Abstract that Professor James G White wrote for his NIH Grant several decades ago best summarizes the type of work that we did at the Platelet Research Laboratory at the University of Minnesota, Medical School, Minneapolis, Minnesota.
“Investigations in this laboratory will continue to be directed toward development of knowledge on blood platelet function in normal hemostasis, its role in the pathogenesis of inherited and acquired bleeding disorders, and its contribution to vascular injury, thrombosis, and atherosclerosis. Analytical, scanning, and transmission electron microscopy, freeze-fracture, cytochemistry, and immunocytochemistry combined with cyclic nucleotide, adenine nucleotide, and prostaglandin biochemistry and advanced physiological techniques, including micropipette elastometry and lumi-aggregometry, will be used to develop new information on these problems and related areas. Particular emphasis has been placed on achievement of six specific aims. New approaches to the study of membrane ultrastructure will be used to identify physical alterations in platelet membranes and membrane systems not evident previously. Giant platelets with normal function from patients with the May-Hegglin anomaly will permit study of platelet membrane deformability before and after activation and surface receptor mobility (capping and patching) for the first time. Freeze-fracture and other sophisticated technology will be used to solve basic problems presented by inherited disorders of platelet function. A new mechanism of membrane modulation regulating platelet activation and the phenomenon of disaggregation and reaggregation of irreversibly aggregated platelets was recently discovered in this laboratory. It offers excellent opportunities to gain new knowledge of platelet biochemistry and physiology. Megakaryocytes can be concentrated and purified. We will recover them from a dog model permitting in-depth study of each stage of maturation and use of the techniques of structural physiology. Accomplishment of these aims will allow us to gain the knowledge required to control or prevent hemorrhagic and thrombotic disease.” http://grantome.com/grant/NIH/R01-HL011880-17.
In the early days of thrombosis research, there was strong emphasis placed on the coagulation mechanisms in hemostatic reactions. In addition, preparation of platelets for studies posed a problem, as they were considered very fragile and very reactive. It was the appreciation that platelets were as important as coagulant proteins in the hemostasis and thrombosis processes that demanded the knowledge of their physiology, pathology, and function.1 A new era of ultrastructural investigation began in the early 1960s. Rapid developments in biochemistry and physiology had created the need for a more critical analysis of platelet fine structure and function. Cytochemical, immunochemical, and 2autoradiographic techniques capable of demonstrating specific chemical constituents in thin section of fixed platelets expanded the scope of ultrastructural investigations. A new concept of platelet anatomy, structural physiology and function was developed from electron microscopic investigations. By that time, I joined Dr White, he had already studied platelet ultrastructure for over a decade and published more than 200 articles on this topic.
Dr White in his “concept paper” on platelet structure, which was his HP Smith award lecture in 1978, emphasized the need to develop knowledge of basic relationships between platelet structure, biochemistry, and function in order to define the mechanisms involved in normal hemostatic activity and pathologic behavior (Figs. 1.1 to 1.5).
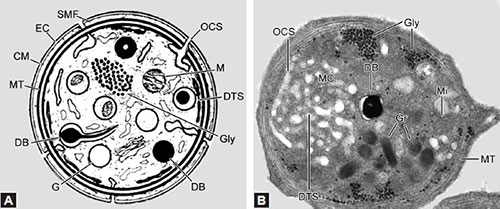
The specialized approach to identify these associations has been termed platelet structural physiology and pathology.1 Platelet in circulating blood has a “disk” or plate-like shape, with smooth convex contours (Fig. 1.5). Figure 1.2A represents a schematic diagram of a cross-section of a platelet showing internal structure and contents essential for executing physiological responses. Figure 1.2B shows cross-section of a platelet as seen in the electron microscope.
Primary purpose for the existence of circulating platelets is to keep a watch on the vessel wall, identify any injury or damage to the blood vessel, interact with the cell matrix of the injured wall, adhere, spread, cover the damaged site, and form an effective hemostatic plug. In order to emphasize this role, Professor White used to use a photograph of a “one in a million” shot of a platelet, which he used to refer as “cross-eyed platelet” (Fig. 1.4). Indeed, this photo of platelet became so famous that several commercial companies wanted to use it for promotional purposes.
I remember one company distributing small replica of “cross-eyed” sticky platelets made of some colored fabric to the participants at the International Society of Thrombosis and Hemostasis (ISTH) meetings.
Fig. 1.1: Schematic representation of platelet ultrastructure shows platelet membrane-associated integrin (GpIIb/IIIa, GpIc/IIa), nonintegrin (GPV, vWF) glycoprotein domains, dense tubular system (DTS), open canalicular system, mitochondria, ACTIN, actin-binding protein, and microtubules. (GPV: Glycoprotein V; vWF: von Willebrand factor).
Figs. 1.2A and B: (A) Schematic representation of a platelet. (B) Cross-section of a platelet as visualized by electron microscopy. (CM: Cell membranes; DB: Dense body; DTS: Dense tubular system; EC: Exterior coat; G: Alpha granules; Gly: Glycogen; MT: Microtubules; OCS: Open canalicular system; SMF: Submembrane filaments; Mi: Mitochondria; Gr: Granules; MC: Membrane channels).
Of course, Dr White did not like the idea and requested them to stop that practice. However, the very thought of a platelet with two eyes must have encouraged or inspired Giantmicrobes, Inc. of Wilmington DE (www.giantmicrobes.com) to produce an attractive soft platelet look-alike with two eyes (Fig. 1.3).
In order to simplify the complicated structural features, internal contents, and relate them to activation mechanism, such as agonist receptor interactions, signal transduction events, biochemical and functional activities, Prof White divided the anatomy of a platelet into four major regions: the peripheral zone, the sol-gel zone, the organelle zone, and the platelet membrane system. Three basic mechanisms govern the activity of platelets in hemostasis: adhesion (aggregation), contraction, and secretion. The peripheral zone mediates all stimuli triggering the platelet response, and conversion from the nonsticky to sticky state takes place in this region.
The peripheral zone comprises the phospholipid membranes and the receptors and glycoprotein-rich domains, which play a major role in cell activation and signal transduction mechanisms. It consists of an exterior coat, or glycocalyx, rich in glycoproteins. Specific agonist receptors as well as integrin and nonintegrin domains, which are important for external stimulus coupling for platelet activation, are located at this zone. This zone is also rich in phospholipids. More than 15% of the dry weight of platelets is lipid of which 80% is phospholipid.2,3 Major lipids include cholesterol (30.8%), phosphatidylcholine (26.3%), phosphatidylethanolamine (8.6%), phosphatidylserine (6.6%), and phosphatidylinositol (2.7%). Recent research in lipidomics revealed the existence of over 8,000 species of lipids in platelets.4 At the time of this writing, the most prominent lipid substrates that played a major role in modulation of platelet and vessel wall functions were arachidonic acid, substrate for cyclooxygenase, thromboxane synthetase, and prostacyclin synthetase enzymes. In addition, a family of cell–cell and cell–surface receptors have been identified on platelet surface membranes.5 The first glycoprotein receptor to be identified was the 11b-111a complex, which when activated can bind four different adhesive proteins: fibrinogen, fibronectin, von Willebrand factor (vWF), and vitronectin. All of these adhesion receptors consist of two noncovalently associated alpha subunit (GPIIb) and beta subunit (GPIIIa). This complex is one of the most abundant receptor proteins present on the platelet surface membranes (> 50,000 copies). Integrin receptors are transmembrane glycoproteins with alpha and beta subunits (GPIIb-IIIa, GPIa-IIa, GPIc-IIa). In addition, there are nonintegrin (GPVI) glycoprotein domains on platelet surface membrane, capable of binding other cell matrix components such as collagen and vWF. In the activation mediated by collagen, integrin alpha-2, beta-1 (GPIIa-Ib), and nonintegrin GPVI are involved.
The sol-gel zone is the matrix of the cytoplasm. It contains fibers and filaments in various states of polymerization. This system of filamentous proteins, capable of polymerization and depolymerization supports the discoid shape in circulating platelets, and provides a contractile system for accomplishing shape change, pseudopod formation, spreading, contraction, and retraction. The contractile system constitutes 30% of the total platelet protein. Significant portion of this system is actin. Other proteins of this system 5include myosin, tropomyosin, actin-binding protein, alpha actinin, gelsolin, profilin, vinculin, and spectrin. In platelets, shape change, pseudopod formation, retraction, and spreading involve dissociation of existing actin structure and reformation of new ones. Thus, it is evident that these structural alterations are dynamic processes and are regulated by a large number of actin-binding proteins. In platelets, some of the proteins identified to play a role in such dynamic process are Arp2/3, cofilin, and capping protein, as well as 2E4/kaptin, gelsolin, VASP, and profilin.6–12 Actin is a globular monomer known to assemble reversibly to form long fibers. Actin fibers, if sufficiently stiff and organized into bundles, could maintain the cell and parts of the cell in particular configurations, for instance, pseudopods of platelets. Working with myosin could generate the contractility needed for spreading, release of granule contents, and clot retraction. Actin filaments could also act as ties for parts of the cell, including its membrane. It has become increasingly clear that action of actin is influenced by enormous number of actin-binding proteins.12–18
The organelle zone consists of dense bodies, which are the storage sites for releasable components, such as ADP, ATP, calcium, and serotonin. Other components of this system include peroxisomes, lysosomes, mitochondria, and glycogen. This zone serves as the storage site for various enzymes, nonmetabolic adenine nucleotides, serotonin, and antioxidants such as taurine, ascorbic acid, and glutathione. On activation, platelets seem to secrete more than 300 active substances from their intracellular granules.13 Proteomic studies have demonstrated that hundreds of bioactive proteins are released from alpha granules.14 Though the role of these granule components was considered to play a role in thrombosis and hemostasis, recent studies have demonstrated their participation in inflammation, atherosclerosis, antimicrobial host defense, wound healing, angiogenesis, and malignancy.14
The membrane system plays a major role in platelet physiology, pathology, and function. The dense tubular system (DTS) has been shown to be the site where calcium, an important bioregulator, is sequestered. The DTS is also the site where enzymes involved in fatty acid metabolism and prostaglandin synthesis are localized.15 Platelet membrane system includes not only structures similar to endoplasmic reticulum (ER), which have been described by White as dense tubular system, but also the boundary or demarcation membranes of a variety of granular organelles within the cytoplasmic matrix: mitochondria, lysosomes, alpha-granules, and dense granules.16 Professor White has described two distinct channel systems in blood platelets.17 The open canalicular system, with its canaliculi, which are continuous with surrounding plasma, serves as a conduit for uptake of plasma-borne substances and as a path for extrusion of endogenous chemicals secreted during the platelet-release reaction.
A series of studies by White and associates have demonstrated that canaliculi of the open canalicular system (OCS) are continuous with the surrounding plasma, particles taken up by the system can be transferred to apparently intact granules, and channels remain open before, and after physical alterations during aggregation and release of secretory products. Existence of the two distinctly different membrane systems in platelets was described first by Behnke.19 White extended these studies to demonstrate a functional role for these membrane systems. On the basis of his extensive studies and that of other researchers working 6in this area, he concluded that platelets were not merely similar to muscle cells, but are muscle cells of the blood, and that contractile physiology dominated the functional activity of platelets in hemostasis.16–18
REFERENCES
- White JG. Current concepts of platelet structure. Am J Clin Pathol. 1979;71:363–78.
- Purdon D. Phospholipid metabolism in platelets. Mod Meth Pharmacol. 1987;4:229–42.
- Marcus AJ. The role of lipids in platelet function: with particular reference to arachidonic acid pathway. J Lipid Res. 1978;19:793–826.
- O'Donnell VB, Murphy RC, Watson SP. Platelet lipidomics: modern day perspectives on lipid discovery and characterization in platelets. Circ Res. 2014;114:1185–203.
- Phillips DR, Charo IF, Parise LV, Fitzgerald LA. The Platelet membrane glycoprotein 11b-111a complex. Blood. 1988;71(4):831–43.
- White JG. The submembrane filaments of blood platelets. Am J Pathol. 1969;56:267–77.
- White JG. Arrangement of actin filaments in the cytoskeleton of human platelets. Am J Pathol. 1984;117:207–17.
- White JG, Rao GHR. Microtubule coil versus surface membrane cytoskeleton in maintenance of platelet discoid shape. Am J Pathol. 1998;152:597–609.
- Schollmeyer JV, Rao GHR, White JG. An actin-binding protein in human platelets. Interactions with alpha-actinin on gelation of actin and the influence of cytochalasin B. Am J Pathol. 1978;93(2):433–46.
- Bearer EL, Prakash JM, Li Z. Actin dynamics in platelets. Int Rev Cytol. 2002;217:137–82.
- Pollard TD, Cooper JA. Actin and actin-binding proteins: a critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035.
- Stossel TP. Contribution of actin to the structure of the cytoplasmic matrix. J Cell Biol. 1984;99(1):S15–S21.
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–62.
- Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–89.
- Gerrard JM, White JG, Rao GH, Townsend D. Localization of platelet prostaglandin production in the platelet dense tubular system. Am J Pathol. 1976;83:283–98.
- Crawford N. Structural and molecular properties of platelet membrane. In: George JN (Ed). Platelet Membrane Glycoproteins. Plenum Press: New York; 1985.
- Cove DH, Crawford. Platelet contractile proteins: separation and characterization of the actin and myosin like components. J Mechanochem Cell Motil. 1975;3(2):123–33.
- White JG. Interaction of membrane systems in blood platelets. Am J Pathol. 1972;66(2): 295–312.
- Behnke O. Electron microscope observations on the membrane systems of the rat blood platelet. Anat Rec. 1967;158:121–37.