatric orthopedics, sports medicine, reconstructive orthopedics (joint replacement), etc.
SOME ORTHOPEDIC LEGENDS AND THEIR CONTRIBUTION
Galen (129–199 bc): Father of sports medicine. He is also credited with describing for first time the use of longitudinal traction for effecting reduction of overlapping bone fragments.
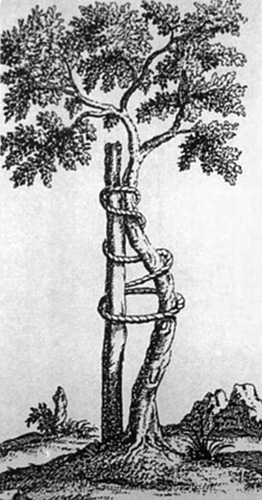
Nicholas Andry (1658–1759) (Fig. 1.1): He published the book “L'Orthopédie” in 1741 which conferred him the title of “Father of Orthopedics”. For correction of curvature deformity of tibia he suggested bandaging the limb to an iron plate (Fig. 1.3). This is the famous engraving of the “crooked tree” which has become the symbol of orthopedics.
Percival pott (1714–1788): Pott's fracture. Potts paraplegia.
2
Jean-Andre Venel (1740–1791): He is considered by some as “Father of Orthopedics”. He established the first Orthopedics institute in the world in Switzerland.
Hugh Owen Thomas (1834–1891): He devised Thomas splint and Thomas test for flexion deformity of hip. He is also know as “Father of British Orthopedics”.
Sir Robert Jones (1855–1933): He was the nephew of great Hugh Owen Thomas. He is known as “Father of Modern Orthopedics”. He described the Jones fracture, Robert Jones bandage. He published first report of use of X-ray in orthopedics.
Albin Lambotte (1866–1955): He coined the term osteosynthesis and regarded as the “Father of Internal Fixation”. He also devised first external fixator.
Martin Kirschner (1879–1942): Who gave the very simple but very useful “K wire” to orthopedics.
Lorenz Bohler (1885–1973): Father of trauma surgery.
Kenji Takagi (1888–1963): Father of arthroscopy.
Austin T Moore (1899–1963): He performed the first metallic hip replacement. He gave the Austin-Moore prosthesis, which is still used today.
Gerhard Kuntscher (1900–1972): His big contribution to orthopedics is intramedullary nail which revolutionized the treatment of diaphyseal fractures of long bones.
Sir Raginald Watson Jones (1902–1972): He devised the Watson Jones approach (anterolateral approach) to hip joint. He was the student of Sir Robert Jones. He was the first editor of Journal of bone and joint surgery (British).
Paul Randall Harrington (1911–1980): Harrington invented the Harrington Rod, a device that has helped more than 1 million patients of scoliosis to keep the spine straighten.
Sir John Charnley (1911–1982) (Fig. 1.4): Father of total hip arthroplasty. He was the great innovator of the modern total hip replacement and use of bone cement in total hip replacement.
Gavriil Abramovich Ilizarov (1921–1992): He gave the famous Ilizarov theory that bone would grow if gradually distracted. His work pioneered the new way of treatment of one of most difficult cases of orthopedics; infected non-union, deformity correction and limb lengthening.
Masaki Watanabe (1911–1995): Father of modern arthroscopy. He performed first arthroscopic partial meniscectomy.
William F Enneking (1926–2014): Father of Orthopedic oncology. He gave a classification for bone tumors.
HIGH-YIELD POINTS
ORTHOPEDIC TERMINOLOGY
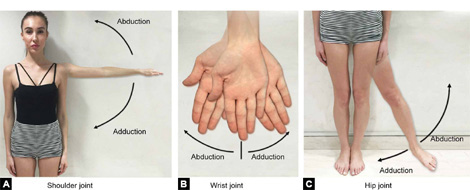
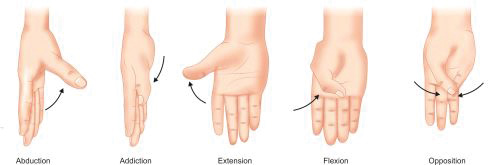
Abduction (Figs. 1.5A to C)—Movement of limb away from mid-sagittal plane of body.
Adduction (Figs. 1.5A to C)—Movement of limb towards the mid-sagittal plane of body.
(Abduction and adduction movement occurs in coronal plane)
Ankylosis (Fig. 1.6A): Stiffness or fusion of a joint due to abnormal adhesions between two joint surfaces.
Arthrocentesis: Joint aspiration (withdrawing synovial fluid/blood from the joint).
Arthrodesis (Fig. 1.6B): Surgically induced fusion of two joint surfaces.
Arthroeresis: It refers to an operation carried on a joint to restrict an undue mobility.
Arthrography: X-ray examination of joint after injecting contrast material (It has largely been replaced by MRI).
Arthropathy: A disease of a joint.
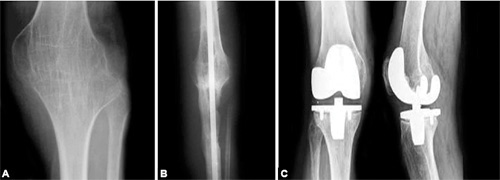
Arthroplasty (Fig. 1.6C): Replacement of a joint with a prosthesis.
Arthrosis: Degenerative wear and tear of cartilage of the joint (Osteoarthritis).
Arthrotomy: A procedure where surgeons cut into the joint (usually done to drain pus from joint).
Calcaneus: Foot fixed in dorsiflexion deformity at ankle joint.
Calcification: Deposition of amorphous calcium phosphate.
Cavus: Exaggeration of longitudinal arch of foot.
Clonus: Successive rhythmic involuntary muscular contraction and relaxation (pathological hyperreflexia of normal deep tendon reflex). More than 5 beats are significant.
Chemonucleolysis: Injection of chymopapain (an enzyme that dissolves part of the disk) into disk space to dissolve the disk (as a treatment of prolapsed disk)
Circumduction (Figs. 1.7A and B): It is a combination of flexion, extension, abduction and adduction. In this movement distal end of a limb makes a conical movement and the apex of the cone is at the proximal end of the limb.
Dorsum: Upper surface of an animal (dorsal surface of hand is surface opposite the palm) or back of human.
Epiphyseal plate/growth plate or physis: Hyaline cartilage present above the proximal metaphysis and below the distal metaphysis separating metaphysis from epiphysis.
Epiphysiodesis: Surgically induced fusion of epiphyseal plate or physis to metaphysis or/and epiphysis.
Equinus: Foot fixed in plantar flexion at ankle joint.
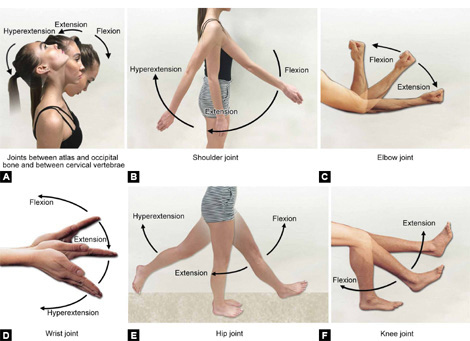
Extension (Figs. 1.8A to F): Movement in sagittal plane which increases angle between two body parts.
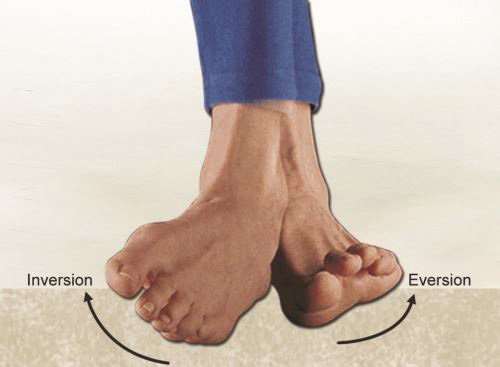
Eversion (Fig. 1.9): Plantar surface (sole) of foot rotates away from mid-sagittal plane.
Flexion (Figs. 1.8A to F): Movement in sagittal plane which decreases angle between two body parts.
Genu: Pertaining to knee.
Hemarthrosis: Bleeding into joint space.
Impacted fracture: When fracture fragments are driven into each other.
Interosseous membrane: A fibrous sheath that separates two bones (between two forearm bones or two leg bones).
Kyphosis: Normal backward convex curvature of spine in thoracic and sacral region.
Laminectomy: Removal of spinal lamina (usually to decompress spinal cord or nerves).
Lordosis: Normal backward concave curvature of spine in cervical and lumbar region.
Leminotomy: Removal of a part of lamina.
Manus: Pertaining to wrist/distal portion of forearm.
Myositis: Inflammation of the muscles.
Neurolysis: Interruption of the transmission of nerve signal (usually for pain relief) by application of physical agents (heat or freezing) or chemicals (such as phenol or alcohol) to a nerve.
Neurorraphy: Surgical suturing of a divided nerve.
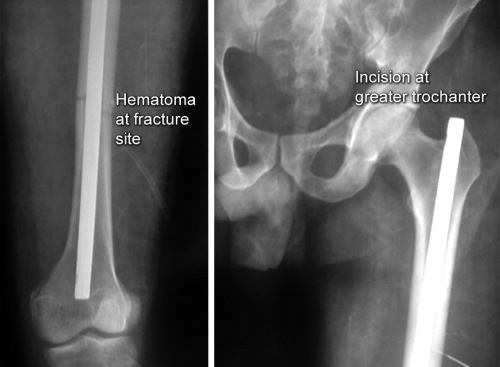
Open reduction: Reduction of fracture fragments by surgical exposure and under direct vision. In open reduction fracture hematoma is drained.
Opposition (apposition) of thumb (Fig. 1.10): A movement unique to thumb in which thumb rotates around its long axis and its palmar surface comes in contact with palmar surface of little finger.
Orthotics: Orthotic is a device that aids/supports a body part and enhances the structural and functional characteristics of the skeletal system.
Ossification: Laying down of new bone or deposition of crystalline calcium phosphate to form new bone.
Osteoclasis: Surgically induced fracture of bone (to correct deformity).
Osteogenesis: Bone formation.
Osteometric devices: Theses are used to measure bone length.
Osteonecrosis: Death of bone tissue.
Osteosynthesis: Stabilization and internal fixation of fracture.
Osteotomy: A surgical procedure in which bone is cut in order to correct deformity.
Plantar surface: Inferior surface of foot which comes in contact of ground.
Plantaris: Equinus that occurs at the fore foot is called plantaris.
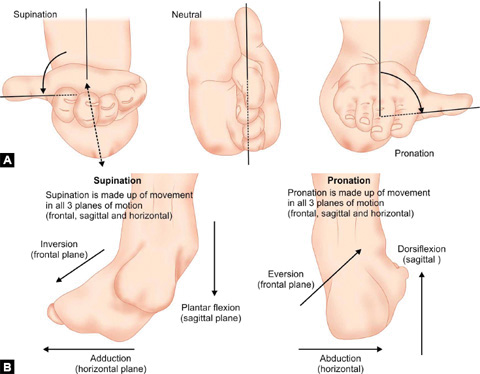
Pronation (Figs. 1.11A and B): Rotation of forearm and hand so that palm faces downward. Pronation of foot is combination of eversion, abduction and dorsiflexion.
Prosthetics: The art and science of developing artificial replacements for body parts.
Recurvatum: Excessive extension deformity of joint (genu recurvatum is hyperextension deformity of knee joint, i.e. knee bends backwards). It is opposite to flexion deformity.
SLAP lesion: Tear of superior labrum of shoulder anteriorly and posteriorly.
Spondylitis: Inflammation of vertebrae.
Spondylolisthesis: Anterior or posterior translation of one segment of spine in relation to the vertebrae below.
Spondylolysis: A defect in pars-interarticularis of vertebral arch. It may progress to spondylolisthesis.
Subluxation: Incomplete dislocation of a joint (articular surfaces remain in contact).
Supination (Figs. 1.11A and B): Rotation of forearm and hand so that palm faces upward. Supination of foot is the combination of inversion, adduction and plantar flexion.
Synovitis: Inflammation of synovium.
Tenodesis: Surgical suturing/anchoring of tendon to bone.
Tenolysis: Surgical release of a tendon from adhesions.
Tenotomy: Surgical division of a tendon.
Tenosynovitis: Inflammation of a tendon and its sheath.
Valgus: Position of distal end of long bone or a joint more lateral than it should be.
Varus: Position of distal end of long bone or a joint more medial than it should be.
(Note: Varus and valgus are coronal plane deformities)Volar: Pertaining to palm or sole (palmar aspect of hand, plantar aspect of foot).
ANATOMY AND COMPOSITION OF BONE AND BONE GROWTH
BONE ANATOMY AND PHYSIOLOGY
Introduction
Bone is the basic unit of the skeletal system of the body. Bones along with ligaments and cartilage provide a strong yet flexible framework on which muscles attach through tendons to generate coordinated movements of the body. Human skeleton is divided into axial and appendicular parts.
Axial skeleton: Skull, vertebral column and thoracic cage.
Appendicular skeleton: Shoulder girdle and upper limbs, pelvic girdle and lower limbs.
HIGH-YIELD POINTS
Functions of Skeleton System
-
Shape and movements: Skeleton framework gives shape to body and in coordination with muscles it allows for body movements.
-
It protects vital organs as ribcage provides protection to lungs and skull provides protection to the brain.
-
Bone is a store house of minerals specially calcium and phosphorus and plays vital role in the calcium metabolism (mineral homeostasis) in the body.
-
In adults flat bones such as the pelvis, sternum, cranium, ribs, vertebrae and scapulae contain the red bone marrow and are the main site of blood production.
Structure of Bone
Bones are made up of bone cells (osteoblast, osteoclast and osteocytes) and intercellular matrix. Bones are densest tissue in the body due to deposition of minerals in the intercellular matrix. Deposition of minerals in the intercellular matrix makes the bones hard but light weight, strong but elastic so that it can efficiently support the movement, walking and running without bending or breaking.
Bone matrix consists of organic and inorganic components. Organic component includes collagen fibers (mostly Type I collagen). Inorganic matter is composed mainly of calcium and phosphorus in a crystalline form called “hydroxyapatite”. Bone also contains other minerals in small amount, i.e. magnesium, potassium, strontium and ferrous salts, etc. Organic matter gives the bones its flexibility and elasticity while inorganic matter gives the strength and hardness to the bones. Composition of bone is given in Box 1.1.
Bone cells: There are three types of bone cells—(1) osteoblast, (2) osteoclast and (3) osteocytes.
Osteoblasts: These are bone forming cells which derive from mesenchymal precursors in the bone marrow. These are mononuclear cells which have well-developed rough endoplasmic reticulum and a large Golgi complex. Osteoblast lays down new matrix which is known as osteoid (uncalcified matrix). These are rich in alkaline phosphatase and produce type I collagen and other noncollagenous bone proteins. Osteoblasts are activated by parathyroid hormone and they control osteoclastic activity. Osteocytes are terminally differentiated stage of osteoblasts which get embedded in osteocytic lacunae.
Osteoclast: These are bone reabsorbing multinucleated giant cells derived from mononuclear precursors of macrophage lineage (specifically monocytes) in the marrow. These cells are activated by osteoblast. Their main function is to resorb bone and is thus involved in bone remodeling. Active osteoclasts are present in excavations in bone formed by them after erosion of matrix, the excavations being called as “Howship's lacunae”.
A typical long bone derives its blood supply from nutrient artery, epiphyseal vessels and periosteal vessels. Nutrient artery supplies the diaphysis and metaphysis and epiphyseal region is supplied by epiphyseal vessels. Periosteum is richly supplied by periosteal vessels which also supply the outer cortex. If nutrient artery is damaged periosteal vessels are usually sufficient to nourish the bone. Bones can be classified based on anatomy and structure (Table 1.1).
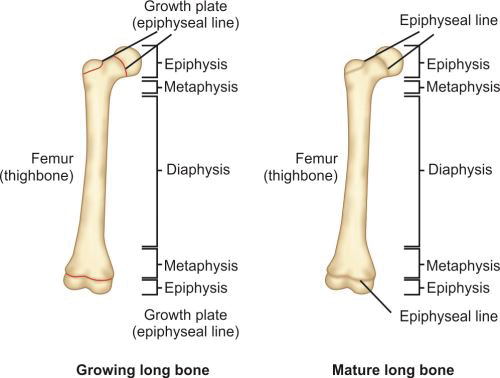
Cortical bones are long bones of the body like femur or humerus and the small bones of hand and foot like metacarpals and metatarsals. Long bones of a child (Fig. 1.12) are divided into epiphysis, physis or growth plate, metaphysis and diaphysis. Epiphysis, physis and metaphysis are present at both ends. In mature bone epiphysis fuses with metaphysis and growth plate gets replaced by bone.
Parts of a growing long bone:
-
Epiphyses: Epiphyses are present on the ends of the bones. It consists of cancellous bone covered by a thin layer of compact bone. On its ends it is covered by articular cartilage and forms the joint. In long bones epiphysis is present on both the ends; it is present on only one end of metacarpal, meta-tarsals and phalanges.
-
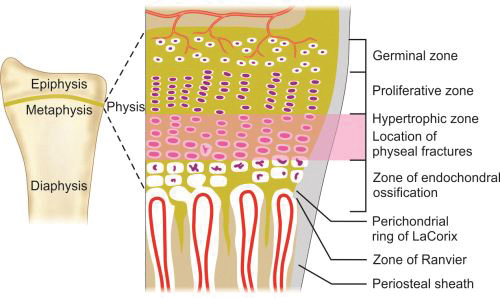
Physes: Physis or growth plate is a thin region of actively growing bone cells between the epiphysis and the metaphysis in a growing bone. This is present on both the end of the long bones and responsible for the longitudinal growth of the bones. It consists of four zones (Fig. 1.13):
- Germinal zone/Resting zone
- Proliferative zone
- Hypertrophic zone
- Zone of endochondral ossification.
Germinal zone provides the developing chondrocytes which divide and get organized into columns in proliferative zone. Both germinal and proliferative zones are rich in extracellular matrix. In hypertrophic zone chondrocytes stop mitoses and undergo hypertrophy. This is the weakest zone of physis and most physeal injuries occur through this plane.
Physis is connected to epiphysis and metaphysis by the zone of Ranvier and perichondral ring of LaCorix. Zone of Ranvier contains germinal cells, which is responsible for circumferential growth of physis. Ring of LaCorix is a fibrous structure that is connected with zone of Ranvier and periosteum of metaphysis.
-
Metaphysis: It is the junction between the growth plate and the diaphysis. It consists of both cancellous and cortical bone.
-
Diaphysis: It is the region between the two metaphyses. It consists of compact cortical bone and has a medullary canal which consists of marrow. This portion of the bone is responsible for the strength of bone for weight bearing and movement.
Cancellous bones or trabecular bone: Small bones of the wrist, bones of the hind and mid-foot like calcaneum, talus, etc. and the epiphyseal and metaphyseal areas of long bones, flat bones (pelvis, ribs, skull, etc.), and vertebrae are cancellous bones. Cancellous bones are more porous, more vascular and have larger surface area than compact bone. It contains sheets of bone called trabeculae which connect open spaces of cancellous bone giving a honeycomb appearance. Red marrow fills the spaces around trabeculae. Trabeculae contain bone cells osteoblasts, osteocytes and osteoclasts. Osteoblasts are the bone-forming cells which secrete hard tissue of bone around them. When they are completely surrounded by layers of hard matrix they get converted into osteocytes which are present into small space called lacuna.
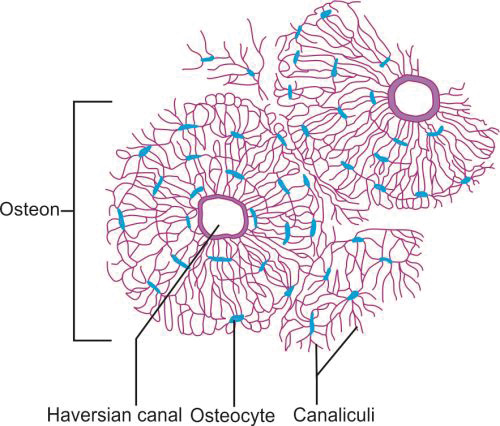
Microscopic Structure of Cortical Bones (Fig. 1.14)
Cortical bone consists of a number of columns of cells called “osteon”. Each osteon has layers of bone cells like osteoblasts, osteocytes and osteoclasts around a central canal called “Haversian canal”. The outer border of an osteon is lined by a cement line which is a region of collagen-poor bone matrix.
The Haversian canal surrounds the blood vessels and nerves cells throughout the bone and communicates with the osteocytes in the lacunae through canaliculi.
“Volkmann's canals” run perpendicular to the “Haversian canals”. They interconnect the Haversian canal with each other and the periosteum. These canals allow for the transfer of nutrients.
Woven bone and lamellar bone: Woven bone is relatively weak and is characterized by random organization of collagen fibers. It is immature bone which is not stress oriented (lamellar bone is stress oriented as it has parallel arrangement of collagen fibers). Woven bone is present in all fetal bone and in initial stage of healing (later it gets replaced by lamellar bone). Woven bone is quickly produced and it has high rate of turn-over compared to lamellar bone. In contrast to woven bone lamellar bone is mechanically stronger as it has a parallel alignment of collagen into sheets (lamellae). Both mature cortical and cancellous bones are lamellar bone.
HIGH-YIELD POINTS
GROWTH OF BONE
The process of formation of bone is called “ossification”. During the fetal stage it occurs either by “intramembranous ossification” or by “endochondral ossification”.
Intramembranous ossification: It occurs in flat bones and in clavicle. Here calcium hydroxyapatite is directly deposited into a preexisting membrane (primitive connective tissue) without any intervening cartilage model stage. During intramembranous ossification mesenchymal cells, derived from neural crest proliferate. Some of these mesenchymal cells differentiate into osteoblasts (ossification center) which secrete collagen and proteoglycan. Calcification occurs in this collagen matrix. During this process of calcification, bony (calcified) spicules are formed. A layer of mesenchymal cells that surround the calcified spicule forms the periosteum.
Endochondral ossification: Endochondral ossification is seen in long bones of the body. It involves the formation 10of cartilage and its subsequent replacement by bone. During endochondral ossification some mesenchymal cells develop into chondrocytes. These chondrocytes proliferate and secrete extracellular matrix which is mineralized to form cartilage model. Now cartilage cells start dying and few cells surrounding cartilage become osteoblast (ossification center) and secrete bony matrix into degrading cartilage. In this way the whole cartilage gets replaced by bone.
Primary ossification centers appear in the cartilage during the fetal development. These are responsible for the formation of diaphysis of the bones. Secondary ossification centers mostly appears after birth and are responsible for the formation of epiphysis of the long bone and the extremities of flat and irregular bones.
Appositional (Increase in Width) and Interstitial Growth (Increase in Length)
In fetal life each long bone is represented by a rod of hyaline cartilage surrounded by perichondrium. Interstitial growth in this cartilage model occurs when chondrocytes within the cartilage divide and secrete new matrix and increase the length of cartilage model. Appositional growth occurs when chondroblasts in the perichondrium produce new matrix at the periphery and increase the width of cartilage model.
In contrast to cartilage, bone grows only by appositional growth. Longitudinal growth which occurs before maturity is due to cartilage proliferation in the epiphyseal and metaphyseal areas of long bones, before subsequently undergoing mineralization to form bone. Inner layer of periosteum (cambium layer) contains osteoprogenitor cells which develop into osteoblast. These osteoblast secret new bone matrix and increase the thickness of bone.
HIGH-YIELD POINTS
FRACTURE TYPES AND CLASSIFICATIONS
FRACTURE TYPES AND CLASSIFICATION SYSTEMS
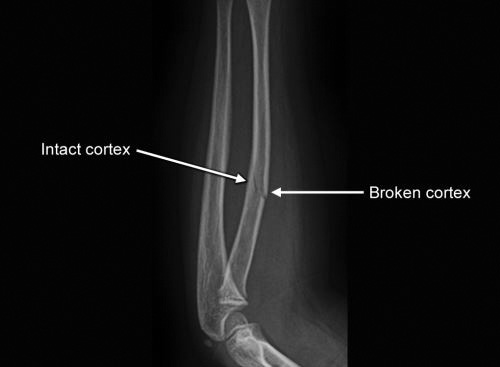
A fracture is a break in the continuity of bone (even a single cortex) with or without displacement. There are many ways to classify fractures (Table 1.2). Classification systems have evolved from description of clinical appearance of fracture (before invention of X-rays, i.e. colles fracture) to radiology based fractures description.
Why a Classification is Needed?
-
It guides the treatment—a common treatment approach is usually followed for same injuries, i.e. interlocking nailing for diaphyseal fracture of femur or tibia.
-
It explains prognosis—open fractures are more likely to carry poor prognosis than close fractures, high chance of avascular necrosis in some fracture, i.e. fracture proximal pole of scaphoid and basal fracture neck of femur.
-
It makes easy to describe a fracture, i.e. to give details of fracture to other surgeons.
Mechanism of injury and fracture pattern (Table 1.3): A fair idea of mechanism of injury can be inferred from X-ray appearance of fracture.
Symptoms and Signs of Fracture
Pain, swelling, tenderness and abnormal mobility are present at the site of fracture. There may be varying soft tissue injury around the fracture.
|
Displaced fracture presents with deformity at fracture site. Patient is not able to use his fracture limb, i.e. weight bearing is not possible on fractured tibia or femur. Crepitus can be elicited at fracture site but is often very painful. Any fracture (especially high velocity injury) may be associated with injury to surrounding vital structures (vessels and nerves).
HIGH-YIELD POINTS
|
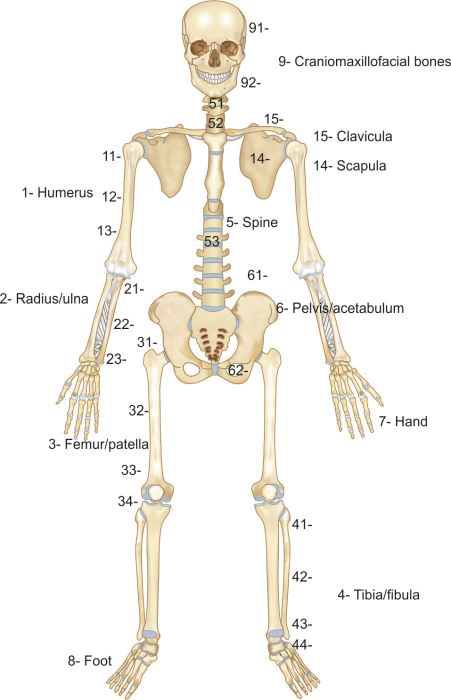
AO classification (Muller AO/OTA classification): This is a unique and comprehensive classification (Boxes 1.2 and 1.3) which can be applied to fracture of all bones. It is an alphanumerical classification, i.e. numbers and alphabets are used to classify a fracture. Recently a pediatric version has also been published.
In AO classification each major bone is given a number (Fig. 1.15) like humerus, forearm bones, femur and leg bones have been assigned 1, 2, 3 and 4 respectively. Each long bone is divided into three parts or segments; proximal segment, diaphysis and distal segment which are assigned 1, 2 and 3 number respectively. Each part is further divided into types (Table 1.4).
Types of proximal and distal segment fractures are classified as:
-
A—Extraarticular
-
B—Partial articular
-
C—Complete articular
Types of diaphyseal fractures are classified as:
-
A—Simple
-
B—Wedge
-
C—Complex
Each type is further divided into groups and subgroups which differ from bone to bone.
|
BIOLOGY OF FRACTURE HEALING
DELAYED UNION AND NONUNION
There is no universal definition of fracture nonunion which is applicable to all fractures. Arbitrarily nonunion fracture is one that has not united and is not expected to unite without intervention. In nonunion fracture gap is filled by fibrous tissue or fibrocartilage.
Delayed union of fracture is defined as when fracture takes more than usual time to unite depending on the type and site of fracture, but shows some progression towards union over time. Most orthopedic surgeons do not consider nonunion of fracture of shaft of long bones to happen before 6 months.
United States Food and Drug Administration panel definition of nonunion: “When union does not take place in 9 months after fracture and fracture shows no visible progressive signs of healing for 3 months”.
Etiology
Causes of nonunion are multifactorial and may be related to patient, injury, fracture or treatment.
Patient or host factors:
-
Age: Old age
-
Malnutrition: Albumin less than 3.5 and lymphocytes less than 1,500 cells/mL indicates poor healing potential
-
Smoking/tobacco abuse
-
Alcohol abuse
-
Systemic diseases: Diabetes, cancer, metabolic bone diseases, osteomalacia, cushing disease.
-
Drugs: Nonsteroidal anti-inflammatory drugs (NSAIDs), antineoplastic drugs, corticosteroid therapy and bisphosphonates
-
Radiation therapy
-
Infection
Local factors (related to injury):
-
Open fractures (excessive periosteal stripping and soft tissue damage)
-
Intraarticular fracture
-
Denervation of bone
-
Bone loss, segmental fracture
-
High velocity injury/severely comminuted fracture
-
Intact fellow bone (intact fibula may prevent apposition of fracture fragments in case of fracture tibia)
-
Soft tissue interposition
Factors related to treatment:
-
Inadequate reduction (Fig. 1.16)
-
Inadequate internal fixation: Implant too short, construct too stiff.
-
Inadequate immobilization: Excessive motion at fracture site leads to disruption of early bridging callus
-
Wrong surgical technique: Excessive periosteal stripping.
Fracture specific factors:
-
Vicarious blood supply: Some fractures are prone to nonunion due to vicarious blood supply of the bone (Box 1.4).
-
Pathological fractures have poor healing potential
-
Neuropathic fractures.
CLINICAL FEATURES
Patient who has been waiting for union of fracture for long time after fracture presents with persistent pain and tenderness at the fracture site with functional disability (inability to use limb). Instability (frank mobility, crepitus) may be present. Patient should be evaluated for signs of infection like swelling, increased local temperature, discharge, etc.
Radiology
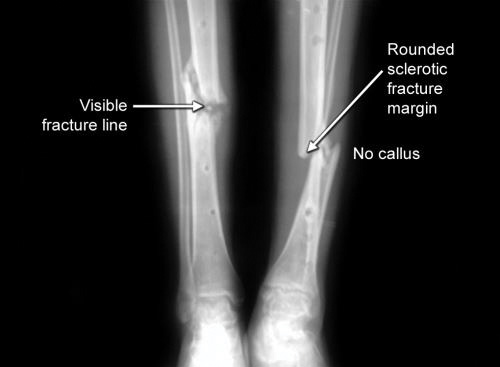
X-ray of fracture nonunion may show following signs (Fig. 1.17): Persistent fracture line, paucity of callus, sclerotic and rounded fracture ends with obliteration of medullary canal and osteopenia in surrounding bone. CT scan may show persistent fracture line in doubtful cases. Scintigraphy helps to distinguish between biologically active (rich blood supply) and nonresponsive nonunion (poor blood supply).
HIGH-YIELD POINTS
Types
Fracture nonunion is classified based on the blood supply of fracture ends (Table 1.5).
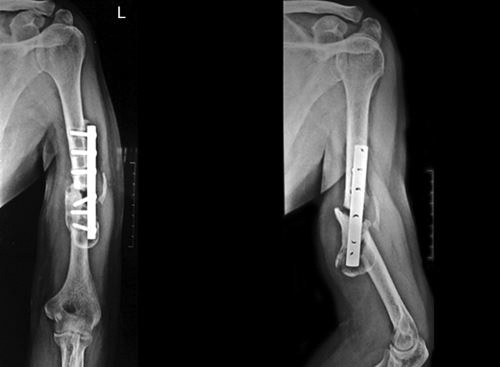
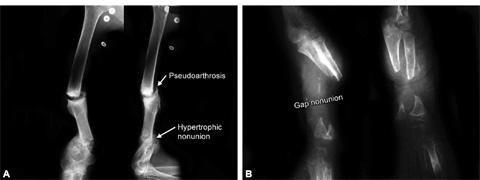
PSEUDOARTHROSIS (FIG. 1.18A)
It is a type of nonunion, characterized by fluid filled cavity (clear fluid) which is lined by a membrane (synovial like cells), between fracture ends. Bone scan shows vascularized bone ends. It occurs due to insecure fixation where excessive motion leads to false joint formation.
Treatment
In hypertrophic nonunion stable internal fixation may promote union but treatment of oligotrophic and atrophic noninfected nonunion is open reduction, debridement of sclerotic fracture ends and bone grafting.
|
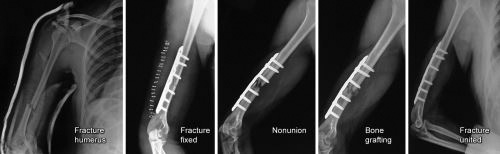
Figs. 1.18A and B: (A) X-rays of humerus AP and lateral views showing pseudoarthrosis and hypertrophic nonunion; (B) X-ray forearm AP and lateral views showing defect nonunion leading to atrophic and osteoporotic bone ends.
Fig. 1.19: X-ray of leg AP and lateral views showing sclerosed fracture ends and scanty callus formation with obliterated medullary canals- typical of atrophic nonunion.
Nonunion with bone loss, shortening and deformity are difficult cases and require distraction osteogenesis using ring fixator based on the Illizarov's principles (page 29).
Apart from surgical methods biophysical methods (Box 1.5) have also been used in treatment of delayed and nonunion with varying success.
BONE GRAFTING
A bone graft is a transplanted bone. Its use is indicated to stimulate bone union, to replace lost bone or to assist in revascularization of avascular segments.
Types
A graft transplanted from one site of skeleton to another site within the same individual is called an autograft/autogenous bone graft.
-
Autogenous bone graft is gold standard. Fresh grafts that are transferred directly from the donor to the recipient site are usually autogenous grafts.
-
Xenografts are transplanted from one species to a member of a different species.
-
An isograft is transplanted from one monozygotic twin to the other.
Additionally, the graft may be described as cortical, cancellous, corticocancellous and osteochondral (bone and cartilage piece).
The graft may be vascularized with its own blood supply (vascularized bone graft) or it may be nonvascularized (free grafts). The vascularized bone grafts can be harvested in two ways. One is to take a bone (usually fibula) and keep its blood vessel intact (free vascularized bone graft) and anastomose the same to a vessel at the recipient site. The other is to take a bone along with an attached pedicle of muscle such that the vessel of the muscle is kept intact (muscle pedicle bone graft). This bone can be transplanted to a nearby site and the muscle's vessel continues to supply the bone.
Properties of Graft
Osteoinduction: Process of inducing pluripotent/primitive mesenchymal cells to differentiate into bone forming cells osteoblasts.
Osteoconduction: Graft acts as scaffold for the growth of new bone on its surface and deep down into pores, when placed in contact with native bone.
Osteogenesis: Graft itself provides bone forming cells osteoblasts.
HIGH-YIELD POINTS
BONE GRAFT SUBSTITUTES
Limited availability of autologous bone graft, donor site morbidity limits the use of autogenous bone graft. Allogenic bone grafts have limited potential of osteoinduction and inferior mechanical strength as compared to autologous bone graft due to freeze-drying used in its storage and sterilization. They also carry a small risk of disease transmission. These shortcomings led to developments of bone graft substitutes. Table 1.6 summarizes the different bone graft substitutes used in orthopedics.
FRACTURE HEALING
Fracture healing is a remarkable process that aims at the restoration of exact anatomy of bone. Although it is a continuous process but divided into five phases which overlap each other. Depending upon the type of fracture and method of fracture fixation and immobilization fracture healing is of two types primary and secondary.
Primary Fracture Healing or Direct Fracture Healing (Healing without Callus)
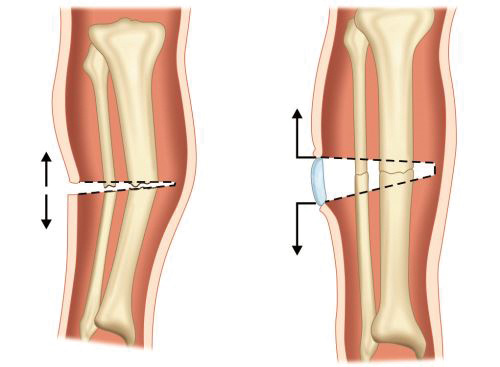
It is less common mode of fracture healing. It is seen in rigid internal fixation of fractures (compression plating) and in unicortical fractures (Greenstick fracture). It is direct attempt of bone to restore its continuity without forming fracture callus. It is of two types, gap healing and contact healing. Gap healing occurs when there is minimal gap in between rigidly fixed fracture ends. Woven bone (immature bone having random arrangement of collagen fibers) is initially formed in transverse orientation between fracture gaps which is later replaced by lamellar b-one (regular parallel alignment of collagen into sheets/lamellae).
Contact healing is seen when fracture ends are closely approximated to each other without any gap. Osteoclasts at one end of fracture cause bone resorption to form so called cutting cones such that the fracture gap widens initially. In these cones osteoclast cut by osteoclasts lay down new bone.
|
Secondary Fracture Healing or Indirect Fracture Healing (Healing by Callus Formation)
This is more common method of fracture healing. It is seen in absence of rigid fixation (cast immobilization, intramedullary nailing and bridge plating for comminuted metaphyseal fractures). It is divided into five phases (see Box 1.6):
-
Stage of hematoma formation: It starts within few hours of fracture with hematoma formation. Periosteal and intramedullary vessel disruption produces a hematoma at fracture site.
-
Stage of granulation tissue/inflammatory phase: Hematoma accumulates beneath periosteum and between fracture ends and act as a source of growth factors and cytokines that initiate cellular events of healing. Inflammatory response peaks at 24 hours with accumulation of neutrophil, platelets, lymphocytes, macrophages, endothelial cells and fibroblasts. These cells produce a number of growth factors and cytokines like fibroblast growth factors (FGFs), vascular endothelial growth factors (VEGFs), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), interleukin-1 and interleukin-6 (IL-1 and IL-6), tumor necrosis factor-α (TNF-α) and bone morphogenetic proteins (BMPs). These factors act as chemoattractants for mesenchymal stem cells derived from periosteum and bone marrow and also induce their differentiation into chondroblast, osteoblast, fibroblast and angioblast.
-
Callus formation: It starts within few days of the end of inflammatory phase and lasts for few weeks. The stem cells that have differentiated into osteoblasts synthesize new bone to effect repair (Repair phase). The phase consists of intramembranous ossification, chondrogenesis and endochondral ossification. As healing progresses PH gradually becomes alkaline from acidic pH of inflammatory phase. In the initial stage of this phase the healing tissue is composed of mixture fibrous connective tissue, cartilage, some woven bone and osteoid called as provisional callus or soft callus. This callus is pliable and weak good enough to prevent shortening at fracture site but not angulation or rotation. Callus becomes visible earliest on radiographs by 3 weeks.
-
Stage of consolidation: Cells from the cambium layer of periosteum are the earliest to produce bone and osteoblast at the periphery of callus also produce bone by intramembranous ossification. Simultaneously chondrocytes derived from stem cells simultaneously lay down cartilaginous matrix. Mineralization of this cartilaginous matrix takes place by these chondrocytes. Ultimately at the end of this phase whole callus is composed of woven bone (mineralized callus but lacking a lamellar structure, also called as hard callus). This woven bone or hard callus is rigid and strong and when it is formed bone is said to have clinically united.
-
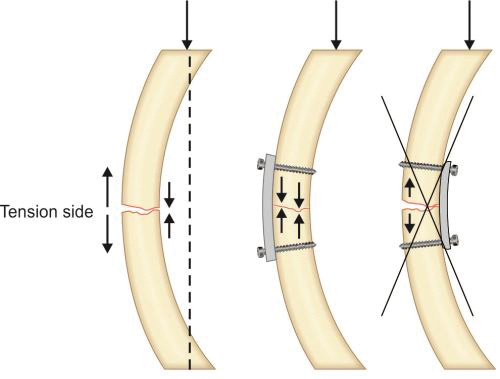
Remodeling phase: This phase continues for the years and this aims at restoration of exact architecture of bone. Some biologists call it the modeling phase where special units called as bone remodeling units consisting of both osteoclasts and osteoblasts are formed on the surface of the bone. The osteoclast reabsorb the woven bone and osteoblast deposit more and more osteoid. In this way the whole woven bone is eventually replaced by lamellar bone. Thicker lamellar bone is laid in the area of high stresses (these are compression sites having more of osteoblastic activity) while in areas with no stress (these are tension sites having more of osteoclastic activity) unwanted bony buttresses are curved away to make the bone attain anatomical shape (Wolf's law).
Figure 1.20 is showing in series radiological progress in a uniting bone.
HIGH-YIELD POINTS
FRACTURE CONSIDERATION IN CHILDREN AND PHYSEAL INJURIES
Pediatric bone is anatomically and biomechanically different from adult bone. It behaves differently from adult bone to injury and also during healing.
UNIQUE FEATURES OF PEDIATRIC BONE
-
Periosteum is thicker than adult bone. Thicker periosteum requires more energy to disrupt than in adults. This is the reason for characteristic greenstick fracture pattern in children.
-
Periosteum is more vascular. This helps in rapid healing of fractures.
-
Pediatric bone is more porous than adult. It prevents peripheral extension of main fracture line, so comminuted fractures are rare in children.
-
Pediatric bone has low bending strength and low modulus of elasticity compared to adult bone (more flexible). Thus pediatric bone absorbs greater energy before failure and plastic deformation is common in pediatric bone.
-
Pediatric bone has more remodeling capacity than adult bone. Deformity in plane of motion remodel to greater extent than deformity in other plane. Rotational deformity remodels less than angular deformity.
-
Open physis: Physeal injury can cause partial or complete closure of physis or growth plate. This may cause growth deformity and shortening.
-
Pediatric bone has low mineral density compared to adult bone.
CHARACTERISTIC FRACTURE PATTERNS OF PEDIATRIC BONE
-
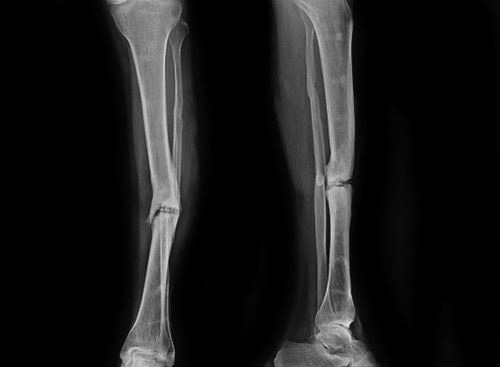
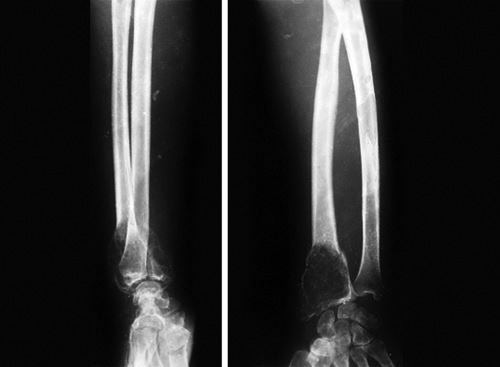
Greenstick fracture: Thicker periosteum resists the deforming forces more than in adult. This causes characteristic greenstick fracture in children. In greenstick fracture one cortex breaks and other cortex remains intact or only deforms (Fig. 1.21). Greenstick fracture is most commonly seen in forearm bones.Treatment of greenstick fracture: Traction is given to align the bone and reduction force is applied directly at the fracture. Overcorrection is often done, this may complete the fracture. After alignment fracture is immobilized in cast.
-
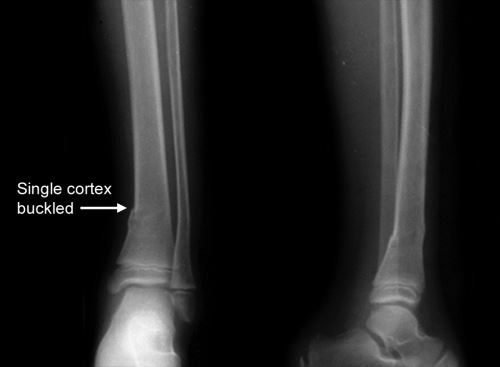
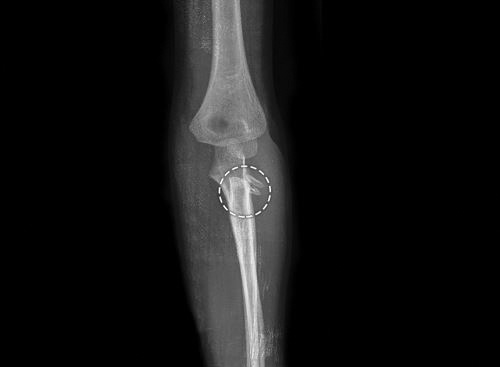
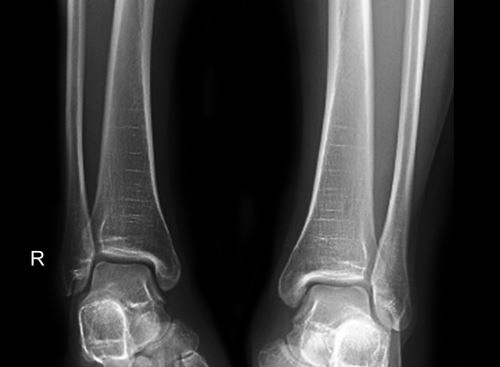
Torus/Buckle fracture (Fig. 1.22): These fractures occur at junction of metaphysis and diaphysis. This is incomplete and stable injury with buckling of one cortex. These fractures result from axial loading with compression of trabeculae. Fracture line is usually not visible and sometimes angulation is the only clue to fracture. Splinting is usually all that is required for treatment.
Fig. 1.23: Plastic deformity—see the angulation without any break.Source: Reproduced from Chee Y (2009). Plastic deformity. EURORAD. DOI:10.1594/EURORAD/CASE.2791.
| ||||||||||||||||||||||
-
Plastic deformation (Fig. 1.23): Bones in children are more flexible than in adult. When force is not sufficient to break the bone it may angulate or bend the bone permanently (beyond elastic limit). Plastic deformation is most common in forearm bone especially in ulna. Severe deformity (angulation more than 20° in older children) requires reduction and splintage.
-
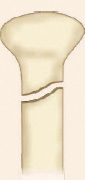
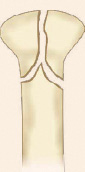
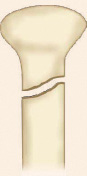
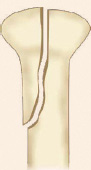
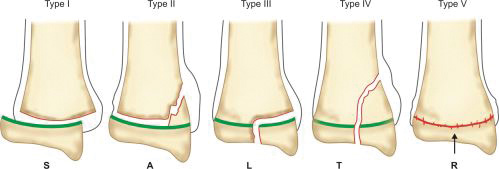
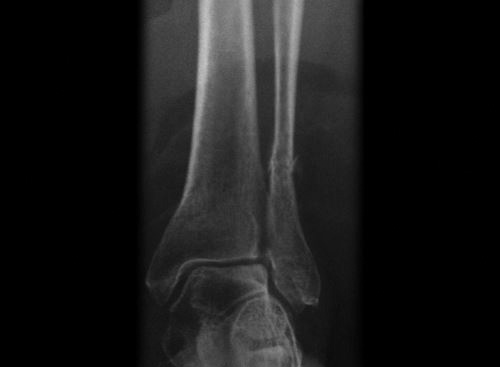
Physeal injury: Open physes are susceptible to injury in children. Theses are common injuries in children especially in adolescents (12–14 years) nearing the growth spurts. Most common site of physeal injury is phalanx followed by distal radius. Salter Harris classification (Table 1.7) (Fig. 1.24) is most commonly used to classify physeal injuries in children. It is based on the location of fracture in one or more of epiphysis, physis and metaphysis. It also predicts the outcome/prognosis. Type I has the best and type V has the worst prognosis.
|
Mnemonic SALTR is useful in remembering this classification (Table 1.8).
Diagnosis of physeal injuries: Child presents with pain and swelling around the joint. Diagnosis of physeal injuries can be made on routine X-rays (Fig. 1.25). Whenever in doubt comparison with X-ray views of normal joint and MRI are very useful.
Treatment: In children fractures heal at a faster rate so timely reduction of physeal injuries is of paramount -importance. Attempt of reduction in cases presenting late can further damage the growth plate. In type I and II fractures reduction should not be attempted after 7–10 days, however type III and type IV fractures must be reduced as they involve the articular cartilage. Once reduced reduction can be secured with pins or/and cast.
Complications: Growth disturbance (angular deformity, shortening) is the single most important complication of physeal injury. Significant deformity may require osteotomy and/or limb lengthening procedures.
HIGH-YIELD POINTS
SPLINTS AND TRACTIONS
SPLINTS
Any “rigid” device “used to immobilize” the injured part of the body is called a splint. Almost any rigid material can be used to splint the injured limb in emergency (Fig. 1.26). The primary purpose of splinting is “preventing further trauma and reducing pain”, until diagnosis and primary management is done. Splints may also be used in “postoperative period for support”, rest or even as a definitive measure to treat an orthopedic injury/deformity in the form of a POP back slab/cast. There are a wide variety of methods to splint an injured limb depending on the site of trauma. A few commonly used splints/braces are mentioned in Table 1.9.
|
Plaster of Paris Splint (Fig. 1.27)
It is a commonly used splint which can be used to support any fracture. It can be molded according to shape of limb. When one molds it to cover three-fourths of circumference of a limb, this splint is called a POP slab. It is often used to support the fractured limb for transportation of patient while the patient is awaiting definitive treatment.
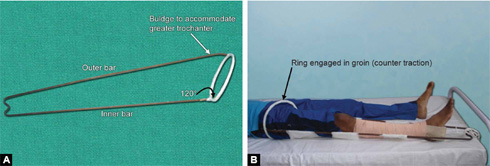
Thomas Splint (Figs. 1.28A and B)
It was designed by H O Thomas for tuberculosis of knee. It has one outer bar, one inner bar and one ring. Ring is at an angle of 120° to the inside bar and the outer bar has a curve to accommodate the greater trochanter.
Figs. 1.29A and B: (A) Cramer wire splint and (B) it can be easily bent to support fractures of limbs.
Size and preparation of Thomas splint: Appropriate length of the Thomas splint should be chosen for proper splintage. Ring size is chosen by adding 2 inches to the thigh circumference at the highest point of groin. Length is measured by adding 6 inches to the length from highest point on the medial side of groin to heel. After having chosen the appropriate size Thomas splint is prepared by padding of it by cotton bandages and cotton. Ring should also be padded well to avoid impingement on the skin.
Use: It is used for immobilization of lower limb in hip and thigh injuries. It is efficient and easy to use tool for transportation of patients with lower limb injuries. Fixed and sliding traction can also be given on Thomas splint.
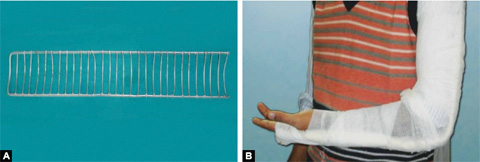
Cramer Wire Splint (Figs. 1.29A and B)
It is made up of two thick and parallel wires with many interlacing wires (ladder splint). It is a flexible splint which can be bent in different shapes to accommodate different body parts. It is used for temporary splintage of fractures of both upper and lower limbs during transportation.
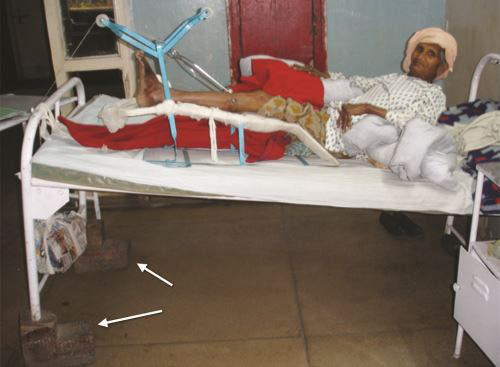
Bohler–Braun Splint (BB Splint) (Figs. 1.30A and B)
Bohler modified the Braun's splint which had only one pulley for tibial traction. Bohler-Braun splint has three pulleys for simultaneous tibial and femoral tractions and to change the angle of traction. Commercially available BB splint has three or four pulleys. It is used for both tibial and femoral fractures.
Functions of Pulleys
Pulley A—Calcaneal/tibial traction
Pulley B—Femoral traction
Pulley C—It is used to change the line/angle of traction. It is also used to prevent equinus deformity of ankle or foot drop.
One disadvantage of BB splint is that ambulation of patient is difficult on it.
HIGH-YIELD POINTS
INDICATIONS
Splints are indicated in initial management of acute musculoskeletal injuries. They are used for short term before the definitive treatment is done. Splints are noncircumferential immobilizers and can accommodate swelling so they are best indicated for injuries where swelling is anticipated.
Indications for use of a splint are as follows:
-
Used for transportation: Splintage of the injured limb provides and makes the transportation of the patient easy and less painful before the definitive treatment. Thomas splint, cramer wire splint are particularly used for this purpose.
-
Used for Traction: Some splints are used for preoperative traction of the fracture limb. This helps in reducing the pain and spasm, correcting deformity and maintaining limb alignment, e.g. BB splint and fixed traction on Thomas splint.
-
Therapeutic use: Splints are also used to maintain deformity correction (e.g. Dennis–Brown splint in CTEV) and to prevent deformity (Cock-up splint in radial nerve palsy). Valgus knee splint is used in medial compartment osteoarthritis for pain relief.
-
To provide rest to acutely inflamed joint as knee brace in TB knee and ankle brace in ankle sprain.
-
They are also used in postoperative period in the form of immobilizers (especially at shoulder and knee) for helping in early cautioned mobilization of patients.
PRECAUTIONS AND CARE
-
Always splint the joint above and below the site of trauma.
-
Use appropriate amount and type of “padding to avoid pressure sores”. Properly pad bony prominences and high-pressure areas before application of a splint.
-
Properly position the extremity before, during, and after application of splints/casts.
-
Always assess the skin condition and dress the wound before splinting. Keep the limb elevated and document the neurovascular status of the affected limb before splintage.
-
Assessment of compartment pressure: Always have an eye on presence/development of compartment syndrome in injured limb. Diagnosis is made on clinical suspicion of tense swelling and pain on passive stretching of limb. Always keep the distal extremity uncovered for serial assessment of neurovascular status.
-
Encourage active toe/finger movements to reduce swelling and cryotherapy postsplinting for pain relief.
TRACTION
Traction is defined as the application of a continuous, well-sustained pulling force on a limb or muscle group in order to achieve a normal anatomical orientation and correction of bony deformity which occurred due to the fracture or dislocation. Traction relieves pain by counteracting the muscle spasm and allows limb to rest in functional position. Commonly used traction systems in orthopedics are listed in Table 1.10.
Countertraction is the pull acting to offset or oppose primary traction force used for the reduction and to maintain the reduction. Depending on the force providing countertraction the traction may be:
-
Fixed (Fig. 1.28B): When the countertraction is produced by the traction system itself. A part of traction system gets purchase on a part of patient's body. For example in Thomas splint ring gets purchase around groin and produces countertraction. Fixed traction cannot obtain reduction but maintain it.
-
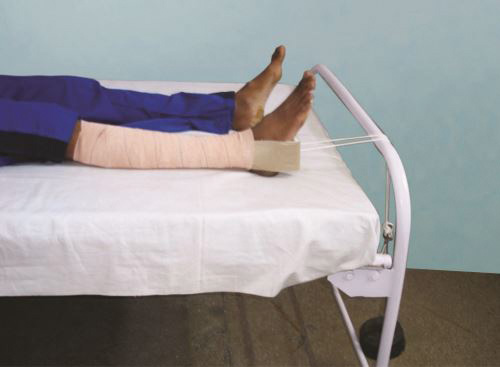
Sliding/Balanced (Fig. 1.31): Where the countertraction is applied by the horizontal component of body weight. Body is kept at an angle by raising the foot end of the bed and gravity/weight of the patient provides the countertraction. Roughly one inch elevation is required for each pound of traction weight.
-
Combined traction: Uses both fixed and sliding tractions.
Depending on the methods of application of traction they are of two types:
|
Skin traction/Buck's traction (Fig. 1.32): It is a noninvasive method used where minimal force (no more than 10 lb) would suffice. It is commonly used in children as muscle mass and spasm is less. It should not be used to obtain or maintain reduction. It can be applied by two methods, adhesive skin traction and nonadhesive skin traction (vent-foam skin traction).
Contraindications to skin traction: It cannot be used where skin condition is poor (wound, allergy, dermatitis, impairment of circulation, venous ulcers, impending gangrene) or in cases of marked shortening/overriding of bony fragments.
Fig. 1.31: Figure showing sliding traction on BB splint where foot end of bed is elevated (arrow) to give countertraction by virtue of gravity.
Complications: Excoriation of skin from slipping of the adhesive strapping, pressure sores around the bony prominences and rarely common peroneal nerve palsy are a few complications.
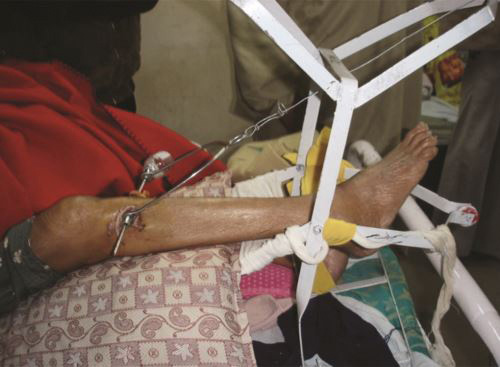
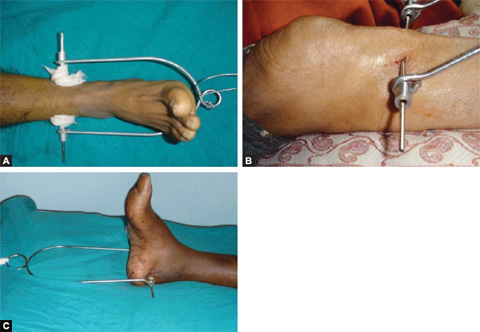
Skeletal traction (Figs. 1.33 and 1.34): It is more definitive form of traction. It is applied by a pin (Steinmann or Denham) through bone and Bohler stirrup. It is used for reducing or maintaining the reduction of a fracture.
Figs. 1.34A to C: Common orthopedic tractions: (A) lower tibial traction; (B) upper tibial traction and (C) calcaneal traction.
Usual sites of skeletal traction are upper third tibia, distal third tibia, supracondylar femur, and the calcaneum in fractures of the lower limb. Olecronon, second and third metacarpals are usual sites in upper limb for passage of pins for skeletal traction.
Complication: Pin site infections, distraction at fracture site, skin necrosis at entry site, damage to the epiphyseal plate in children and rarely osteomyelitis are the few complications of skeletal traction.
Indications of Traction
-
To regain “anatomical alignment” and relation in cases of fractures and dislocations when surgery is delayed or not possible due to medical reasons.
-
To “reduce muscle spasm”, deformities and relieve pain, e.g. poliotic/spastic limb, TB hip, psoas abscess.
-
For “decompression of nerve root impingement” (sciatica, spondylolisthesis, compression fractures of spine.
Contraindications
-
“Active” stage of inflammatory (rheumatoid) or infective arthritis
-
Spinal instability
-
Signs of “vascular disease”, i.e. ischemia
-
Increased pain or worsening of symptoms with traction
-
Fractures with metastatic bone disease
-
Pregnancy.
Precaution and Care
-
Limb should be “comfortably placed” and “adequate weight” should be applied depending on the site of fracture/deformity and the built of the patient. Up to 20 kg weight can be put on skeletal traction.
-
The “weight” should “never touch the ground” and counter should always be in place if the traction is sliding. The ropes should be in the pulley only.
-
Proper “pin site care” and daily cleaning and dressing is must to minimize chance of pin site infection.
-
God nursing care is very important to avoid complications of recumbency, i.e. frequent turning in bed to avoid pressure sores, active and passive physiotherapy to avoid joint stiffness and muscle wasting.
-
Frequent documentation of neurovascular status is very important. Swelling of toes may indicate tight skin traction. Any tingling/paresthesia may indicate towards excessive traction causing traction palsy of nerve.
-
Regular X-ray of the limb should be done to see the reduction of fracture.
HIGH-YIELD POINTS
GENERAL PRINCIPLES OF FRACTURE FIXATION
PRINCIPLES OF FRACTURE MANAGEMENT
Management of a fractured bone should aim not only at restoration of bony anatomy but functional rehabilitation of the limb also. Diagnosis of a fracture with modern diagnostic modalities is usually straightforward. Management of a fracture can be summarized in the following headings:
-
Resuscitation: Based on advanced trauma life support (ATLS) guidelines
-
Management of soft-tissue injury
-
Fracture reduction
-
Maintenance of reduction by fracture immobilization: Cast immobilization, internal fixation or external fixation
-
Rehabilitation
Orthopedic trauma patients are often victims of high-velocity road traffic accidents and may sustain multiple injuries. Some injuries may be life threatening and need immediate intervention before the definitive treatment of fracture is begun (save life, then save limb, then save joint, then save function). ATLS guidelines provide comprehensive and speedy management of such injuries (see Chapter 2 for detail).
Management of soft-tissue injury: Traumatic fracture of bone is almost always associated with injury to its soft-tissue cover.
Tscherne graded the soft-tissue injury associated with closed fracture in four grades (Table 1.11).
Soft-tissue injury does not mean injury to only skin and muscles. Look also for blood vessels injury (signs of 27ischemia), nerve injury (paresthesia, sensory loss, motor weakness), ligament injury (joint instability), etc.
|
In Grade 0 or I injuries the focus is on treatment of fracture only. However in Grade II or III injuries outcome of fracture management depends upon timely and effective dealing with soft-tissue injury. Limb should be splinted to provide rest to injured tissues and put on traction to keep the fracture aligned. Elevation of the limb helps in subsidence of swelling. Appearance of wrinkles around fracture (Wrinkle sign) is a good sign of soft-tissue recovery and time for definitive fracture fixation. Always keep an eye with high suspicion on the development of compartment syndrome in both closed and open fractures. Out of proportion pain and pain on passive stretching of muscles are the earliest features. Timely management is the only key to save the limb in compartment syndrome. Management of open fractures is discussed in detail in Chapter 2.
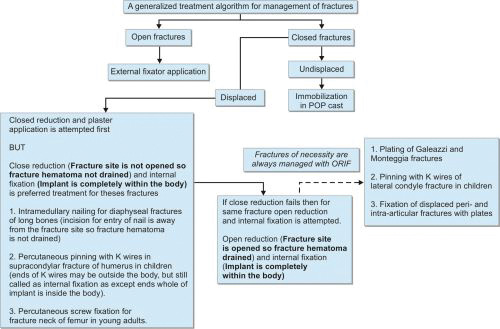
METHODS OF FRACTURE REDUCTION
Not all fractures require reduction. Undisplaced or minimally displaced fractures are usually amenable to direct immobilization. Criteria for acceptable reduction depend on many factors like site of fracture, age, etc. As a general rule, acceptability criteria are more generous for children than adults and for extraarticular fractures than intra-articular fractures. In some fractures like clavicle fracture and fractured neck of humerus in elderly, conservative treatment often gives good outcome as slight malunion does not affect the function result.
Two main methods of fracture reduction are closed reduction and open reduction.
-
Closed reduction: In closed reduction, fracture site is not opened so fracture hematoma is retained. Reduction is done under general or regional anesthesia but sometimes IV sedation or local anesthesia may suffice. Manual traction is given to disimpact the fracture ends and then direct forces are applied in opposite direction of displacement to bring the fracture ends in close approximation in both anteroposterior (AP) and lateral views which is confirmed in image intensifier.Closed reduction by mechanical traction: In some fractures displacing force of muscles is too much to reduce the fracture manually, e.g. in fractured shaft of femur, cervical fractures, etc. These fractures require mechanical traction before manual manipulation for achieving reduction. Whatever be the reduction technique it must be gentle and atraumatic.
-
Open reduction: In open reduction, fracture site is opened so fracture hematoma is drained. Reduction is done under direct vision with help of reduction forceps and other instruments. Heroical efforts for anatomical reduction (excessive use of reduction forceps and other tools) may cause excessive periosteal stripping and may damage the blood supply to bone. So they should be avoided as they are actually detrimental to healing.
Fractures that mostly require open reduction are listed in Box 1.8.
IMMOBILIZATION METHODS
There are four methods of fracture immobilization and maintaining the reduction achieved.
-
Cast immobilization: Mostly used for fractures that have been reduced closed.
-
Continuous traction: Used in cases where one cannot apply cast or fix a fracture with an implant.
-
External fixation: Primarily a method of immobilization for open fractures.
-
Internal fixation: It is a rule for fixing fractures that have been reduced by open incisions. In special situations, implants are used to fix fractures reduced in closed manner (see below).
Cast Immobilization
Plaster of Paris cast immobilization is still the standard method of immobilization for most fractures that have been reduced closed. In acute fractures, casts are applied as slabs (plaster that covers three-fourths circumference of limb) and not as full circumference plasters due to risk of swelling and development of compartment syndrome. When swelling subsides usually after 1 week, slab is converted into full cast.
Fiber cast is now available, which is light weight, radiolucent and impervious to water but costlier than POP cast. In acute displaced fractures, plaster cast is preferred because molding of plaster cast according to body contours is easier than fiber cast.
Some common fractures where cast is usually a preferred method of immobilization are given in Box 1.9. Commonly used cast methods are tabulated in Table 1.12.
Wedging of a cast (Fig. 1.36): This is reduction technique used in long bone diaphyseal fractures with angular malalignment. In this technique, a window is cut in the cast at fracture level leaving a hinge intact on the apex of the deformity. For example in valgus deformity (distal fragment in valgus) window is cut on lateral side leaving a hinge on medial side and varus force is applied distal to the fracture. Once correction is obtained, more cast is applied to maintain the reduction.
|
Precautions and Care of Cast
-
Adequate padding of cotton or stockinette and synthetic wool should be done before applying cast.
-
Plaster cast is a circumferential splint and well-fitted cast does not accommodate swelling. If swelling appears after casting it can hamper arterial blood flow. Keep a careful watch for signs and symptoms of compromised circulation. Excessive swellings of digits and out of proportion pain are alarming signs and if present plaster should be cut and limb should be assessed for development of compartment syndrome.
-
Limb should be kept elevated to prevent development of swelling.
-
There should not be any indentations on the cast as it may impinge upon underlying soft tissues. Similarly edges of the cast should not impinge upon skin.
HIGH-YIELD POINTS
Functional Cast Bracing (Fig. 1.37)
Functional cast bracing concept was popularized in late 1960s by Sarmiento. Initially functional cast bracing was done for tibial fractures but later on for fractured femur and upper limb fractures also. The technique of functional cast bracing consists of applying a splint (called brace) to the fractured limb that while supporting the fracture allows early weight bearing and movement of nearby joints. Early mobilization in this manner encourages the osteogenesis, union and tissue healing. Functional bracing cast is accurately molded around limb in segments which are connected by hinges around joint to allow joint motion while conventional cast bracing immobilizes joints above and below the fracture and restricts their movement.
Functional cast bracing provides less support to fracture than conventional cast so it is applied after 2–3 weeks when fractured ends become sticky and pain and swelling subsides. Early weight bearing is allowed with painless minor movement at fracture site. Thus functional cast bracing prevents joint stiffness, speeds-up rehabilitation and promote osteogenesis and union.
Continuous Traction
Role of traction for immobilization in modern orthopedic practice is very limited. Only valid indication for which continuous traction is still widely practiced is cervical fractures and dislocations. In some fractures like fractured shaft of femur, proximal femoral fractures, acetabular fractures, comminuted pilon fractures and fracture dislocation of hip joint, if surgery is delayed or postponed due to medical reasons and no other options are available, patient is put on continuous skeletal traction to maintain the limb aligned and achieve union in acceptable position. Cast immobilization is not able to hold the fragments in proper positions in these fractures.
Immobilization by External Fixation
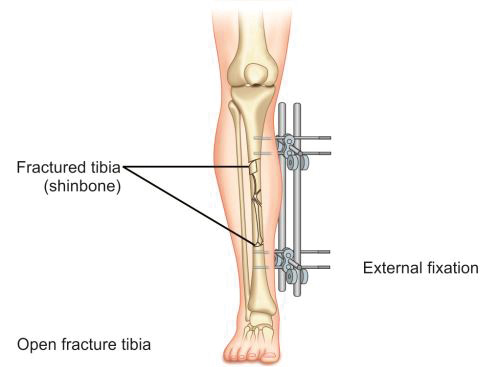
In this method, fractured fragments are anchored to an external bar with help of pins inserted into proximal and distal fragments of bone. Two or three pins are inserted into each fragment and connected to a rod or bar with help of clamps (Fig. 1.38). This method is mainly applied in cases of open and infected fractures where internal fixation carries a high risk of infection or its exacerbation. External fixator in this situation provides a stabilizing assembly that simultaneously allows dressing of the wound and since most of its assembly is outside the skin, there are least chances of infection. Commonly used external fixator frames are one plane (monolateral) frame, two plane (bilateral) frame and ring fixator.
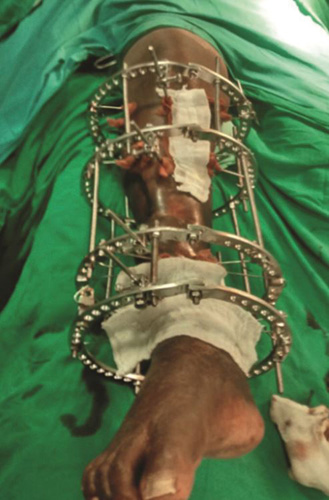
Ilizarov Ring Fixator
Russian surgeon, Gavriil Ilizarov pioneered the revolutionary technique of bone and soft-tissue regeneration based on distraction osteogenesis in 1960s.
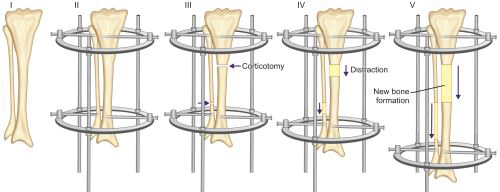
Principle: It is based on the principle of distraction histogenesis which states that gradual distraction of bone at the rate of 1 mm/day, regenerates new bone at the distraction site. At the site of distraction, fibroblast-like cells become metabolically active and secrete collagen. Dormant mesenchymal cells at the site get converted into osteoblasts and secrete osteoid. Growth changes are also seen in soft tissues with cellular hypertrophy and hyperplasia in myocytes and capillary formation and30 development of nerves in direction of tension vector, so all tissues including bone are lengthened.
Assembly and technique (Figs. 1.39A and B): The Ilizarov external fixator is a special modified external fixator that has a complex assembly of metal rings, threaded rods, and Kirschner wires. Wires are passed through skin and soft tissue and drilled through both bony cortices. Wires are attached under tension to half and full metal rings encircling the bone. Assembly is completed by connecting the rings to threaded rods. Assembly can be angulated using hinges if deformity correction is planned. After fixation of the assembly, corticotomy (cutting the cortices of bone while leaving a posterior hinge of periosteum intact for vascular supply) is done in the bone to be lengthened. Corticotomy is usually done at metaphysis because of high potential for osteogenesis in metaphyseal cancellous bone. Gradual distraction is started after few days of corticotomy. Few days are given as a latency period for hematoma to form and organize at site of corticotomy. Rate of distraction is kept slow at 1 mm/day at a rhythm of 0.25 mm every 6 hours. New bone is formed by gradual distraction at the corticotomy site.
Uses of Ilizarov method are given in Box 1.10.
HIGH-YIELD POINTS
Complications: Ilizarov technique is a complex procedure and should be done by experts only.
-
Patient compliance: Ilizarov frame needs to be kept for long time (approximate 1 cm of bone is formed per month). This often leads to social isolation of the patient and also it is cumbersome to carry the heavy frame.
-
Complications related to procedure are muscle contracture, neurovascular insult, pin site infection, and premature or delayed consolidation at corticotomy site.
31Pin site infection is most common complication. Daily pin care with saline cleaning and betadine dressing of the pin tract should be done. In severe infection, pin is removed and new pin is inserted elsewhere. Thorough knowledge of neurovascular anatomy is necessary to avoid neurovascular injury during pin insertion. During distraction if sensory symptoms appear, rate of distraction is slowed or even stopped until symptoms disappear. Proper splinting and physiotherapy is necessary to prevent soft-tissue contractures. Regular radiological examination of the whole limb should be done to judge the consolidation at corticotomy site and also for earliest detection of subluxation or dislocation of adjacent joints.
Internal Fixation
In modern orthopedic practice, trend has been changed in favor of internal fixation (fixing fracture with implants applied inside the skin) of most fractures. Benefits of internal fixation are early mobilization preventing joint stiffness and more anatomical fracture alignment.
Internal fixation is the rule for all fractures that are treated by open reduction where the complete treatment is called as open reduction and internal fixation (ORIF).
However, in some special situations internal fixation can also be done in some fractures that have been reduced closed [e.g. while nailing a long bone (Fig. 1.40)]. Here the treatment is called as closed reduction and internal fixation (CRIF) as skin incision being away from fracture site does not drain the hematoma (so closed reduction) while the fracture is fixed with an implant that lies inside the skin (internal fixation).
Classical examples of closed reduction (hematoma not drained) and internal fixation (implant inside the skin) include:
-
Nailing of a long bone
-
Fixing of a neck femur fracture with multiple screws*
-
Pinning (putting K wires) in supracondylar humerus fractures*
*In the latter two situations the skin incisions are minimal such that hematoma is not drained so closed reduction but since an implant has been put within the body to fix fracture, it is internal fixation.
Various methods of internal fixation available include:
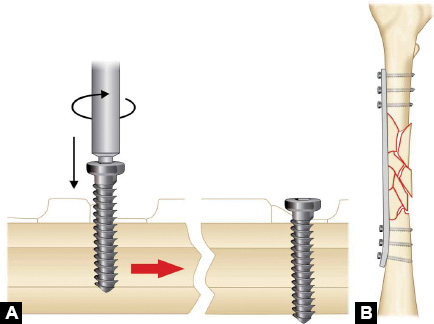
-
Screws: They are inserted either directly across the fracture (lag screw) to compress it or put through the plates to fix a fracture. Common types of screws are cortical, cancellous and locking screws (see page 406 for details).
-
Kirschner wires (K wires): These are stainless steel wires which are available in 1–3 mm in diameters. These are used mainly in pediatric fractures or in fractures of small bones of hands and feet.
-
Plates: These are available in different designs and contour for different fractures. Five basic principles on which plates work are listed in Table 1.13.
|
Some Special Plates
Dynamic compression plate (Fig. 1.41A): In dynamic compression plating tightening of eccentrically placed screws across plate cause axial compression of fracture.
In dynamic compression plate slots for compression have a sloping surface at one end. Centrally placed screw compress the plate against bone. Now in opposite fragment screws are placed eccentrically and when tightened plate moves and compresses the fracture (Newton's third law of motion).
32
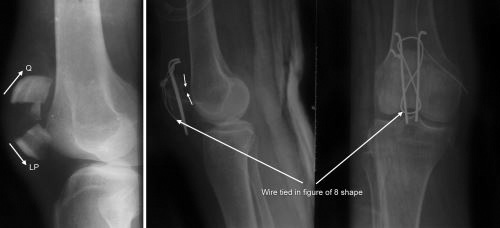
Fig. 1.43: Tension band wiring of patella fracture (Q—pull by quadriceps and LP—pull by ligamentum patellae).
Locking plate: In this plate, screw heads have threads which get locked into plate holes at different angles thus providing angular stability. It is particularly useful for comminuted metaphyseal fractures, osteoporotic fractures and periprosthetic fractures where screw hold in bone may not be good. Here the screws hold to the plate by virtue of them getting locked to it and provide reasonably good stability.
-
The tension band principle: Tensile/distractive forces are converted into compressive forces by applying device (either plate or tension band wire) on convex surface or tension surface of a fractured bone (Fig. 1.43). Here the patellar fragments are being distracted by quadriceps above and ligament patellae below. A wire has been tied on the convex side of patella. This will get stretched due to patellar distraction by quadriceps and just like a stretched rubber band it will reciprocally exert compression pull upon this stretch to compress the fracture (Newton's third law of motion).
Fractures where tension band principle is commonly used are listed in Box 1.11.
Intramedullary nail: First human intramedullary nail was done by Gerhard Küntscher in 1939.
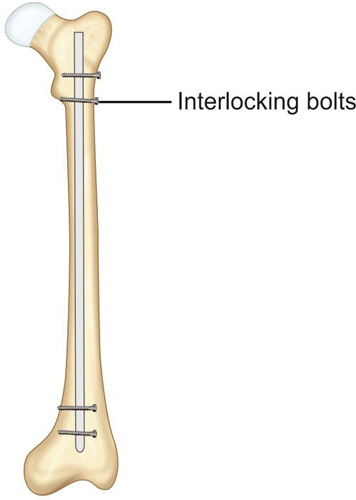
It was a V-shaped steel nail which was later changed to hollow clover leaf model. This nail relied on a frictional fit between nail and bone. Since then intramedullary nailing has seen many changes in design as well as in technique. In 1942, Fischer introduced the use of intramedullary reamers to increase the contact area between the nail and host bone thus increasing the stability of fracture. Later Modney invented the interlock nails that get locked in bones to provide additional rotational stability in case of comminuted fractures (Fig. 1.44). Then in the 1960s, development of image intensifiers allowed surgeons to do intramedullary nailing with better confidence and with closed reduction (as explained in Fig. 1.40).
Principle of intramedullary nailing: Fracture is reduced under image intensifier and after reaming of medullary cavity nail is inserted into the medullary cavity which act as an internal splint to resist bending. Interlocking nails are provided with slots for locking bolts which prevent rotation and shortening.
Advantages of intramedullary nailing: It is ideal implant for long bone diaphyseal fractures. It can be implanted by minimally invasive technique without exposing the fracture hematoma. Thus it is a biological method of fracture fixation.
A treatment algorithm for management of fracture is given in Flowchart 1.1.
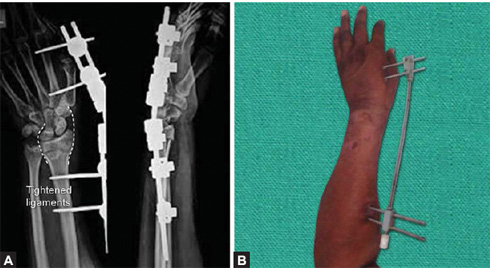
Figs. 1.45A and B: (A) X-ray of wrist joint AP and lateral views showing fixation of distal radius fracture by external fixator (tightened ligaments are shown by broken white lines). (B) clinical picture of distractor used for distal end radius fracture.
HIGH-YIELD POINTS
STRESS FRACTURES, PATHOLOGICAL FRACTURES AND PERIPROSTHETIC FRACTURES
STRESS FRACTURES
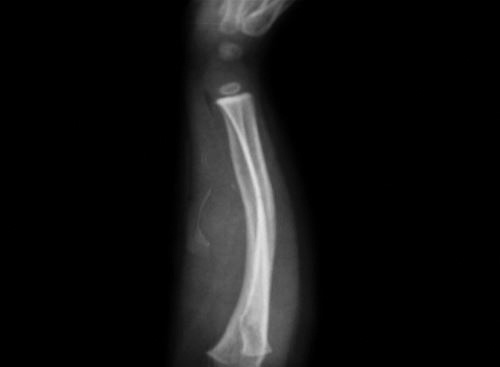
Stress fractures occur when normal bone is subjected to excessive and unaccustomed stress of lower magnitude than required for acute traumatic fracture (abrupt increase in duration and/or frequency and/or intensity of work). Stress fractures are common in lower limb bones in dancers, runners, jumpers, gymnast and military recruits. Stress fracture of nonweight bearing bones (upper limbs) may also occur following repetitive stress, i.e. olecranon is the most common site of stress fracture in baseball players. Box 1.12 shows common sites of stress fractures.
Pathophysiology
Repetitive cyclical loading alters bone's microstructure and causes microfractures which lead to increase in osteoblastic and osteoclastic activity.
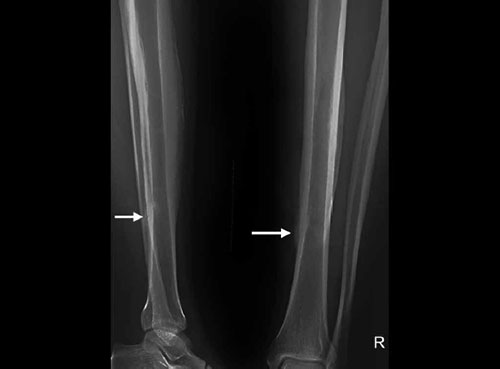
Fig. 1.46: X-ray of leg AP and lateral views showing stress fracture of distal third of tibia (arrow marks, in AP view periosteal eaction can be seen due to healing of stress fracture).
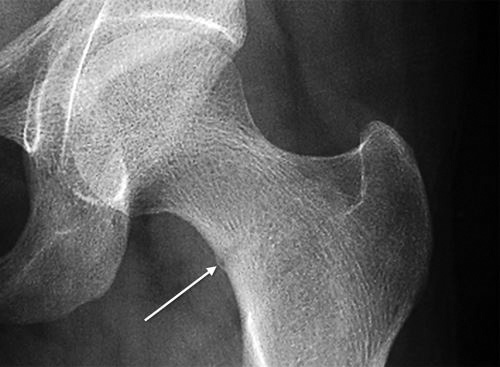
Fig. 1.48: Compression side femoral neck stress fracture, see the periosteal new bone formation at the site of stress fracture (arrow mark).
When repetitive loading occurs at a rate at which body does not have time to recover, bone formation (osteoblastic activity) lags behind the bone resorption (osteoclastic activity). If the stress continues, fatigued bone may fail.
Risk Factors for Stress Fracture
-
Alteration in training program: Sudden increase in duration/intensity/frequency of training
-
Alteration in biomechanics: Stiff ankle (decreased ankle dorsiflexion), increased hip external rotation
-
Limb length discrepancy.
Clinical Features
After a period of stressful activity (athletic training, unaccustomed activity), patient presents with complaints of gradual development of pain at the site of stress fracture. Patient describes it as activity-related pain and that he gets relief with rest. On examination focal bony tenderness suggests the diagnosis of stress fracture. Little swelling over affected region may be present.
Radiology
It takes 2–3 weeks for the stress fracture to become visible on X-ray. Low-density cortical area (gray cortex) is the earliest sign. Later a radiolucent line extending across the cortex appears at the site of fracture. Periosteal new bone formation, linear sclerotic line or frank fracture line are late stage features (appear 2–3 months after the stress fracture). Bone scan [technetium-99m methylene diphosphonate (MDP) bone scan] shows increased activity and 36can show stress fracture in early stage. MRI is the investigation of choice (IOC) for detection of stress fractures.
Stress fractures must be differentiated from Harris lines/Park lines/growth arrest lines (Fig. 1.49) which are bilateral symmetrical dense trabecular metaphyseal lines mostly seen in rapidly growing bone ends.
Treatment
Activity modification is enough for most of the stress fractures. For stress fractures of foot bones (metatarsals, navicular), nonweight bearing below knee cast immobilization for 4–6 weeks is the treatment of choice. High-grade (MRI showing wide or transcortical increased signal intensity) tension-side (superior) femoral neck stress fracture requires prophylactic internal fixation with cancellous screws.
HIGH-YIELD POINTS
PATHOLOGICAL FRACTURES
Pathological fracture (Fig. 1.50) occurs in a bone which is abnormally weak either by systemic affection or by a localized disease. Trivial trauma/stress which would have left the normal bone intact can cause pathological fracture in weak bone. Osteoporosis is the most common cause of pathological fracture in elderly. Box 1.13 shows the causes of pathological fractures.
Fig. 1.50: X-ray wrist with forearm AP and lateral views shows pathological fracture in a giant cell tumor (GCT) of distal end of radius.
HIGH-YIELD POINTS
(CBC: Complete blood count; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; DEXA: Dual-energy X-ray absorptiometry; PTH, parathyroid hormone; PET: Positron emission tomography).
Diagnosis
Pathological fracture should be suspected when fracture occurs:
-
In an elderly who is a known patient of cancer
-
Spontaneously or after trivial trauma
-
In a patient with history of irradiation
-
With complaint of pain or limp preceding the fracture
-
With unusual fracture pattern.
A detailed workup (Box 1.14) of the patient with suspected pathological fracture should be done including search for occult primary. If investigations fail to reveal the cause of pathological fracture, biopsy should be done to establish the diagnosis.
Pathophysiology of Bone Metastasis
Metastatic bone lesions are both osteoblastic and osteolytic. Tumor cells in some cancers (prostate) produce some signaling molecules that stimulate bone formation by increasing osteoblast activity. They include TGF-β, BMPs, and endothelin-1. Whereas in other metastatic cancers like breast cancer, tumor cells secrete parathyroid hormonerelated protein (PTHrP) and IL-6, which are powerful mediators of osteoclast activation and participate in osteolysis by stimulating the production of receptor activator of nuclear factor kappa-B ligand (RANKL) by osteoblasts and stromal cells.
HIGH-YIELD POINTS
| |||||||||||||||||||||||||
Management
Management of pathological fracture should focus on pain relief, management of cause of pathological fracture, and fracture stabilization. Often a combination of NSAIDs and narcotics is required for adequate pain relief. Bisphosphonates and radiotherapy are also used for pain relief and to halt the progression of bone destruction in metastatic bone cancer. Radionuclide therapy is the recent addition in treatment for palliative pain relief from metastatic bone disease. Commonly used agents are phosphorus-32 orthophosphate and strontium-89 chloride.
Fracture management: Conventional methods (cast immobilization or ORIF) are usually enough for pathological fractures secondary to cystic lesions of bone, benign neoplasm and due to generalized systemic disease (osteoporosis, Paget's disease, osteogenesis imperfecta, etc.). Fracture in case of osteomyelitis requires primary management of infection.
Metastatic pathological fractures often fail to unite and require internal fixation with intramedullary rod or long plate with addition of bone cement to fill the defect or replacement of affected bone with prosthesis. Joint arthroplasty is a favorable option for lesion near the joint. For involvement of large area of bone often replacement of whole bone with tumor prosthesis (megaprosthesis) is required.
Impending Pathological Fracture
A fracture is likely to occur in a large lytic lesion of bone. Prophylactic internal fixation of bone is warranted if risk of fracture is high. Mirel's criteria (Table 1.14) are used to quantify the risk of impending fracture.
PERIPROSTHETIC FRACTURES
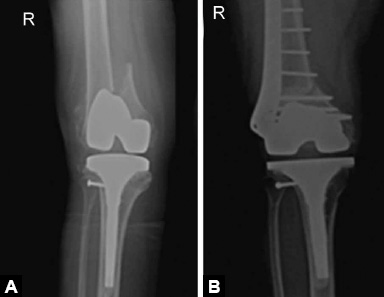
Fractures around joint replacement prostheses are called periprosthetic fracture. These usually result from low energy fall. Patients present with pain around prosthesis and difficulty in weight bearing following a fall. Diagnosis is made on X-rays (Figs. 1.51A and B).
Figs. 1.51A and B: X-ray knee AP view showing periprosthetic distal femoral fracture around total knee arthroplasty and its management with ORIF with distal femoral locked plate.
Treatment of these fractures is challenging due to poor bone stock and presence of prosthesis poses difficulty in internal fixation. Displaced fractures with stable prosthesis are usually treated with ORIF with locking plates. Loose prosthesis needs to be replaced by revision arthroplasty.
HIGH-YIELD POINT
IMAGING IN ORTHOPEDICS
Radiology plays a pivotal role in management of orthopedic patients. X-rays, ultrasonography (USG), CT scan, MRI and nuclear imaging all have specific roles in different orthopedic pathologies.
X-RAYS
It was discovered by Wilhelm Conrad Röntgen in 1895. Electromagnetic radiations are produced when high-energy electrons strike the tungsten target in a special X-ray tube. These electromagnetic radiations are sent by X-ray machine through various tissues of body giving their images on X-ray films. Mostly the wavelength of these radiations ranges from 0.01 nm to 10 nm.
Uses of X-ray in orthopedics are given in Box 1.15.
-
Advantages: Cheap, easily available even in peripheral centers.
-
Drawbacks: Radiation risk (avoid in pregnant women), less sensitivity as compared to CT/MRI, minimal details about cartilage/soft tissues.
ULTRASOUND
These are high-frequency sound waves that are transmitted and received by a transducer. These are based on piezoelectric effect. USG transducers contain a piezoelectric crystal made of lead zirconate titanate (PZT), which changes shape when electric current is passed through it which results in production of sound waves. These sound waves are made to pass through body tissues and their differential passage through various tissues gives their images.
High-frequency high-resolution transducer is used (8–13 MHz) for musculoskeletal ultrasound. A Doppler ultrasound can identify vascular flow in channels and also measure the speed.
Uses of ultrasound in orthopedics are given in Box 1.16.
-
Advantages: No radiation risk, easily available, high sensitivity for soft tissues, very good to detect presence of fluid in any plane, dynamic examination (patient can be asked to move a particular limb to see the status of specific muscles/tendons), useful in evaluating patients with metallic implants which limits the evaluation by CT and MRI.
-
Drawbacks: Status of bone not well seen, operator-dependent investigation, limited field of view.
CT SCAN
It was discovered by Godfrey Hounsfield. It consists of a rotating X-ray tube and a series of detectors in a gantry, with a table that moves in and out of the gantry as required. It generates cross-sectional images of the scanned portion of body.
Uses of CT scan in orthopedics are given in Box 1.17.
-
Advantages: Takes less time, high sensitivity for bone pathologies (but not for marrow), 3D reconstruction of datasets is possible (useful in planning surgeries), multiplanar reconstruction (coronal, sagittal, axial and oblique).
-
Drawbacks: High radiation exposure, expensive, poor image quality and artifacts in metallic implants (due to beam hardening), poor resolution for soft tissues.
MRI
It images the body structures with the use of powerful magnets that produce radiofrequency pulses. The intensity of different organs appears according to their proton density.
-
Advantages: Excellent soft-tissue details, marrow details, no radiation
-
Drawbacks: Expensive, time consuming (high chances of motion artifacts)
-
Contraindications: Permanent pacemaker, metallic implants (presently MRI compatible titanium implants are being used in most places), neurostimulators, metallic auditory implants, claustrophobic patients (open MRI is preferred in claustrophobic patients and MAVRIC MRI has recently been introduced to reduce artifacts in MRI scanning of patients with metallic implants).
Uses of MRI in orthopedics are given in Box 1.18.
Basic MRI Pulse Sequences
-
T1W: Useful for obtaining anatomic detail, detecting fat and hemorrhage. Fat and hemorrhage are hyperintense (bright) on T1W images.
-
T2W: Useful for pathology—water is hyperintense on T2W (pneumonic: World War 2—WW2, water is white on T2) and thus sites of inflammation shine out.
-
PD-proton density images: Useful for meniscal pathology and anatomic detail.
-
Gradient echo (T2) images: Loose bodies and hemorrhage (appear hypointense) are better identified. Fibrocartilage—meniscus in knee and labrum in shoulder can be visualized better. 3D reconstruction can be made.
-
FAT-SAT images: Marrow and soft-tissue pathology identification.
PET-CT
It is combination of two modalities—positron emission tomography (PET) and CT scan. A substrate molecule is infused and its uptake by sites of high metabolism is detected by CT imaging.
18F-FDG (fluorodeoxyglucose) is the most commonly used agent to study metabolism while 13N ammonia is most commonly used agent to study perfusion. In orthopedics PET-CT is mainly used for finding occult primary in suspected patients with bone tumors, in follow up of oncologic imaging to assess treatment response and for assessing distant/widespread metastasis.
BONE SCAN
Technetium Tc 99m-labeled methylene diphosphonate is usually used to scan the bones. Areas of uptake represent increased osteoblastic activity.
Activity in bone scan is recorded in two phases:
- Early perfusion phase: Shortly after injection when the isotope is still in the blood.
- Delayed bone phase: After 2–4 hours when isotope has been taken up by bone.
Uses of bone scan are given in Box 1.19.
HIGH-YIELD POINTS