CORONARY ARTERY DISEASE
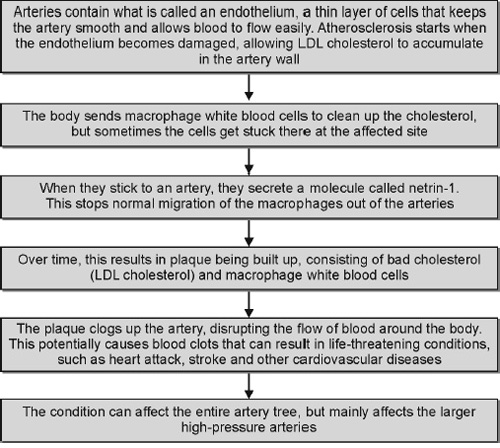
Coronary artery disease (CAD) is characterized by atherosclerosis in the epicardial coronary arteries.
Atherosclerotic plaques, the hallmark of atherosclerosis, progressively narrow the coronary artery lumen and impair antegrade myocardial blood flow. The plaque becomes thick, calcified and solid, which causes obstruction in the coronary blood flow. The reduction in coronary blood flow may be symptomatic or asymptomatic, occur with exertion or at rest, and culminate in a myocardial infarction, depending on obstruction severity and the rapidity of development.
Etiology and Risk Factors
Nonmodifiable Major Risk Factors
- Heredity (including race): Children whose parents had heart disease are at higher risk of CAD. This increased risk related to genetic predisposition to hypertension, elevated lipid levels, diabetes, and obesity, all of these conditions increase the risk of CAD.
- Increasing age: Age influences both the risk and the severity of CAD. Symptomatic CAD appears predominantly in people older than 40, and four of five people who die of CAD are aged 65 years or older.
- Gender: CAD is the number one killer of both men and women. In 1999, mortality from CAD was almost equal for men and women. Although, men are at higher risk for heart attacks at younger ages. The risk for women increases significantly at menopause, so that the CAD rates in women after menopause are two to three times that of women the same age before menopause.
Modifiable Major Risk Factors
- Cigarette smoking: Both active and passive smoking have been strongly implicated as a risk factor in the development of CAD. Smoking triples the rate of heart attacks in women and doubles in men. Nonsmokers who are exposed to second-hand tobacco smoke at home or work may also have higher mortality rate from CAD.
- Hypertension: High blood pressure increases the workload of heart by increasing afterload, enlarging and weakening the left ventricle over time. As blood pressure increases, the risk of serious cardiovascular event also escalates.
- Elevated serum cholesterol level: The risk of CAD increases as blood cholesterol level increases. In an adult, total cholesterol levels of 240 mg/dl are classified as ‘HIGH’ and levels ranging from 200 to 239 mg/dl are classified as ‘BORDERLINE HIGH’.
- Physical inactivity: Those who exercise reduce their risk of CHD because they have:
- High HDL level
- Lower LDL cholesterol, triglyceride and blood sugar levels
- Lower blood pressure
- Lower body mass index
- Obesity: Obesity places an extra burden on the heart. In addition, it also increases the risk because it is often associated with elevated serum cholesterol and triglyceride levels, high bold pressure and diabetes.
- Diabetes: A fasting blood glucose level of more than 126 mg/dl or routine blood glucose level of 180 mg/dl and glucosuria signal the presence of diabetes. Clients with diabetes have a 2 to 8-fold higher prevalence, incidence and mortality.
Contributing Factors include
- Response to stress: A person's response to stress may contribute to the development of CAD. Some researchers have reported a relationship between CAD risk and stress level, e.g.:- some people response to stress by overeating or by starting or increasing smoking. Stress is also associated with elevated blood pressure.
- Inflammatory responses: A newly identified risk factor currently being researched is the presence of any chronic inflammatory state that leads to increase in the body's production of CRP (C-reactive protein). Too much CRP tends to destabilize plaque inside too artery walls. When plaque lesions break, a clot is formed and this may lead to heart attack. So, this means that clients with chronic inflammatory disease, such as arthritis and autoimmune deficiency may be at higher risk for heart attack.
- Menopause: The incidence of CHD increases among women after menopause. Before menopause, estrogen is thought to protect against CHD risk by releasing HDL and lowering LDL levels.
Pathophysiology
- CAD is a chronic process that begins during adolescence and slowly progresses throughout life. Independent risk factors include a family history of premature CAD, 4cigarette smoking, diabetes mellitus, hypertension, hyperlipidemia, sedentary lifestyle, and obesity. These risk factors accelerate or modify a complex and chronic inflammatory vascular process that ultimately manifests as fibrous atherosclerotic plaque.
- The most widely accepted theory of atherosclerosis states that the process represents the body's attempt to heal in response to an endothelial injury. The first step in the atherosclerotic process is the development of fatty streaks, which contain atherogenic lipoproteins and macrophage foam cells. These streaks form between the endothelium and internal elastic lamina. Over time, an intermediate lesion composed of an extracellular lipid core and layers of smooth muscle and connective tissue matrix eventually forms a fibrous cap. The edge of the fibrous cap plays a critical role in the development of acute coronary syndromes. The shoulder region is the site where most plaques lose their integrity or rupture. Plaque rupture exposes the underlying thrombogenic core of lipid and necrotic material to circulating blood and its thrombogenic particulates. This exposure results in platelet adherence, aggregation, and progressive luminal narrowing, which can rapidly progress and–often in the absence of coronary artery collateral development–are associated with acute coronary syndromes.
- Vascular inflammation has emerged as a critical and established component of atherosclerosis genesis, activity, and potential plaque instability. Patients with established CAD who possess a confluence of risk factors known as the metabolic syndrome remain at particularly high risk for a future vascular event, such as an acute MI or cerebrovascular accident. Biochemical markers such as elevated levels of high sensitivity or ultra-sensitive C-reactive protein in the absence of systemic inflammation are thought to signal an increased likelihood of vascular inflammation and to portend a higher risk of vascular events. This marker may also signal more rapidly advancing CAD and the need for aggressive preventive measures.
Signs and Symptoms
The most common symptom of coronary artery disease is angina or chest pain. Angina can be described as a discomfort, heaviness, pressure, aching, burning, fullness, squeezing, or painful feeling in your chest. It can be mistaken for indigestion or heartburn. Angina may also be felt in the shoulders, arms, neck, throat, jaw, or back.
Other symptoms of coronary artery disease include:
- Shortness of breath.
- Palpitations (irregular heartbeats, or a ‘flip-flop’ feeling in your chest).
- A faster heartbeat.
- Weakness or dizziness.
- Nausea
- Discomfort, pressure, heaviness, or pain in the chest, arm, or below the breastbone.
- Discomfort radiating to the back, jaw, throat, or arm.
- Fullness, indigestion, or choking feeling (may feel like heartburn).
- Sweating, nausea, vomiting, or dizziness.
- Extreme weakness, anxiety, or shortness of breath.
- Rapid or irregular heartbeats.
ANGINA
Angina (chest pain) that occurs regularly with activity, after heavy meals, or at other predictable times is termed as ‘stable angina’ and is associated with high-grade narrowings of the coronary arteries.
Stable Angina
Angina pectoris is said to be stable when its pattern of frequency, intensity, ease of provocation or duration does not change over a period of several weeks. Identification of activities that provoke angina and the amount of sublingual nitroglycerin required to relieve symptoms are helpful indicators of stability versus progression. A decrease in exercise tolerance or an increase in the need for nitroglycerin suggests that the angina is progressing in severity or transitioning to an accelerating pattern.
Accelerating Angina
Angina pectoris is said to be accelerating when there is a change in the pattern of stable angina. This may include a greater ease of provocation, more prolonged episodes, and episodes of greater severity, requiring a longer recovery period or more frequent use of sublingual nitroglycerin. This suggests a transition and most likely reflects a change in coronary artery blood flow and perfusion of the myocardium. This frequently portends unstable angina or an acute coronary syndrome, such as an acute MI.
Unstable Angina
Unstable angina pectoris occurs when the pattern of chest discomfort changes abruptly. Signs of unstable angina are: symptoms at rest, a marked increase in the frequency of attacks, discomfort that occurs with minimal activity, and new-onset angina of incapacitating severity. Unstable angina usually is related to the rupture of an atherosclerotic plaque and the abrupt narrowing or occlusion of a coronary artery, representing a medical emergency with an incipient acute coronary syndrome and an MI to follow. Immediate medical attention is mandatory.
Variant Angina
Variant angina is also known as Prinzmetal's angina. Variant angina can occur while you are resting or sleeping. It can be relieved by taking appropriate medicines. It occurs usually between midnight and morning.
Microvascular Angina
Microvascular angina can be more severe and last longer than other types of angina. Medicine may not relieve this type of angina.
Decubitus Angina
The term ‘decubitus’ is derived from the latin word ‘decumbere’ meaning, ‘to lie down’. The angina decubitus means chest pain while lying down. It usually occur at night. It occurs because the fluid in the body are redistributed in this position due to gravity and the heart has to work harder. It occurs when the affected person assumes the left lateral decubitus position or basically lying on the left side with hypertrophied heart. The muscles are already at risk of ischemia. When the blood flow reduces, the angina occurs.
Risk Factors
Modifiable
- Hyperlipidemia
- Smoking
- Hypertension
- Diabetes
- Stress
- Inactivity
- Obesity
Nonmodifiable
- Age
- Gender
- Heredity
Etiology
- Imbalance between myocardial oxygen supply and demand.
- Obstruction of coronary blood flow due to arthrosclerosis, and coronary artery spasm.
Pathophysiology
Diagnostic Evaluation
The initial diagnostic approach for CAD encompasses a detailed patient history including compiling a comprehensive list of CAD risk factors, a thorough physical examination to include an assessment of all peripheral pulses which, when abnormal, may signal the presence of underlying peripheral arterial disease, and an electrocardiogram. Once this initial evaluation is performed, laboratory blood tests, stress testing, and a cardiac catheterization may be necessary to obtain further diagnostic insight.
History
The history should include any current symptoms. An inventory of cardiac risk factors, and a complete family history are essential components. The history should also include information about the character and location of discomfort, radiation of discomfort, associated symptoms, and precipitating, exacerbating, or alleviating factors. The importance of the family history should not be underestimated.
Physical Examination
The results of the physical examination of a patient with stable or unstable angina may be entirely normal. The presence of multiple risk factors or atherosclerosis in the carotid or peripheral arteries increases the likelihood that a chest pain syndrome is related to myocardial ischemia. Evaluation should include measurements of blood pressure and the ankle-brachial index. Examination of the carotid arteries should include auscultation for bruits. Examination of the chest wall, neck, and shoulders for deformities and tenderness may be helpful in diagnosing musculoskeletal chest discomfort. Cardiac auscultation may detect murmurs caused by aortic stenosis or hypertrophic cardiomyopathy, either of which can cause angina in the absence of epicardial CAD. Assessment of the abdominal aorta for an aneurysm and palpation of lower extremity pulses is necessary to evaluate for peripheral vascular disease. Careful palpation of all peripheral pulses and assessment of symmetry 9versus diminution are also valuable noninvasive approaches for assessing the integrity of the arterial circulation. Finally, examination for xanthelasmas, tendon xanthomas, retinal arterial abnormalities, and peripheral neuropathy can be helpful.
Diagnostic and Imaging Studies
Electrocardiography
A resting 12-lead electrocardiogram should be obtained on all patients with suspected CAD. Electrocardiographic results are normal in approximately 50% of patients with chronic stable angina, and they can remain normal during an episode of chest discomfort. Importantly, a normal electrocardiogram does not exclude coronary artery disease. When abnormal, especially when Q-waves are present in a regional myocardial territory of diagnostic duration, it can signify the presence of a past MI with high accuracy.
Chest Radiograph
The usefulness of a routine chest radiograph in a patient with chest discomfort has not been established. Calcification of the aortic knob is a common finding in older patients and is a nonspecific indicator of flow-limiting obstructive coronary disease. Coronary calcification may also be present. A widened mediastinum may signify an aortic aneurysm and represent the first clue of unstable aortic disease as the cause of chest discomfort.
Cardiac Computed Tomography Angiography
A noninvasive imaging assessment of coronary atherosclerosis is now possible in the form of cardiac computed tomography angiography. When negative, this test possesses a high negative predictive value. The positive predictive value is also high, but exact stenosis quantification can be complicated. Associated calcification can cause a blooming artifact, resulting in an overestimation of stenosis.
Echocardiography
Echocardiography is recommended for patients with stable angina and physical findings suggesting valvular heart disease. It is invaluable for assessing the patient with suspected hypertrophic cardiomyopathy. It is also recommended for the assessment of global and regional left ventricular systolic functions in patients who have been diagnosed 10with congestive heart failure, complex ventricular arrhythmias, or a history of MI. The echocardiogram is in many ways an ideal test when assessing a patient with known CAD.
Laboratory Studies
Routine laboratory measurements recommended as a part of the initial evaluation of patients with CAD should include determination of fasting glucose and fasting lipid levels (total cholesterol, high-density lipoprotein [HDL] cholesterol, triglycerides, and calculated low-density lipoprotein [LDL] levels). Other markers, such as lipoprotein(a) and high-sensitivity C-reactive protein may be useful in assessing cardiac risk. High-sensitivity C-reactive protein is gaining greater prominence in assessing the inflammatory level of vascular disease and predicting future risk of vascular events, such as MIs and cerebrovascular accidents.
Stress Testing
Stress testing is another method for determining the presence of flow-limiting, functionally significant coronary artery disease. All stress-testing techniques include electrocardiography and blood pressure monitoring.
Absolute and Relative Contraindications to Exercise Stress Testing
Absolute Contraindications |
|
|
|
|
|
|
|
|
Relative Contraindications |
|
|
|
|
|
|
|
|
|
Cardiovascular stress testing takes 2 forms, exercise and pharmacologic administration. The preferred method of cardiovascular stress testing is exercise, using a treadmill or bicycle. Through aerobic exercise, a higher rate pressure product (peak systolic blood pressure multiplied by peak pulse rate), and, therefore, greater cardiovascular stress, can be obtained. This permits an assessment of a patient's functional capacity, providing prognostic data using the sole parameter of attained metabolic equivalents or oxygen uptake. Heart rate 11recovery—how fast the heart rate decreases after exercise cessation—is also a proven and prognostically important parameter. The most common pharmacologic agents used for nonexercise stress testing are dobutamine, dipyridamole, and adenosine or one of its derivatives.
Coronary Arteriography
Cardiac catheterization remains the gold standard for determining the presence of obstructive CAD. A cardiac catheterization yields a 2-dimensional rendering of the coronary artery circulation. To assist in circumventing the limitations of a 2-dimensional depiction of 3-dimensional anatomy, multiple views from varying angles are obtained with the extent of CAD severity, typically ascribed to the angulation with the greatest stenosis severity within the particular coronary arterial segment.
Treatment
Once a cardiac catheterization has been performed, the three most common therapeutic options are medical therapy, including lifestyle modification, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG).
Lifestyle Modification
Patients with documented CAD should actively pursue lifestyle modifications that reduce the risk of future cardiovascular events.
Smoking
Tobacco use is one of the most important reversible contributors to recurrent cardiovascular events. Tobacco use induces endothelial dysfunction, reduces coronary vasoreactivity, increases circulating carbon monoxide levels, impairs functional status, and raises blood pressure.
Exercise
Functional capacity is a strong predictor of major adverse cardiac events. Functional capacity can be improved by following an exercise program that includes at least 30 minutes of exercise 3 or 4 days a week (a daily regimen is considered optimal).
Weight Control
The best weight management strategy is diet and exercise. Ideal benchmarks are a body mass index between 19 and 25 kg/m2 and a waist circumference ≤ 40 inches for men and ≤ 35 inches for women. Weight loss has a favorable effect on the metabolic syndrome and associated cardiac risk factors, including hypertension, high LDL level, low HDL level, blood pressure, endothelial function, vascular inflammation, and glucose intolerance.
Pharmacologic Therapy
Antiplatelet Agents
Aspirin is the mainstay of antiplatelet therapy for patients who have known CAD or symptoms suggestive of CAD. Aspirin inhibits both cyclo-oxygenase and the synthesis of thromboxane A2.
Antianginal Agents
Beta blockers, calcium channel blockers, and nitrates are the mainstays of antianginal therapy. Unless contraindications exist, all patients who have a history of angina pectoris should carry sublingual nitroglycerin. Beta blockers are recommended as first-line therapy for the management of stable angina in all patients with established CAD.
Patients who have a history suggestive of vasospastic angina should be treated with a calcium channel blocker or a long-acting nitrate as an initial therapy. Either treatment option can also serve as a substitute for a beta blocker in the presence of traditional angina when intolerable beta blocker effects ensue.
Nitrates improve exercise tolerance and prolong the time of onset of angina in patients with exertional angina. They are contraindicated in patients who have severe aortic stenosis or hypertrophic cardiomyopathy because they can adversely alter hemodynamics and exacerbate symptoms. Ranolazine may be useful for treating refractory angina pectoris. Unlike beta blockers, calcium channel blockers, nitrates, and ranolazine have not been demonstrated to reduce cardiac event rates or cardiac mortality.
Risk Factor Management
Hypertension
Management of hypertension in patients with CAD is exceedingly important. Control of blood pressure reduces myocardial oxygen consumption and thereby reduces angina, and it also lowers the incidence of cardiovascular events.
Beta blockers devoid of intrinsic sympathomimetic activity represent first-line antihypertensive therapy for patients with a history of MI or coronary artery disease with angina. Angiotensin-converting enzyme (ACE) inhibitors are indicated for all patients with diabetes mellitus or a history of MI with impaired left ventricular systolic function.
Calcium channel blockers are useful for patients with hypertension and angina despite maximum administration of beta blockers. The long-acting dihydropyridines are preferred; short-acting preparations should be avoided because they are suspected of increasing the risk of cardiac events via precipitous blood pressure reduction and induction of the coronary steal phenomenon, diverting coronary arterial blood flow from flow-limited myocardial regions.
Hyperlipidemia
Guidelines of the National Cholesterol Education Program (NCEP) have recommended an LDL cholesterol level > 70 mg/dl for all patients with coronary artery or other atherosclerotic diseases. Patients whose LDL levels are > 100 mg/dl should start pharmaceutical therapy. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are the recommended first-line agents for patients who have CAD and elevated total and LDL cholesterol levels.
Diabetes Mellitus
Diabetic patients with CAD have a particularly high risk for recurrent cardiovascular events, and they should be targeted for aggressive risk-factor modification. The American Diabetes Association recommends enhanced blood glucose control and monitoring with a hemoglobin A1c level lower than 7%.
Surgical Management: Revascularization
The primary revascularization options are PCI and CABG surgery. The most common PCI techniques are percutaneous transluminal coronary angioplasty and coronary stenting. A major limitation of PCI is restenosis at the intervention site. This represents the body's response to local injury with an exaggerated neointimal proliferative response. The use of drug-eluting stents, aspirin, clopidogrel, and glycoprotein IIb/IIIa inhibitors lowers the rate of restenosis to < 10% at 6 months in optimal circumstances.
The most common conduits for CABG are the saphenous vein and the internal thoracic (mammary) artery. The long-term patency rates of internal thoracic artery grafts are superior to those of venous grafts.
Percutaneous Transluminal Coronary Angioplasty (PTCA)
Percutaneous transluminal coronary angioplasty (PTCA) is performed to open blocked coronary arteries caused by coronary artery disease (CAD) and to restore arterial blood flow to the heart tissue without open-heart surgery. A special catheter (long hollow tube) is inserted into the coronary artery to be treated.
This catheter has a tiny balloon at its tip. The balloon is inflated once the catheter has been placed into the narrowed area of the coronary artery. The inflation of the balloon compresses the fatty tissue in the artery and makes a larger opening inside the artery for improved blood flow.
The use of fluoroscopy assists the physician in the location of blockages in the coronary arteries as the contrast dye moves through the arteries. A small sample of heart tissue (called a biopsy) may be obtained during the procedure to be examined later under the microscope for abnormalities.
A technique called intravascular ultrasound (IVUS), that uses a computer and a transducer that sends out ultrasonic sound waves to create images of the blood vessels, may be used during PTCA.
The use of IVUS provides direct visualization and measurement of the inside of the blood vessels and may assist the physician in selecting the appropriate size of balloons and/or stents, to ensure that a stent, if used, is properly opened, or to evaluate the use of other angioplasty instruments.
The physician may determine that another type of procedure is necessary. This may include the use of atherectomy (removal of plaque) at the site of the narrowing of the artery. In atherectomy, there may be tiny blades on a balloon or a rotating tip at the end of the catheter.
When the catheter reaches the narrowed spot in the artery, the plaque is broken up or cut away to open the artery. Atherectomy is used when the plaque is calcified, hardened, or if the vessel is completely closed. Another type of atherectomy procedure uses a laser, which opens the artery by ‘vaporizing’ the plaque.
Procedure Completion, Both Methods
- The sternum will be pushed back together and sewn together with small wires.
- The skin over the sternum will be sewn back together.
- Tubes will be inserted into your chest to drain blood and other fluids from around the heart. These tubes will be connected to a suction device to keep fluids pulled away from the heart.
- A tube will be inserted through your mouth or nose into your stomach to drain stomach fluids.
- A sterile bandage or dressing will be applied.
Coronary Artery Bypass Graft Surgery (CABG)
Coronary artery bypass graft surgery (CABG) is a procedure used to treat coronary artery disease in certain circumstances. Coronary artery disease (CAD) is the narrowing of the 15coronary arteries (the blood vessels that supply oxygen and nutrients to the heart muscle), caused by a buildup of fatty material within the walls of the arteries. This buildup causes the inside of the arteries to become narrowed, limiting the supply of oxygen-rich blood to the heart muscle.
One way to treat the blocked or narrowed arteries is to bypass the blocked portion of the coronary artery with another piece of blood vessel. Blood vessels, or grafts, used for the bypass procedure may be pieces of a vein taken from the legs or an artery in the chest. At times, an artery from the wrist may also be used. One end of the graft is attached above the blockage and the other end is attached below the blockage. Thus, the blood is rerouted around, or it bypasses the blockage through the new graft to reach the heart muscle. This bypassing of the blocked coronary artery can be done by performing coronary artery bypass surgery.
Risks of the Procedure
Possible risks associated with coronary artery bypass graft surgery include:
- Bleeding during or after the surgery
- Blood clots that can cause heart attack, stroke, or lung problems
- Infection at the incision site
- Pneumonia
- Breathing problems
- Cardiac dysrhythmias/arrhythmias (abnormal heart rhythms)
Coronary Artery Bypass Graft Surgery-on-Pump Procedure
- In order to sew the grafts onto the very small coronary arteries, the heart must be stopped to allow the doctor to perform the very delicate procedure. Tubes will be inserted into the heart so that the blood can be pumped through your body by a cardiopulmonary bypass machine.
- Once the blood has been diverted into the bypass machine for pumping, the heart will be stopped by injecting it with a cold solution.
- When the heart has been stopped, the doctor will perform the bypass graft procedure by sewing one end of a section of vein over a tiny opening made in the aorta, and the other end over a tiny opening made in the coronary artery just below the blockage. If the internal mammary artery inside your chest is being used as a bypass graft, the lower end of the artery will be cut from inside the chest and sewn over an opening made in the coronary artery below the blockage.
- You may have more than one bypass graft performed, depending on how many blockages you have and where they are located. After all the grafts have been completed, the doctor will examine them to make sure they are working.
- Temporary wires for pacing may be inserted into the heart. These wires can be attached to a pacemaker and your heart can be paced, if needed, during the initial recovery period.
Coronary Artery Bypass Surgery-off-Pump Procedure
- Once the chest has been opened, the area around the artery to be bypassed will be stabilized with a special type of instrument.
- The rest of the heart will continue to function and pump blood through the body.
- The cardiopulmonary bypass machine and the perfusionist who runs it may be kept on stand-by, should the procedure need to be completed on bypass.
- The doctor will perform the bypass graft procedure by sewing one end of a section of vein over a tiny opening made in the aorta, and the other end over a tiny opening made in the coronary artery or internal mammary artery just below the blockage.
- You may have more than one bypass graft performed, depending on how many blockages you have and where they are located.
- Before the chest is closed, the doctor will examine the grafts to make sure they are working.
CABG Pre- and Post-operative Care
The Preoperative Phase
Education: Preoperative preparation of patients and significant others is a well-established protocol in most institutions. Research has shown that education of the patient prior to surgery assists with recovery, increases patient contentment, and decreases postoperative complications. Appropriate timing of preoperative preparation is helpful for the patient's information retention because impending open heart surgery is anxiety-provoking to most patients.
- Assessment of learning ability: It is imperative for the nurse to assess the patient for individual learning needs and provide the information in a timely manner to minimize as much anxiety as possible. The skilled professional nurse individualizes preoperative instructions to meet the specific needs of that patient.
- It has been suggested that anxiety state levels are lower 5 to 14 days prior to CABG surgery, which makes this an ideal time for teaching. A high anxiety level is not conducive to retention of information.
- Some patients went to hospital for preadmission testing several days before surgery and completing the preoperative teaching during this time may be effective.
- Some patients want specific details about the perioperative experience, whereas others seem to need only the reassurance that a knowledgeable and compassionate caregiver will provide the needed perioperative care.
- Information: Focus Points for Preoperative Patient Education:
- Sights and sounds in the perioperative environment
- Insertion of monitoring lines
- Preoperative medications and anticipated sensations
- Length of the operation
- Expectations related to postoperative environment
- Availability of postoperative pain medication and nursing staff: Assure the patient that a competent caregiver will be in close proximity during the immediate postoperative recovery period and will be able to anticipate and provide for needs.
- Effectiveness of splinting incision for pain control.
- Postoperative presence of an endotracheal tube: Patients should be informed that an endotracheal tube will probably be in place postoperatively, resulting in a temporary inability to speak.
- Anticipated time of intubation: The patient should be assured that the endotracheal tube will be removed as soon as it is no longer needed.
- Communication issues: The significant other may be anxious and this may intensify as his/her loved one is taken to surgery. Separation is inevitable, but communication with the significant other during the intraoperative period is helpful to minimize anxiety.
- Postoperative activity: Pulmonary care is an important part of the postoperative care of the patient after CABG surgery.
- Preoperative practice with the equipment (such as an incentive spirometer) that will be used postoperatively is helpful.
- Teaching in the preoperative period assists the patient to comprehend the necessity of coughing effectively in spite of incisional pain to achieve positive outcomes postoperatively.
- Preoperative teaching might include information related to the potential for mobilization to a chair during the first evening postoperatively. Early mobilization is effective in improving postoperative pulmonary outcomes.
- Preparation of the significant other:
- Nursing interventions important for significant others include teaching them about the expected patient appearance. The patient may appear pale, cool, and edematous.
- The nurse should also discuss equipment that will be connected to the patient. This equipment will include the ventilator, chest tubes, nasogastric tube, invasive lines, and urinary catheter.
Risk Stratification
Various tests should be done to find and categorize risks.
Low-risk (< 1%) | Intermediate-risk (1–5%) | High-risk (> 5%) |
|---|---|---|
|
|
|
|
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Intraoperative Phase
The intraoperative events during cardiac surgery influence nursing care postoperatively:
- Insertion of a large-bore peripheral intravenous catheter, an arterial line, and a pulmonary artery catheter. These are needed so intravenous fluids can be administered and hemodynamics monitored during the operation and in the postoperative period.
- After the insertion of the invasive lines, anesthesia will be administered.
- After the patient is anesthetized, there will be a head-to-toe surgical preparation and insertion of a urinary catheter.
- Heparin is administered to promote anticoagulation. The activated clotting time is measured during surgery to determine the effectiveness of the anticoagulation and, therefore, guide the amount of heparin that is administered.
- The patient may receive protamine to reverse the heparin at the end of the operation.
- The patient's postoperative body temperature may be lower than a patient who was on bypass because the heat exchanger on the pump cannot be utilized for warming. Because of the reduced body temperature, bleeding may be exacerbated. So, it is the duty of a nurse to maintain the patient's body temperature.
Postoperative Phase
- Postoperative care of the cardiac surgery patient is challenging in that changes can occur rapidly. The preoperative condition of the patient as well as intraoperative events should be considered in postoperative care.
- It is essential for the nurse to anticipate the possible complications so that appropriate interventions are initiated in a timely manner in order to ensure a positive outcome for the patient.
- There is a flurry of activity as the patient enters the recovery room/ICU and the admitting nurse connects the patient and the invasive lines to the monitoring equipment while another staff member connects drainage devices appropriately and draws admission blood work.
- The operating room nurse and the anesthesiologist report the patient's condition to the receiving nurse.
Postoperative Pulmonary Management: Pulmonary dysfunction and hypoxemia may occur in 30% to 60% of patients after CABG.
- Patient history and intraoperative factors must be considered in the postoperative pulmonary management. These are:
- A history of smoking, obstructive pulmonary disease, steroid use, gastroesophageal reflux disease, heart failure, and poor nutrition may increase postoperative pulmonary complications.
- There is potential for an increase in postoperative complications when patients are intubated longer than 24 hours. The length of hospital stay may also increase with longer intubation times.
- The current trend is to extubate patients within the first 12 hours after surgery.
- Routine postoperative care to promote oxygenation and ventilation involves prevention and treatment of atelectasis and pulmonary infection as well as maintenance of effective gas exchange and breathing patterns.
- 19Nurse should assess several factors during heart surgery that increase the potential for pulmonary complications postoperatively. The length of the surgery and resultant increase in the amount of needed anesthetic agents, the amount of fluids administered during the intraoperative period, and prolonged time in the supine position increase the potential for pulmonary complications.
- Nurse should be conscious about atelectasis. Atelectasis can be related to cardiopulmonary bypass, surfactant inhibition, and stimulation of the inflammatory response. Atelectasis as well as the inflammatory mediators inhibit diffusion of oxygen and carbon dioxide across the alveolar capillary membrane and impairs effective gas exchange.
- Prolonged pump time causes fluid shifts, potentially increasing the amount of fluid in the pulmonary tissue, thus increasing the possibility of pulmonary complications.
- Pain caused from the sternotomy can impair breathing patterns.
- Some patients shiver after heart surgery and this response may lead to an increase in the carbon dioxide level or lead to lactic acidosis. Shivering may increase the body's oxygen consumption. Therefore, oxygen levels should be monitored and adjusted accordingly.
- Shivering may be the result of the body compensating for the surgically induced hypothermia or a reaction to anesthetic agents. Shivering is usually managed by administration of sedation and neuromuscular blocking agents while the patient is being mechanically ventilated.
- Postoperative management includes:
- Accurate and frequent physical assessment
- Arterial blood gas analysis
- Continuous pulse oximetry
- Pulmonary care (including suctioning while the patient is intubated and coughing and incentive spirometry after extubation)
- Early mobilization
- Control of pain and shivering.
- Most protocols require a chest X-ray after heart surgery to determine placement of the endotracheal tube, thermodilution catheter, and nasogastric tube as well as information about the width of the mediastinum, amount of atelectasis, presence of hemothorax or pneumothorax, and size of the heart.
- Pain control is usually achieved with intravenous narcotics while the patient is intubated. Oral and/or intravenous narcotics may be used after extubation. The nurse must balance the need for pain control without respiratory depression with the patient's need to have his/her pain minimized to allow an effective cough.
- The nurse must assess the patient for readiness for early extubation. Extubation should be considered when the patient is arousable, able to follow commands, hemodynamically stable, and initiating spontaneous ventilations without excessive respiratory effort.
- Typical intensive care protocols for the cardiac surgery patient include preprinted orders that facilitate the weaning process. As the patient is being weaned from the ventilator, ventilatory support is gradually withdrawn and the patient must sustain spontaneous ventilations.
- 20Physical assessment of effective ventilation, and laboratory analysis of arterial blood gases and specific ventilatory parameters must be completed prior to extubation. Protocols may vary, but some standards require a PO2 80 mm of Hg on a FiO2 of 0.40 or less, a PCO2 less than 45 mm Hg, a pH between 7.35 and 7.45, and an oxygen saturation (SaO2) 99%. Ventilatory parameters include a maximum inspiratory pressure of at least 20, a tidal volume of at least 5 mL/kg body weight, and a minute volume of at least 5 liters per minute.
- During the weaning process, the nurse should assess the patient for an increase in respiratory and/or heart rates, use of accessory muscles, fatigue, and color changes because these findings may indicate the patient is not ready for extubation.
- An increase in pulmonary artery pressures can indicate an increase in PCO2 and give the nurse an early indication prior to arterial blood gas analysis that the patient is not ready for extubation. Early extubation is desirable but if parameters are not met and/or the patient is hemodynamically unstable, there may be detrimental effects of early extubation.
Postoperative Management of Hemodynamics: Movement of the patient from the operating room to the recovery room/ICU can create hemodynamic instability, and, thus, reconnection to the monitoring equipment in a timely manner is of the essence.
BP: A cuff BP is usually taken to provide correlation of the BP obtained from the arterial line.
- The nurse must continually assess the patient for cardiac dysfunction and hemodynamic instability because intraoperative myocardial ischemia is a potential cause of low cardiac output (CO) during the immediate postoperative period. The receiving nurse must intensively monitor the interrelationship between heart rhythm and rate, preload, afterload, contractility, and myocardial compliance to achieve this outcome.
- Blood pressure must be maintained within ordered parameters to provide tissue perfusion and prevent disruption of the surgical anastomoses.
- The nurse must monitor the volume in the system, which is reflected by the right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP).
- If the BP is too low, there is either too little volume (preload), a decrease in contractility, or the SVR is too low (the patient's blood vessels are dilated).
- If the BP, CO, and RAP/PCWP are all low, the patient probably needs volume.
- Volume is generally replaced as needed with a colloid solution unless the hematocrit is low and then volume may be replaced with packed red blood cells.
- If the BP and CO are low but the PCWP is high, the patient may be experiencing decreased contractility and inotropic support may be instituted with an agent, such as dopamine or dobutamine.
- If the BP is low and the CO is adequate or elevated, the systemic vascular resistance may be low and the patient may need a constrictive agent, such as phenylephrine. Low BP can be temporarily increased by turning off positive end-expiratory pressure (to decrease intrathoracic pressure and augment preload) and by position changes.
- The patient should be put in the supine position with legs elevated to allow the BP to increase until the cause of the low BP can be determined and corrective measures are taken. The Trendelenburg position can offer symptomatic relief from low BP, especially in the early postoperative phase, by shifting volume from the legs to the chest and increasing preload.
- The nurse has to carefully monitor the patient for high BP and quickly intervene per institution protocol. Nitroprusside, a vasodilator, is often administered to lower the BP to the ordered parameter. Nitroglycerine, a nitrate, may also be used to cause vasodilation and lower the BP. These medications should be started slowly so patient response can be evaluated. The patient must be monitored closely as the BP may drop as the patient's body temperature increases.
- The nurse must rewarm the patient after surgery if hypothermia persists. The negative effects of hypothermia include depression of the myocardium, ventricular dysrhythmias, vasoconstriction, and depression of clotting factors (increasing the risk of bleeding postoperatively). Rewarming may be accomplished by the use of warm blankets, warm humidified oxygen, convective air mattresses, and other individual institutional approaches.
- Vasoconstriction induced by hypothermia may increase BP. Because of the potential for issues with graft anastomoses and the importance of maintaining BP within the reference range, a vasodilator may be needed while the patient is rewarming. As normothermia is achieved, if the patient's systemic vascular resistance decreases significantly, additional intravenous fluids may need to be administered.
- The nurse should carefully monitor the pulmonary artery pressures and the CO as well as the BP when interventions are instituted to assess the effect. Some references suggest that hemodynamic parameters be rechecked every 30 to 60 minutes after each intervention during the early postoperative period. It is important to maintain effective CO after open heart surgery to provide adequate tissue perfusion.
Ventricular dysrhythmias are more common in the early postoperative period and supraventricular dysrhythmias are more likely in 24 hours to 5 days postoperatively. The incidence of atrial fibrillation ranges from 10% to 65% depending on many factors.
These factors include:
- Patient history
- Preoperative medications, and type of surgery
- Hypothermia
- Inhaled anesthetics
- Electrolyte disturbances (i.e. hypocalcemia, hypercalcemia, hypomagnesium, and hypokalemia)
- Metabolic disturbances (such as acidosis)
- Manual manipulation of the heart
- Myocardial ischemia
- Increase in catecholamine levels secondary to pain, anxiety, and inadequate sedation.
- Management depends on the type of dysrhythmia and the patient's clinical response. The nurse must treat the patient effectively.
- Atropine may be given to increase the heart rate in the absence of epicardial pacing wires.
- Tachydysrhythmias are usually controlled pharmacologically. The specific medication utilized will depend on hospital protocols and physician preference.
- The critical care nurse should utilize standing orders in the institution as well as current advanced cardiac life support protocols.
Postoperative Management of Bleeding: The postoperative period may be complicated by excessive bleeding. Many factors should be considered when assessing the patient's potential for bleeding.
- Patients who were on anticoagulants and antiplatelet agents prior to surgery are at an increased risk of postoperative bleeding. Potential sites for bleeding include the internal mammary site, the chest wall, and chest tube sites.
- Induced hypothermia, the use of the CPB machine, and the administration of heparin for anticoagulation can all contribute to postoperative bleeding.
- The nurse should be aware that heparin can be stored in adipose tissue and some patients may have an increase in bleeding 4 hours postoperatively, depending on the body's adipose composition.
- Some surgeons utilize an intravenous infusion of aprotinin intraoperatively to minimize the risk of postoperative bleeding. This drug is a protease inhibitor that inhibits fibrinolysis. Aprotinin may also have some anti-inflammatory effects and, therefore, be beneficial to the patient after CABG.
- The nurse should monitor the patient for signs of bleeding from the chest tubes and the surgical sites as well as clinical signs of hypovolemia related to blood loss.
- Hemoglobin and hematocrit should be monitored at regular intervals during the postoperative period.
- If bleeding is an issue, drugs such as protamine sulfate (to reverse the effects of heparin) or antifibrinolytic agents, such as aminocaproic acid or desmopressin (DDAVP) may be ordered.
- Blood products such as fresh frozen plasma and platelets may also be ordered.
- When bleeding occurs, there is potential for the blood to accumulate in the pericardium, and, therefore, the nurse must be cognizant of the potential for cardiac tamponade.
- The clinical manifestations of cardiac tamponade include lack of chest tube drainage, decreased BP, narrowed pulse pressure, increased heart rate, jugular venous distention, elevated central venous pressure, and muffled heart sounds.
- Emergency reoperation would be required.
Postoperative Neurologic Management: Patients who require coronary artery bypass surgery are at an increased risk for neurologic complications.
- Stroke can be caused by hypoperfusion or an embolic event during or after surgery.
- Manipulation of the aorta has been implicated in embolic events. Other risk factors for stroke may include age, previous stroke, carotid bruits, and hypertension.
- The nurse should be particularly astute to neurologic assessment in the postoperative period.
- Pupils should be assessed initially; however, normal size and reactivity may not return until agents utilized intraoperatively have been metabolized.
- Over the first few hours after surgery, the results of the neurologic assessment should improve gradually.
- By the time the patient is ready for extubation, he/she should follow commands and have equal movement and strength of the extremities with neurologic function approaching the patient's normal condition.
- It is difficult for significant others during this time because waiting during the awakening process can be anxiety-provoking. Patients and significant others are informed prior to surgery of the risk for stroke and want that to be definitively ruled out as soon as the patient returns to the intensive care unit.
- The nurse should provide the needed comfort but not give false hope, as the neurologic status cannot be completely assessed until the patient is fully awake and extubated.
- At that time, the patient should be assessed for orientation to person, place, time, and circumstance. A motor and sensory assessment should also be performed.
- A positive result is a good indication that an intraoperative stroke can be ruled out.
- Neurologic assessments must continue because the risk of stroke does not end with the operation.
Postoperative Renal Management: There is a potential for renal dysfunction in the postoperative cardiac surgery patient.
- Renal insufficiency may be related to advanced age, hypertension, diabetes, decreased function of the left ventricle, and length of time on the CPB.
- One indicator of effective CO is adequate renal perfusion as evidenced by urinary output of at least 0.5 mL/kg/h.
- The nurse must monitor the urinary output at least hourly during the early postoperative period.
- The urine should be assessed for color and characteristics as well as amount.
- Diuresis is likely in the postoperative period when renal function is adequate, as the fluids mobilize from the interstitial to the intravascular space.
- The patient's potassium level should be monitored at least every 4 to 6 hours for the first 24 hours, as potassium is lost with diuresis.
- Intravenous potassium replacement should be administered to keep the serum potassium levels within the normal limits.
- The patient should be astutely monitored for cardiac dysrhythmias if the serum potassium level is abnormal.
- Other laboratory values that should be monitored at least daily are the blood urea nitrogen and serum creatinine.
Postoperative Gastrointestinal Management: Gastrointestinal complications include peptic ulcer disease, perforated ulcer, pancreatitis, acute cholecystitis, bowel ischemia, diverticulitis, and liver dysfunction.
- If the gastroepiploic artery is used as a conduit for bypass, this may also increase the risk of gastrointestinal dysfunction.
- Anesthetic agents, analgesics, and hypoperfusion of the gut during surgery can also contribute to gastrointestinal dysfunction.
- The nurse should monitor the patient for bowel sounds, abdominal distention, and nausea and vomiting.
- The intubated patient will have a nasogastric tube to low intermittent suction.
- Placement and patency should be assessed as well as amount, color, and characteristics of the drainage.
- Prior to extubation, if bowel sounds are present, the nasogastric tube will be discontinued and the nurse should continue to assess the patient for potential gastrointestinal disturbances.
- The nurse should administer antiemetic agents as ordered.
- Some surgeons order a histamine blocker to minimize acid secretion until normal dietary patterns are resumed.
- When the nasogastric tube is removed, the patient will be started on a clear liquid diet and this can be advanced as tolerated by the patient.
Postoperative Pain Management: Dependent upon surgical approach, the patient may have a median sternotomy incision, leg incision(s), and/or a radial incision.
- Manipulation of the chest cavity, use of retractors during surgery, and electrocautery may all contribute to postoperative pain.
- In addition, positioning on the operating room table and length of time of the surgery may also be factors in pain experienced postoperatively.
- Poorly controlled pain can stimulate the sympathetic nervous system and lead to cardiovascular consequences.
- The heart rate and BP can increase and the blood vessels can constrict, causing an increase in the cardiac workload and myocardial oxygen demand.
- Effective pain control is essential for patient comfort, hemodynamic stability, and prevention of pulmonary complications.
- Nurses must individualize pain assessment and control for each patient as responses vary among individuals.
- Opioid analgesics, positioning, mobilization, distraction, and relaxation techniques are among some of the methods of pain control.
- Keeping serum levels of opioid analgesics in the therapeutic range is beneficial.
- Nonsteroidal anti-inflammatory agents may be used in conjunction with opioid agents to control pain and minimize the amount of narcotics needed.
- Pulmonary care is more effective for the patient when pain is effectively managed.
- Teaching the patient to splint the incision when coughing and moving improves pain control.
- The nurse should evaluate the effectiveness of pain management interventions regularly.
- Another source of pain for the patient after CABG is the removal of the chest tubes. This usually occurs 24 to 48 hours postoperatively when the amount and characteristics of chest tube drainage meet ordered parameters as long as there is no air leak noted in the water seal chamber.
- Pain medication should be administered prior to removal of chest tubes per institution protocol to minimize the trauma of the procedure.
Postoperative Management of Infection: The incidence of infection of sternal and leg incisions after cardiac surgery is less than 3%.
- Risk factors for infection include diabetes, malnutrition, chronic diseases, and patients requiring emergent surgery or prolonged surgery.
- Assessment for, and prevention of, infection is part of the nurse's role in the postoperative period.
- The patient should be assessed for local and systemic signs of infection.
- Postoperative antibiotics may be ordered.
- Dressings should be removed and incision care should be completed according to institution protocols.
- Control of blood glucose level may help with the prevention of infection. It is desirable to control blood glucose levels of greater than 150 mg/dL with a continuous intravenous infusion of insulin versus intermittent subcutaneous insulin injections. This practice is thought to be helpful in the prevention of deep sternal wound infection.
- Some surgeons order corticosteroids postoperatively. When used, these drugs are intended to minimize the potential risks of inflammation after heart surgery.
- Patients should be monitored for suppression of the immune system, as this can be an adverse effect of corticosteroid administration.
- Patients need to be taught how to slowly discontinue the medication after discharge as per physician's orders. The other potential effect of corticosteroid administration is an elevation in serum glucose levels.
- A sliding scale insulin order may be needed to maintain blood glucose levels within normal limits while the patient is in the hospital.
Additional Postoperative Management: The nurse must intensively care for the patient in the early postoperative period.
- This intensive monitoring and postoperative discomfort can interfere with the patient's need for sleep. There is a potential for sleep disturbance as the patient is recovering from CABG.
- Lack of sleep may negatively affect postoperative outcomes. Organization of needed care and provision of time for uninterrupted sleep cycles is important for effective outcomes.
- Some of the postoperative confusion experienced by patients may be minimized and positive outcomes maximized when time for sleep is provided.
- Hospital routines and too many visits by well-meaning significant others may add to the sleep deprivation problem.
- It can be frightening for significant others to visit the patient during the early postoperative period because of the monitoring equipment and appearance of their loved one. Explanations regarding the equipment and physical appearance may be helpful.
- Often significant others need to overcome fear of touching the patient postoperatively and receive reassurance from the professional nurse that no harm will come from the touch.
Nursing Care
Acute pain related to an imbalance of oxygen supply to myocardial demands.
Outcome: The patient will express pain decreased
Intervention:
- Assess pain location, duration, radiation, occurrence, a new phenomenon.
- Review of previous activities that cause chest pain.
- Create a 12-lead ECG during anginal pain episodes.
- Assess signs of hypoxemia, give oxygen therapy if necessary.
- Give analgesics as directed.
- Maintain a rest for 24–30 hours during episodes of illness.
- Check vital signs during periods of illness.
Decreased cardiac output related to electrical factors (dysrhythmias), Decrease in myocardial contraction, structural abnormalities (papillary muscular dysfunction and ventricular septal rupture).
Outcome: The patient will demonstrate a stable or better cardiac condition.
Intervention:
- Maintain bed rest with head elevation of 30 degrees during the first 24–48 hours.
- Assess and monitor vital signs and hemodynamic per 1–2 hours.
- Monitor and record ECG continue to assess the rate, rhythm, and order to each change per 2 or 4 hours.
- Review and report signs of CO reduction.
Anxiety related to the needs of the body is threatened.
Objectives: The patient will demonstrate reduced anxiety after nursing actions.
Intervention:
- Assess signs and verbal expressions of anxiety.
- Take action to reduce anxiety by creating a calm environment.
- Accompany patient during periods of high anxiety.
- Provide an explanation of procedures and treatments.
- Encourage patients to express feelings.
- Refer to the spiritual adviser if necessary.
CARDIOMYOPATHY
Cardiomyopathy is a weakening of the heart muscle or associated with other problems with the heart muscle. It may be associated with heart failure, endocarditis or other heart problems which alter the normal architecture of heart. Most patients with cardiomyopathy have heart failure.
Etiology
In the broadest sense, ‘cardiomyopathy’ (CM) refers to heart disease resulting from a primary abnormality of the myocardium (heart muscle). There are 3 types:
- Dilated cardiomyopathy (also called ‘congestive’ cardiomyopathy)
- Restrictive cardiomyopathy
- Hypertrophic cardiomyopathy
- Dilated cardiomyopathy is a condition in which the heart becomes weak and the chambers get large. As a result, the heart cannot pump enough blood out to the body. The heart with dilated cardiomyopathy is striking in appearance. Dilation (enlargement) of all 4 chambers (both atria and both ventricles). The total size of the heart is typically huge (cardiomegaly). The myocardium becomes ‘flabby’ and loses its ability to contract. Naturally, the heart chambers will lose their pumping function and the heart will ultimately undergo failure. Since blood flow within the chambers is sluggish, intracardiac mural thrombi are prone to form on the inner walls of the atria and ventricles. Pieces of these thrombi may break off and embolize to the lungs (pulmonary emboli), or any other organ and tissue (systemic emboli). This may lead to infarction of these organs.
- Hypertrophic cardiomyopathy (HCM) is a condition in which the heart muscle becomes thick. The thickening makes it harder for blood to leave the heart. This type of cardiomyopathy is usually passed down through families. This is a disease of younger people (mean age 26). It is a genetically inherited disease. The classic anatomic feature is the profound hypertrophy of the myocardium of the left ventricle. The part of the LV wall that forms the interventricular septum (IVS) is more hypertrophic than the lateral part of the LV wall. This extra-thickened interventricular septum is referred to as asymmetric septal hypertrophy (ASH). The IVS can become so hypertrophied that it bulges into the lumen of the LV, thereby decreasing the volume of the LV chamber.
- Restrictive cardiomyopathy is a group of disorders. Restrictive cardiomyopathy can either be idiopathic or can be caused by diseases that deposit abnormal substances within the myocardium. The classic example is amyloidosis, whereby the abnormal amyloid protein accumulates within the myocardium, resulting in stiffness.
- Peripartum cardiomyopathy occurs during pregnancy or in the first 5 months afterwards.
Signs and Symptoms
- Breathlessness with exertion or even at rest
- Swelling of the legs, ankles and feet
- Fatigue
- Irregular heartbeats that feel rapid, pounding or fluttering
- Dizziness, lightheadedness and fainting
- Palpitations (fluttering in the chest due to abnormal heart rhythms)
- Fainting (usually caused by irregular heart rhythms or abnormal responses of the blood vessels during exercise
- Chest pain or pressure (occurs usually with exercise or physical activity but can also occur with rest or after meals)
Diagnostic Evaluation
- The health care provider may hear abnormal sounds, called murmurs, when listening to your heart with a stethoscope.
- A physical exam may also reveal:
- Enlarged spleen and enlarged heart size with atrophy
- The following tests may be performed:
- Blood culture and sensitivity (to detect bacteria)
- Chest X-ray
- Complete blood count (may show mild anemia)
- CT scan of the chest
- Echocardiogram (ultrasound of the heart)
- ECG
Treatment
- When possible, the cause of cardiomyopathy is treated. Medicines and lifestyle changes are often needed to treat the symptoms of heart failure, angina, and abnormal heart rhythms.
Different procedures or surgeries may also be used:
- A defibrillator sends an electrical pulse to stop life-threatening abnormal heart rhythms.
- A pacemaker treats a slow heart rate or helps both sides of your heart beat at the same time.
- Coronary artery bypass (CABG) surgery or angioplasty can improve blood flow to the damaged or weakened heart muscle.
- Heart transplant is used when all other treatments have failed.
Management
- The overall goals of treatment for cardiomyopathy are to manage your signs and symptoms, prevent your condition from worsening, and reduce your risk of complications.
- Angiotensin-converting enzyme (ACE) inhibitors to improve your heart's pumping capability, such as enalapril (Vasotec), lisinopril (Zestril, Prinivil), ramipril (Altace) and captopril (Capoten).
- Angiotensin receptor blockers (ARBs) for those who can not take ACE inhibitors, such as losartan (Cozaar) and valsartan (Diovan).
- Digoxin (Lanoxin). This drug, also referred to as digitalis, increases the strength of your heart muscle contractions. It also tends to slow the heartbeat. Digoxin reduces heart failure symptoms and improves your ability to live with cardiomyopathy.
- Diuretics: Often called water pills, diuretics make you urinate more frequently and keep fluid from collecting in your body. Commonly prescribed diuretics for heart failure include bumetanide (Bumex) and furosemide (Lasix). The drugs also decrease fluid in your lungs, so you can breathe more easily. One diuretic, spironolactone (Aldactone), may also be helpful in treating scarring of your heart tissue.
- Another option for some people with dilated cardiomyopathy is a special pacemaker that coordinates the contractions between the left and right ventricles (biventricular pacing). In people who may be at risk of serious arrhythmias, drug therapy or an implantable cardioverter-defibrillator (ICD) may be an option. An ICD is a small device—about the size of a box of matches—implanted in your chest to continuously monitor your heart rhythm and deliver electrical shocks when needed to control abnormal, rapid heartbeats. The device can also work as a pacemaker.
Surgical Management
- Septal myectomy: This is an open-heart operation in which the surgeon removes part of the thickened, overgrown heart muscle wall (septum) that separates the two bottom heart chambers (ventricles). Removing the part of this overgrown muscle improves blood flow and reduces mitral regurgitation. Myectomy is used if medications do not relieve symptoms. Most people who have symptoms and undergo myectomy have no further symptoms. This type of surgery is available only in medical centers that specialize in the treatment of hypertrophic cardiomyopathy.
- Septal ablation: Also called septal alcohol ablation, this is a treatment in which a small portion of the thickened heart muscle is destroyed by injecting alcohol through a catheter into the artery supplying blood to it. There are possible complications with this procedure, including heart block — a disruption of the heart's electrical system — which requires implantation of a pacemaker. The long-term success of this procedure is not yet known, but it is becoming more commonly used.
- Pacemaker implantation: A pacemaker is a small electronic device inserted under your skin that sends electrical signals to your heart to monitor and regulate your heartbeat. Surgery to implant the pacemaker is usually performed during local anesthesia and typically takes less than three hours. Pacemaker implantation is generally not as effective as surgical options, but it is sometimes used in older people who want to avoid more invasive procedures.
- Implantable cardioverter-defibrillator (ICD): This is a pager-sized device implanted in your chest like a pacemaker. An ICD continuously monitors your heartbeat. If a life-threatening arrhythmia occurs, the ICD delivers precisely calibrated electrical shocks to restore a normal heart rhythm. A small number of people with hypertrophic cardiomyopathy are at risk of sudden cardiac death because of abnormal heart rhythms. In these high-risk individuals, many doctors recommend the implantation of an ICD.
- 30Heart transplant and ventricular assist devices (VADs): If you have severe cardiomyopathy and medications can not control your symptoms, a heart transplant may be an option. Because of the shortage of donor hearts, even people who are critically ill may have a long wait before having a heart transplant. In some cases, a mechanical heart assist device can help critically ill people as they wait for an appropriately matched donor. These devices, known as ventricular assist devices (VADs), can help blood circulate through your heart for months or even years.
Nursing Management
- Acute pain related to an impaired ability of blood vessels to supply oxygen to the tissues.
- Activity intolerance related to compromised oxygen transport system secondary to heart muscle dysfunction.
- Risk for ineffective breathing pattern related to decreased respiratory depth secondary to pain.
Interventions
- Bed rest is important because it reduces myocardial oxygen demand and usually continues until the following criteria are met:
- Temperature remains normal without the use of salicylates.
- Resting pulse rate remains less than 100 beats/min.
- ECG tracings show no manifestations of myocardial damage.
- Pericardial friction rub is not present.
- Administer analgesics as needed and use salicylates around the clock. Balance rest and activity according to the degree of pain and activity tolerance.
- Provide psychosocial support while patient is confined to hospital or home with restrictive intravenous therapy.
- If patient received surgical treatment, provide postsurgical care and instruction.
- After surgery, monitor patient's temperature; a fever may be present for weeks.
- A high-protein, high-carbohydrate diet helps maintain adequate nutrition in the presence of fever and infection.
- Oral hygiene every 4 hours; small, attractive meal servings and foods that are not overly rich, sweet or greasy stimulate the appetite.
- Instruct the client about how to reduce exposure to infection as follows:
- Take good care of the teeth and gums, obtain prompt dental care for cavities and gingivitis
- Prophylactic medication may be needed before invasive dental procedures, and individualized evaluation for prophylaxis medication is also needed.
- Avoid people who have an upper respiratory tract infection.
- Assess for signs and symptoms of organ damage, such as stroke (CVA, brain attack), meningitis, heart failure, myocardial infarction, glomerulonephritis, and splenomegaly.
- Instruct patient and family about activity restrictions, medications, and signs and symptoms of infection.
- Refer to home care nurse to supervise and monitor intravenous antibiotic therapy at home.
CONGESTIVE HEART FAILURE
The heart's pumping power is weaker than normal blood moves through the heart and body at a slower rate, and thus pressure in the heart increases. As a result, the heart cannot pump enough oxygen and nutrients to meet the body's needs. The chambers of the heart may respond by stretching to hold more blood to pump through the body or by becoming stiff and thickened.
Etiology
- Coronary artery disease: Coronary artery disease (CAD), a disease of the arteries that supply blood and oxygen to the heart, causes decreased blood flow to the heart muscle. If the arteries become blocked or severely narrowed, the heart becomes starved for oxygen and nutrients.
- Heart attack: A heart attack occurs when a coronary artery becomes suddenly blocked, stopping the flow of blood to the heart muscle. A heart attack damages the heart muscle, resulting in a scarred area that does not function properly.
- Cardiomyopathy: Damage to the heart muscle from causes other than artery or blood flow problems, such as from infections or alcohol or drug abuse.
Categories of Heart Failure
- Acute failure
- Chronic failure
- Left-sided heart failure
- Right-sided heart failure
- Forward failure
- Backward failure
- High-output failure
- Low-output failure
- Acute heart failure: It occurs in response to a sudden decrease in CO, resulting in rapid decrease in tissue perfusion. It can occur due to:
- Chronic heart failure: It occurs when body adjusts to decrease in CO through compensatory mechanisms, results in systemic congestion. It develops slowly as in:
- Left-sided heart failure versus right-sided heart failure: Right-sided heart failure compromises pulmonary flow to the lungs. Left-sided heart failure compromises aortic flow to the body and brain. Mixed presentations are common. Left-sided heart failure often leads to right heart failure in the longer term.
Left-sided heart failure:
- Left ventricle fails as effective pump
- Left ventricle cannot eject blood delivered from right heart through pulmonary circulation
- Blood backs up into pulmonary circulation
- Increased pressure in pulmonary capillaries forces blood serum out of capillaries into interstitial spaces and alveoli
- Increased respiratory work and decreased gas exchange occur.
Pathophysiology
Signs and Symptoms
Congested lungs: Fluid backup in the lungs can cause shortness of breath with exercise or difficulty breathing at rest or when lying flat in bed. Lung congestion can also cause a dry, hacking cough or wheezing.
Fluid and water retention: Less blood to your kidneys causes fluid and water retention, resulting in swollen ankles, legs, abdomen (called edema), and weight gain. Symptoms may cause an increased need to urinate during the night. Bloating in your stomach may cause a loss of appetite or nausea.
Dizziness, fatigue, and weakness: Less blood to your major organs and muscles makes you feel tired and weak. Less blood to the brain can cause dizziness or confusion.
Rapid or irregular heartbeats: The heart beats faster to pump enough blood to the body. This can cause a rapid or irregular heartbeat.
- Anxiety, confusion, restlessness
- Persistent cough
- Pink, frothy sputum
- Tachycardia
- Tachypnea
- Noisy, labored breathing
- Rales, wheezing (‘cardiac asthma’)
- Dry hacking cough
- Cyanosis (late)
- Third heart sound (S3)
- Dyspnea on exertion
- Paroxysmal nocturnal dyspnea
- OrthopneaRight-sided heart failure:
- Right ventricle fails as effective pump
- Right ventricle cannot eject blood returning through vena cava
- Blood backs up into systemic circulation
- Increased pressure in systemic capillaries forces fluid out of capillaries into interstitial spaces
- Tissue edema occurs.
Compensatory Mechanism During CHF
- Arterial blood pressure falls. This destimulates baroreceptors in the carotid sinus and aortic arch. This center in the brain increases sympathetic activity, releasing catecholamines into the bloodstream. Binding to alpha-1 receptors results in systemic arterial vasoconstriction. This helps restore blood pressure but also increases the total peripheral resistance, increasing the workload of the heart. Binding to beta-1 receptors in the myocardium increases the heart rate and makes contractions more forceful, in an attempt to increase cardiac output. This also, however, increases the amount of work the heart has to perform.
- Increased sympathetic stimulation also causes the hypothalamus to secrete vasopressin, which causes fluid retention at the kidneys. This increases the blood volume and blood pressure.
- Reduced perfusion (blood flow) to the kidneys stimulates the release of renin – an enzyme which catalyzes the production of the potent vasopressor angiotensin. Angiotensin and its metabolites cause further vasoconstriction, and stimulate increased secretion of the steroid aldosterone from the adrenal glands. This promotes salt and fluid retention at the kidneys, also increasing the blood volume.
Diagnostic Evaluation
- Blood tests: Blood tests are used to evaluate kidney and thyroid function as well as to check cholesterol levels and the presence of anemia. Anemia is a blood condition that 36occurs when there is not enough hemoglobin (the substance in red blood cells that enables the blood to transport oxygen through the body) in a person's blood.
- B-type Natriuretic Peptide (BNP) blood test: BNP is a substance secreted from the heart in response to changes in blood pressure that occur when heart failure develops or worsens. BNP blood levels increase when heart failure symptoms worsen, and decrease when the heart failure condition is stable.
- Chest X-ray: A chest X-ray shows the size of your heart and whether there is fluid build-up around the heart and lungs.
- Echocardiogram: This test is an ultrasound which shows the heart's movement, structure, and function.
- Ejection Fraction (EF): It is used to measure how well heart pumps with each beat to determine if systolic dysfunction or heart failure with preserved left ventricular function is present.
- Electrocardiogram (EKG or ECG)
- Cardiac catheterization
- Stress test: Noninvasive stress tests provide information about the likelihood of coronary artery disease.
Major criteria
- Cardiomegaly on chest radiography
- S3 gallop (a third heart sound)
- Acute pulmonary edema
- Paroxysmal nocturnal dyspnea
- Crackles on lung auscultation
- Central venous pressure of more than 16 cm H2O at the right atrium
- Jugular vein distension
- Positive abdominojugular test
- Weight loss of more than 4.5 kg in 5 days in response to treatment
Minor criteria
- Tachycardia of more than 120 beats per minute
- Nocturnal cough
- Dyspnea on ordinary exertion
- Pleural effusion
- Decrease in vital capacity by one-third from maximum recorded
- Hepatomegaly
Management
General measures
- Quit smoking
- Exercise regularly
- Reach and maintain your healthy weight
- Treat high blood pressure
- Discontinue alcohol or illegal drug use
- Restrict dietary sodium (salt)
- Monitor weight
- Restrict fluids
- Drugs that worsen the condition should be discontinued
- Cardiac resynchronization therapy (biventricular pacemaker) may be recommended
- An implantable cardiac defibrillator (ICD) may be recommended.
Pharmacological Management
- Nonsteroidal anti-inflammatory medications, such as Motrin or Aleve. For relief of aches, pains or fever, take Tylenol instead.
- Some antiarrhythmic agents, lidocaine, quinidine, etc.
- Calcium channel blockers, amlodipine, naverapin, etc.
- Some nutritional supplements, such as salt substitutes, and growth hormone therapies
- Antacids that contain sodium (salt)
- An angiotensin-converting-enzyme inhibitor (ACE inhibitor), enalapril
- Beta blockers
- Diuretics (water pills) and digoxin
- An aldosterone inhibitor
- Decongestants.
Surgical Management
- Biventricular pacing: Cardiac resynchronization therapy (CRT) is used to treat the delay in heart ventricle contractions that occur in some people with advanced heart failure. The CRT pacing device (also called a biventricular pacemaker) is an electronic, battery-powered device that is surgically implanted under the skin. The device has 2 or 3 leads (wires) that are positioned in the heart to help the heart beat in a more balanced way. The leads are implanted through a vein in the right atrium and right ventricle and into the coronary sinus vein to pace the left ventricle. The CRT device can be implanted using the endocardial or epicardial approach. With the endocardial (transvenous) approach, a local anesthetic (pain-relieving medication) is injected to numb the area. The epicardial approach may also be used to place the CRT if you are already having surgery to treat another heart condition.
- Coronary artery bypass grafting surgery: The most common surgery for heart failure caused by coronary artery disease is bypass surgery. Although surgery is more risky for people with heart failure, new strategies before, during and after surgery have reduced the risks and improved outcomes.
- Heart valve surgery: Diseased heart valves can be treated both surgically (traditional heart valve surgery) and nonsurgically (balloon valvuloplasty).
- Implantable left ventricular assist device (LVAD): The LVAD is known as the ‘bridge to transplantation’ for patients who have not responded to other treatments and are hospitalized with severe systolic heart failure. This device helps your heart pump blood 38throughout your body. It allows you to be mobile, sometimes returning home to await a heart transplant. It may also be used as destination therapy for long-term support in patients who are not eligible for transplant.
- Stem cell transplantation: Stem cell transplantation represents a new therapeutic opportunity for such patients. Peripheral stem cells were mobilized and collected by apheresis. They were transplanted into areas of injury with open-heart surgery. To increase blood flow to the engrafted areas, coronary artery bypass surgery was also performed. This approach opens a new window in the treatment of ‘no hope’ patients with congestive heart failure.
- Heart transplantation.
Complications
- Cardiac asthma
- Nonproductive cough due to lung congestion
- Hemoptysis
- Dysphagia due to distension of the pulmonary venous atrium or
- Containment of systemic veins–JVP increased
- Hepatomegaly
- Peripheral edema
- Ascites and anasarka
- Increase in body weight due to water retention and sodium.
Nursing Management
- Assessment: The nurse should assess the client for the clinical manifestations of CHF, especially in high-risk clients.
- Impaired gas exchange related to fluid in the alveoli
- Auscultation of breath sounds every 4 hours
- Encourage to turn cough and deep breath
- Maintain Fowler's position
- Administer oxygen
- Monitor ABG
- Intubation and mechanical ventilation
- Decreased cardiac output related to heart failure and Dysrhythmias
- Vital signs every hour
- Lung and heart sounds every 2 hours
- Administer oxygen
- Hourly urine output
- Assess changes in mental status
- Small meals
- Fluid volume excess related to reduced cardiac output and Na and water retention
- Decreased peripheral tissue perfusion related to reduced cardiac output
- Monitor peripheral pulses
- Color and temperature of skin
- Keep extremities warm
- Assess for thrombophlebitis
- Active or passive ROM
- High-risk for impaired skin integrity related to reduced peripheral tissue perfusion
- Change position every 2 hours
- Pressure mattress
- Heel protectors
- High-risk for digitalis toxicity related to impaired excretion
- Assess for hypokalemia, heart block
- Serum digitalis levels and potassium
- Activity intolerance related to fatigue due to a decrease of cardiac output and pulmonary congestion as manifested by dyspnea, tachycardia, and feelings of weakness and shortness of breath.
- Encourage alternative rest as well as activity periods to reduce cardiac workload.
- Provide emotional and physical rest to reduce oxygen consumption and to relieve dyspnea and tiredness.
- Observe cardiorespiratory response to activity to establish amount of activity that can be performed.
- Educate patient and significant other particular approaches of self-care to decrease much needed oxygen consumption.
- Assist to decide on activities in keeping with physical, psychological and social capabilities to establish the amount of activity that can be carried out.
ISCHEMIC HEART DISEASE
Ischemic heart disease is caused by an imbalance between the myocardial blood flow and the metabolic demand of the myocardium. Reduction in coronary blood flow is related to progressive atherosclerosis with increasing occlusion of coronary arteries. Blood flow can be further decreased by superimposed events, such as vasospasm, thrombosis, or circulatory changes leading to hypoperfusion. This will further cause sudden loss of blood supply to an area of the heart, causing permanent heart damage or death called Acute Myocardial Infarction (AMI).
Etiology and pathophysiology: The most common cause is atherosclerotic disease of coronary arteries, also arterial thrombi, spasm, and rarely coronary emboli. Myocardial ischemia can also occur if myocardial oxygen demands are abnormally increased, as in severe ventricular hypertrophy due to hypertension or aortic stenosis.
- A reduction in the oxygen-carrying capacity of the blood: As in extremely severe anemia or in the presence of carboxyhemoglobin, is a rare cause of myocardial ischemia.
- Occlusive intracoronary thrombus: A thrombus overlying a plaque causes 75% of myocardial infarctions, with superficial plaque erosion present in the remaining 25%.
- Vasospasm: With or without coronary atherosclerosis and possible association with platelet aggregation.
- Emboli: From left-sided mural thrombosis, vegetative endocarditis, or paradoxic emboli from the right side of heart through a patent foramen ovale.
Pathophysiology
- High-plasma LDL, low-plasma HDL, cigarette smoking, diabetes mellitus, and hypertension → dysfunction of vascular endothelium and an abnormal interaction with blood monocytes and platelets → subintimal collections of abnormal fat, cells, and debris (i.e. atherosclerotic plaques) → segmental reductions in cross-sectional area. When the luminal area is reduced by more than approximately 80 percent, blood flow at rest may be reduced, and further minor decreases in the stenotic orifice can reduce coronary flow dramatically and cause myocardial ischemia.
- Severe coronary narrowing and myocardial ischemia are frequently accompanied by the development of collateral vessels, especially when the narrowing develops gradually.
The gross morphologic appearance of a myocardial infarction can vary. Patterns include:
- Transmural infarct: Involving the entire thickness of the left ventricular wall from endocardium to epicardium, usually the anterior free wall and posterior free wall and septum with extension into the RV wall in 15–30%. Isolated infarcts of RV and right atrium are extremely rare.
Clinical Manifestation
- Chest pain that occurs suddenly and constantly does not subside. Usually above sterna region and upper abdomen, this is a major symptom.
- The severity of pain can increase settled until unbearable pain.
- Pain is very severe, such as punctured pain that can spread to shoulder and continue down to arm (usually left arm).
- Pain starts spontaneously (does not occur after emotional disturbance or activity).
- Pain does not disappear with the help of rest or nitroglycerin.
- Pain spread to jaw and neck.
- Pain is accompanied by shortness of breath, pale, cold, sever diaphoresis or head floating and nausea and vomiting.
- Stomach, back and abdominal pain.
- Shortness of breath and difficulty in breathing.
- Unexplained anxiety.
- Weakness and fatigue.
- Palpitations.
Diagnostic Evaluation
- Creatine Kinase: The total CK is a simple and inexpensive test that is readily available using many laboratory instruments. However, an elevation in total CK is not specific for myocardial injury, because most CK is located in skeletal muscle, and elevations are possible from a variety of noncardiac conditions.
- Creatine Kinase–MB Fraction: Creatine kinase can be further subdivided into three isoenzymes: MM, MB, and BB. The MM fraction is present in both cardiac and skeletal muscles, but the MB fraction is much more specific for cardiac muscle: about 15 to 40% of CK in cardiac muscle is MB, while less than 2% in skeletal muscle is MB. The BB fraction (found in brain, bowel, and bladder) is not routinely measured. The creatine kinase-MB 42fraction (CK-MB) is part of total CK and more specific for cardiac muscle than other striated muscles. It tends to increase within 3 to 4 hours of myocardial necrosis, then peak in a day and return to normal within 36 hours. It is less sensitive than troponins.
- Troponin I and T are structural components of cardiac muscle. They are released into the bloodstream with myocardial injury. They are highly specific for myocardial injury—more so than CK-MB. Troponins will begin to increase following MI within 3 to 12 hours. Troponins will remain elevated longer than CK—up to 5 to 10 days for troponin I and up to 2 weeks for troponin T. This makes troponins a superior marker for diagnosing myocardial infarction.
- Myoglobin: Myoglobin is a protein found in skeletal and cardiac muscle which binds oxygen. It is a very sensitive indicator of muscle injury. However, it is not specific for cardiac muscle, and can be elevated with any form of injury to skeletal muscle. The rise in myoglobin can help to determine the size of an infarction.
- CRP: C-reactive protein (CRP) is an acute phase protein elevated when inflammation is present. Since inflammation is part of atheroma formation, then CRP may reflect the extent of atheromatous plaque formation and predict risk for acute coronary events.
Management
- Explanation and reassurance.
- Reduction of risk factors (secondary prevention): The discontinuance of cigarette smoking is vital. The risk of coronary events is low when the total plasma cholesterol is less than 200 mg/100 mL, intermediate when it is 200 to 240 mg/100 mL, and abnormally increased when the plasma cholesterol is over 240 mg/100 mL.
- Ideal weight should be attained and maintained. Aggravating factors (e.g. endocrine disorders, hypertension, and drugs such as glucocorticoids) should be treated and eliminated when possible. Diabetes mellitus and hypertension, when present, should be treated.
- The administration of estrogen to postmenopausal women appears to provide significant protection with a reduction in coronary events.
- Sensible adaptations of activities to minimize anginal attacks. Patients should reduce their energy requirements in the morning and immediately after meals. It may be necessary to recommend a change in employment to avoid physical stress.
Pharmacological Treatment
- Nitrates act by causing systemic vesodilation, thereby reducing myocardial wall tension and oxygen requirements, as well as by dilating the epicardial coronary vessels and increasing blood flow in collateral vessels. Nitroglycerin is administered sublingually in tablets of 0.4 or 0.6 mg. Patients with angina should be instructed to take the medication both to relieve an attack and also in anticipation of stress (exercise or emotional). If relief is not achieved after the first dose of nitroglycerin, a second or third dose may be given at 5-minute intervals. These preparations can be swallowed, chewed, or administered as a patch or paste by the transdermal route.
- Beta-adrenoceptor blockers reduce myocardial oxygen demand by inhibiting the increases in heart rate and myocardial contractility caused by adrenergic activity. Beta blockage reduces these variables most strikingly during exercise while causing only 43small reductions in heart rate, cardiac output, and arterial pressure at rest. Propranolol is usually administered in an initial dose of 20 to 40 mg four times a day.
- Calcium antagonists: Verapamil (80 to 120 mg tid), diltiazem (30 to 90 mg qid) and other calcium antagonists are all coronary vasodilators that produce variable and dose-dependent reductions in myocardial oxygen demand, contractility, and arterial pressure
- Aspirin is an irreversible inhibitor of platelet cyclooxygenase activity. Chronic administration of 100 to 325 mg orally per day reduces coronary events.
- ACE inhibitors: Lower blood pressure, so the heart does not have to work as hard to pump blood. It is very useful in post heart-attack care.
- Digitalis glycosides, such as digoxin, may be prescribed in some cases to strengthen heart muscle contraction.
- Dopamine or dobutamine may be administered to increase blood flow to the heart and strengthen the heartbeat.
- Thrombolytic, or clot-dissolving, drugs, such as tissue plasminogen activator (tPA), streptokinase or urokinase may be injected immediately to dissolve arterial blockage. This technique is most effective within three hours of the onset of a heart attack.
Surgical Management
Percutaneous Transluminal Coronary Angioplasty (PTCA)
- Percutaneous transluminal coronary angioplasty (PTCA) is performed to open blocked coronary arteries caused by coronary artery disease (CAD) and to restore arterial blood flow to the heart tissue without open-heart surgery. A special catheter (long hollow tube) is inserted into the coronary artery to be treated.
- This catheter has a tiny balloon at its tip. The balloon is inflated once the catheter has been placed into the narrowed area of the coronary artery. The inflation of the balloon compresses the fatty tissue in the artery and makes a larger opening inside the artery for improved blood flow.
- The use of fluoroscopy assists the physician in the location of blockages in the coronary arteries as the contrast dye moves through the arteries. A small sample of heart tissue (called a biopsy) may be obtained during the procedure to be examined later under the microscope for abnormalities.
- A technique called intravascular ultrasound (IVUS), a technique that uses a computer and a transducer that sends out ultrasonic sound waves to create images of the blood vessels, may be used during PTCA.
- The use of IVUS provides direct visualization and measurement of the inside of the blood vessels and may assist the physician in selecting the appropriate size of balloons and/or stents, to ensure that a stent, if used, is properly opened, or to evaluate the use of other angioplasty instruments.
- The physician may determine that another type of procedure is necessary. This may include the use of atherectomy (removal of plaque) at the site of the narrowing of the artery. In atherectomy, there may be tiny blades on a balloon or a rotating tip at the end of the catheter.
- When the catheter reaches the narrowed spot in the artery, the plaque is broken up or cut away to open the artery. Atherectomy is used when the plaque is calcified, hardened, or if the vessel is completely closed. Another type of atherectomy procedure uses a laser, which opens the artery by ‘vaporizing’ the plaque.
Procedure Completion, Both Methods
- The sternum will be pushed back together and sewn together with small wires.
- The skin over the sternum will be sewn back together.
- Tubes will be inserted into your chest to drain blood and other fluids from around the heart. These tubes will be connected to a suction device to keep fluids pulled away from the heart.
- A tube will be inserted through your mouth or nose into your stomach to drain stomach fluids.
- A sterile bandage or dressing will be applied.
Coronary Artery Bypass Graft Surgery (CABG)
Coronary artery bypass graft surgery (CABG) is a procedure used to treat coronary artery disease in certain circumstances. Coronary artery disease (CAD) is the narrowing of the coronary arteries (the blood vessels that supply oxygen and nutrients to the heart muscle), caused by a buildup of fatty material within the walls of the arteries. This buildup causes the inside of the arteries to become narrowed, limiting the supply of oxygen-rich blood to the heart muscle.
One way to treat the blocked or narrowed arteries is to bypass the blocked portion of the coronary artery with another piece of blood vessel. Blood vessels, or grafts, used for the bypass procedure may be pieces of a vein taken from the legs or an artery in the chest. At times, an artery from the wrist may also be used. One end of the graft is attached above the blockage and the other end is attached below the blockage. Thus, the blood is rerouted around, or bypasses, the blockage through the new graft to reach the heart muscle. This bypass of the blocked coronary artery can be done by performing coronary artery bypass surgery.
Nursing Management
- Acute pain related to an imbalance of oxygen supply to myocardial demands.Intervention:
- Assess pain location, duration, radiation, occurrence, a new phenomenon.
- Review of previous activities that cause chest pain.
- Create a 12-lead ECG during anginal pain episodes.
- Assess signs of hypoxemia; give oxygen therapy, if necessary.
- Give analgesics as directed.
- Maintain a rest for 24–30 hours during episodes of illness.
- Check vital signs during periods of illness.
- Decreased cardiac output related to electrical factors (dysrhythmias), decrease in myocardial contraction, structural abnormalities.Intervention:
- Anxiety related to the needs of the body is threatened.Intervention:
- Assess signs and verbal expressions of anxiety
- Take action to reduce anxiety by creating a calm environment
- Accompany the patient during periods of high anxiety
- Provide an explanation of procedures and treatments
- Encourage patients to express feelings
- Refer to the spiritual adviser, if necessary
ENDOCARDITIS
Endocarditis is an infection of the heart's valves or its inner lining (endocardium). It is most common in people who have a damaged, diseased, or artificial heart valve, caused by bacterial infection
Etiology
Endocarditis is caused by bacteria that enter the bloodstream and settle on the inside of the heart, usually on the heart valves. Bacteria can invade in the bloodstream in the many ways, including during some dental and surgical procedures. If one does not take care of teeth, then chances of endocarditis increase. Streptococcus viridans is responsible for about 50% of all bacterial endocarditis cases. This is why dental procedures increase chances for developing this condition. Other common agents include Staphylococcus aureus and Enterococcus. Staphylococcus aureus can infect normal heart valves and is the most common cause of infectious endocarditis in intravenous drug users.
Risk Factors
- Had endocarditis in the past.
- Hemodialysis for kidney failure.
- Abnormal or damaged heart valves.
- An artificial heart valve.
- A congenital heart defect.
- Hypertrophic cardiomyopathy.
- Injected illegal drugs using dirty needles or without cleaning the skin.
- HIV
- Coronary artery bypass graft surgery (bypass surgery).
- Previous rheumatic fever without heart valve damage.
- A pacemaker or an implantable cardioverter-defibrillator (ICD).
- A heart attack without other complications.
- Mitral valve prolapse without mitral valve regurgitation or unusually thickened valve leaflets.
- A coronary artery stent.
Pathophysiology
Signs and Symptoms
- Chills and fever
- Fatigue
- Weight loss
- Night sweats
- Painful joints
- Persistent cough and shortness of breath
- Bleeding under the fingernails
- Tiny purple and red spots under the skin
- Nail abnormalities (splinter hemorrhages under the nails)
- Night sweats (may be severe)
- Paleness
- Red, painless skin spots on the palms and soles (Janeway lesions)
- Red, painful nodes (Osler's nodes) in the pads of the fingers and toes
Diagnostic Evaluation
A physical examination may reveal:
- Enlarged spleen
- Splinter hemorrhages in the fingernails
- A history of congenital heart disease raises the level of suspicion. An eye examination may show bleeding in the retina a central area of clearing. This is known as Roth's spots.
The following tests may be performed:
- Blood culture and sensitivity (to detect bacteria)
- Chest X-ray
- CT scan of the chest
- Echocardiogram (ultrasound of the heart)
- Erythrocyte sedimentation rate (ESR)
- Transesophageal echocardiogram
Management
The American Heart Association recommends preventive antibiotics for people at risk for infectious endocarditis before:
- Certain dental procedures
- Surgeries on respiratory tract or infected skin, skin structures, or musculoskeletal tissue
Antibiotics are more likely to be recommended to those with the following risk factors:
- Artificial heart valves
- Certain congenital heart defects, both before or possibly after repair
- History of infective endocarditis
- Valve problems after a heart transplant
Nursing Management
Nursing Diagnosis
- Hyperthermia related to infection of cardiac tissue as evidenced by temperature elevation, diaphoresis, chills, malaise, tachycardia, and tachypnea.Intervention
- Monitor temperature as appropriate to determine effectiveness of therapy and to prevent treatment-induced hypothermia.
- Administer antipyretic medication as appropriate or as ordered to reduce fever.
- Administer medications as appropriate to treat the cause of the fever.
- Monitor white blood cell count to evaluate a patient's response to treatment.
- Monitor vital signs to assess cardiorespiratory response to fever.
- Encourage intake of oral fluids to replace fluids lost as a result of fever.
- Activity intolerance related to generalized weakness, arthralgia, and alteration in oxygen transport secondary to valvular dysfunction.Intervention
- Monitor cardiorespiratory response to activity (e.g. vital signs) to plan or alter activities.
- Monitor patient for evidence of excess physical (e.g. tachycardia, hypertension, diaphoresis, dyspnea) or emotional fatigue to plan for changes in activity level.
- Instruct patient/caregiver to recognize signs and symptoms of fatigue that require reduction in activity (e.g. pulse increases > 20 beats/min; no increase in activity if resting pulse > 100 beats/min) since these signs indicate excessive cardiac effort.
- Encourage alternate rest and activity periods to reduce cardiac workload.
- Deficient knowledge related to lack of experience and exposure to information about disease and treatment process.
- Review patient's and caregiver's knowledge about condition to identify teaching needs.
- Discuss lifestyle changes that may be required to prevent future complications and/or control the disease process (e.g. avoiding persons with infection, taking prophylactic antibiotics before dental procedures) to reduce the risk of recurrent infective endocarditis.
- Teaching: Prescribed Medication
- Provide the patient and caregiver with information about the action, purpose, and side effects of the medications to promote safe medication therapy.
MYOCARDITIS AND PERICARDITIS
Introduction
Any of the heart's layer is affected by an infectious process. The infections are named for the layer of the heart most involved in the infectious process.
Definition of Myocarditis
Myocarditis or inflammatory cardiomyopathy is the inflammation of heart muscles (myocardium).
Or
Myocarditis is the inflammatory process involving the myocardium, can cause heart dilation, thrombi on the heart wall (mural thrombi), infiltration of circulating blood cells around the coronary vessels and between the muscle fibers, and degeneration of the muscle fibers themselves.
Etiology
- Infections
- Viral (HIV, rubella virus, poliovirus, cytomegalovirus, human herpesvirus 6 and possibly hepatitis C)
- Protozoan (Toxoplasma gondii)
- Bacterial (Brucella, Corynebacterium diphtheriae)
- Fungal (Aspergillus)
- Parasitic (Ascaris, Echinococcus granulosus)
- Toxins
- Drugs (ethanol, anthracyclines and some other forms of chemotherapy, and antipsychotics)
- Immunological causes:
- Allergic (acetazolamide, amitriptyline)
- Rejection after a heart transplant
- Autoantigens (scleroderma, systemic lupus erythematosus)
- Toxins (arsenic, toxic shock syndrome toxin, carbon monoxide, or snake venom)
- Heavy metals (copper or iron)
-
- Electric shock, hyperpyrexia, and radiation
Pathophysiology
Signs and Symptoms
- Chest pain (often described as ‘stabbing’ in character)
- Palpitations
- Fever and other signs of infection, including headache, muscle aches, sore throat, diarrhea or rashes
- Fatigue
- Dyspnea
- Joints pain or swelling
- Leg swelling
- Dysrhythmias can also occur
- Fainting (often related to irregular heart rhythms)
- Low urine output.
Diagnostic Evaluations
Physical examination may reveal the following:
- Abnormal heartbeat or heart sounds (murmurs, extra heart sounds)
- Fever
- Fluid in lungs
- Rapid heartbeat (tachycardia)
- Swelling in the legs.
- Chest X-ray: It shows enlarged cardiac silhouette resulting from ventricular enlargement or pericardial effusion.
- Blood Test: It shows moderate leukocytes.
- Elevated cardiac enzymes.
- Antibodies are found against the heart muscle and the body itself.
- ECG
- It helps to identify heart chamber size and ventricular functioning.
- Biopsy: It shows features of myocardial interstitium with abundant edema and inflammatory infiltrates, rich in lymphocytes and macrophages. Focal destruction of myocytes explains the myocardial pump failure.
- Cardiac Magnetic Resonance Imaging (cMRI or CMR): It helps in diagnosing myocarditis by visualizing markers for inflammation of the myocardium.
Treatment
- As most viral infections cannot be treated with directed therapy, symptomatic treatment is the only form of therapy for those forms of myocarditis.
- In the acute phase, supportive therapy, including bed rest, is indicated.
- For symptomatic patients, digoxin and diuretics provide clinical improvement.
- For patients with moderate to severe dysfunction, cardiac function can be supported by use of inotropes such as Milrinone in the acute phase, followed by oral therapy with ACE inhibitors (Captopril, Lisinopril) when tolerated.
- People who do not respond to conventional therapy are given bridge therapy with left ventricular assist devices. Heart transplantation is reserved.
- For patients who fail to improve with conventional therapy, heart transplantation is reserved. Others: Anti-embolism stockings and passive and active exercises should be used because embolization from venous thrombosis and mural thrombi can occur, especially in patients on bed rest.
Complications
- Cardiomyopathy
- Pulmonary congestion
- Pericarditis
- Heart failure
- Sudden death
Prevention
Prevention of infectious diseases by means of appropriate immunizations (e.g. influenza, hepatitis) and early treatment appear to be important in decreasing the incidence of myocaridtis.
PERICARDITIS
Definition
Pericarditis is an inflammation of the pericardium (the fibrous sac surrounding the heart).
Or
- Pericarditis is inflammation of the pericardium, which causes its two layers to rasp and rub against each other as the heart contracts and relaxes.
- Classification: It is of two types:
- Acute pericarditis.
- Chronic pericarditis.
Classification on the Basis of Etiological Factors
- Acute nonspecific (idiopathic)
- Infective:
- Bacterial
- Viral
- Other infections
- Immunologic:
- Rheumatic fever
- Other connective tissue disorders
- Neoplastic
- Metabolic:
- Uremic
- Myxedema
- Gout.
- Traumatic (including after cardiac surgery)
- Associated with myocardial infarction.
Infective
- Bacterial pericarditis: This condition is potentially fatal without prompt medical treatment. Most cases of bacterial pericarditis are triggered by infections somewhere else in the body. For example, a person with pneumonia (lung infection) may be vulnerable to bacterial pericarditis if the bacteria access the pericardium directly or via the bloodstream. Some of the dangerous complications include:
- Cardiac tamponade: Fluid builds up between the two layers of the pericardium. The heart is compressed and can not function properly.
- Abscess: A buildup of pus either within the heart or in the pericardium.
- Spread of infection: As with any infection, the infection can spread to other areas.
- Constrictive pericarditis: The pericardium is scarred by the inflammation. Scar tissue does not stretch, so the heart can not function properly.
- Viral pericarditis: Viral infections that cause cold or pneumonia, such as the echovirus or coxsackie virus (which are common in children) as well as influenza.
- Chronic effusive pericarditis: Long-term inflammation causes a gradual buildup of fluid within the two layers of the pericardium. In most cases, the reasons for this are unknown. Some of the known causes of chronic effusive pericarditis include tuberculosis and hypothyroidism (underactive thyroid gland).
- Constrictive pericarditis: As the pericardium recovers from injury or inflammation, scar tissue may form. Scar tissue makes the pericardium stiff and hard, so that the heart is unable to fill properly with blood. Symptoms include unexplained weight loss, fatigue, breathlessness, swelling of the abdomen and heart murmurs. Without treatment, constrictive pericarditis can lead to a range of complications including:
- Irregular heartbeat (heart arrhythmia)
- Heart failure
- Liver damage.
- Traumatic: Heart surgery or trauma to the chest, esophagus or heart.
- Pericarditis following heart surgery: Pericarditis may be a complication of heart surgery. Operations on the heart involve opening the pericardium in order to apply coronary artery bypass grafts, open or replace heart valves, or undertake other corrective procedures.
- Post-heart attack pericarditis: While pericarditis does not cause or contribute to heart attack, the injury to cardiac tissue caused by a heart attack can sometimes lead to pericarditis. This is known as postmyocardial infarction (post-MI) pericarditis. The symptoms may not appear for some weeks or months after the heart attack, and can include relatively mild chest pain, joints pain and fever. It is not possible to prevent post-MI pericarditis.
- Acute pericarditis: It is the inflammation of the pericardium characterized by chest pain, pericardial friction rub and serial of ECG changes (ST evelations).
Types include: Pericarditis can be classified according to the composition of the inflammatory exudates.
- Serous: This form usually consists of 50 to 200 ml of slowly accumulating exudates. Characteristically produced by nonbacterial involvement, including rheumatic fever, systemic lupus erythematosus, tumors, uremia and primary viral infection (e.g. coxsackie).
- Purulent: This is due to bacteria, fungus or parasitic action. Infection reaches directly, through blood or through lymphatic route from the nearby areas of infection, e.g. pneumonia, empyema, lung abscess, etc. Most common causative organisms are Staphylococci, Streptococci, and Pneumococci. Purulent pericarditis is composed of 400 to 500 ml of a thin to creamy pus. The patient presents with fever, rigor and a friction rub. It usually organizes and may produce mediastinopericarditis or constrictive pericarditis.
- Fibrinous: In this, exudates will be completely resolved or be organized, causing adhesive pericarditis.
- Caseous: This form is due to tuberculosis by direct extension from neighboring lymph nodes.
- Hemorrhagic: This is composed of an exudate of blood mixed with fibrinous to suppurative effusion. Most commonly, it occurs after cardiac surgeries.
Causes
- Infectious: Pericarditis may be caused by:
- Viral (coxsackievirus, influenza)
- Bacterial (Pneumococcus, tuberculous, Staphylococcus or Streptococcus)
- Fungal (Aspergillus, Candida, and Coccidioides)
Other
- Idiopathic: No identifiable etiology found after routine testing.
- Immunologic conditions including systemic lupus erythematosus (more common among women) or rheumatic fever
- Myocardial infarction (Dressler's syndrome)
- Uremia (uremic pericarditis)
- Malignancy (as a paraneoplastic phenomenon)
- Side effects of some medications, e.g. isoniazide, cyclosporine, warfarin, and heparin
- Radiation-induced
- Aortic dissection
- Tetracyclines
- Postpericardiotomy syndrome: Usually after CABG surgery
Pathophysiology
Signs and Symptoms
- Chest pain
- Dry cough
- Fever
- Chills
- Fatigue
- Malaise
- Anxiety
- Joints pain
- Anorexia
- Increased heart rate (depends upon the degree of fever and anxiety)
- Weight loss
Characteristic/Parameter | Pericarditis | Myocardial infarction |
|---|---|---|
Pain description | Sharp, pleuritic, retrosternal (under the sternum) or left precordial (left chest) pain | Crushing, pressure-like, heavy pain. Described as ‘elephant on the chest’. |
Radiation | Pain radiates to the trapezius ridge (to the lowest portion of the scapula on the back) or no radiation | Pain radiates to the jaw, or the left arm, or does not radiate |
Exertion | Does not change the pain | Can increase the pain |
Position | Pain is worse in the supine position or upon inspiration (breathing in) | Not positional |
Onset/duration | Sudden pain that lasts for hours or sometimes days before a patient comes to the ER | Sudden or chronically worsening pain that can come and go in paroxysms or it can last for hours before the patient decides to come to the ER |
Diagnostic Evaluations
- ECG: It indicates tachycardia but with underlying heart disease or uremia, bradycardia can occur.
- Laboratory investigation shows:
- Elevated ESR
- Elevated WBC count
- Cardiac enzymes are usually normal but may be elevated
- Physical Examination: It reveals the classical symptom, that is friction rub.
- Acute Complications: Pericarditis can progress to:
- Pericardial effusion
- Cardiac tamponade
- Pulsus paradoxus (decrease of at least 10 mm Hg of the systolic blood pressure upon inspiration), hypotension (due to decreased cardiac index), JVD (jugular vein distention) from right-sided heart failure and fluid overload.
- Treatment: The treatment in viral or idiopathic pericarditis is with Aspirin, or non-steroidal anti-inflammatory drugs (NSAIDs, such as naproxen). Severe cases may require:
- Antibiotics to treat tuberculosis or other bacterial causes.
- Steroids are used in acute pericarditis but are not favored because they increase the chance of recurrent pericarditis.
- Colchicine is a very effective treatment option. If aspirin and NSAIDs are not sufficient, colchicine should be added to the regimen.
Surgical Management
Pericardiocentesis is done to treat pericardial effusion/tamponade. (Pericardiocentesis is a procedure in which puncture of pericardial sac is done and some of the pericardial fluid is removed to relieve cardiac tamponade, which restricts normal heart action. During the procedure, the patient is monitored by ECG and central venous pressure measurements are made. A defibrillator is turned on and other emergency resuscitative equipment should be kept ready).
CHRONIC PERICARDITIS
Definition: Chronic pericarditis is a condition in which there is chronic inflammatory thickening of the pericardium that changes the pericardium into thick fibrous band of tissues. Thus, the tissues encircle, encase and compress the heart and prevent it from expanding to normal size, causing restriction of ventricular filling.
Types
- Adhesive pericarditis
- Adhesive mediastinopericarditis
- Adhesive pericarditis: Chronic pericarditis with adhesions between visceral and parietal pericardium.
Causes
- Shortness of breath
- Pain: It may be steady and constant or it may occur in paroxysms, usually after unusal effort or after mental excitement or a fit of anger.
- Pulse: Rapid and feasible; pulse tension and pressure greatly reduced; irregular pulse.
- Palpitations
- Ventricles become dilated and hypertrophied with its concomitant symptoms—dropsy, vertigo and venous stasis are present.
- Treatment: It needs most careful and continuous oversight. Effusion should be retarded and its absorption and removal should be promoted by rational measures.
- Adhesive mediastino pericarditis: Here pericardial sac is obliterated due to adhesion between two layers of pericardium as well as between parietal pericardium and surrounding mediastinal structures, chest wall and diaphragm.
- Constrictive pericarditis: Constrictive pericarditis is a late sequela of an inflammatory condition of the pericardium. The inflammatory condition is usually an infection that involves the pericardium, but it may also occur after a heart attack or after heart surgery.
Almost half the cases of constrictive pericarditis in the developing world are idiopathic in origin. In regions where tuberculosis is common, it is the cause in a large portion of cases. Causes of constrictive pericarditis include:
- Infectious:
- Tuberculosis
- Incomplete drainage of purulent pericarditis
- Fungal and parasitic infections
- Inflammatory and autoimmune:
- Chronic pericarditis
- Postviral pericarditis
- Postsurgical
- Following pericarditis associated with acute myocardial infarction
- Following postmyocardial infarction (Dressler's) syndrome
- In association with pulmonary asbestosis
- Prior mediastinal radiation therapy
- Chronic renal failure
- Connective tissue disorders
- Neoplastic pericardial infiltration
Pathophysiology
Diagnostic Evaluation
- Imaging will demonstrate a thickened pericardium. In contrast with restrictive cardiomyopathy, there is an increased resistance to ventricular filling due to increased myocardial stiffness. Imaging features of restrictive cardiomyopathy demonstrate an increased left ventricular thickness with infiltration of the myocardium.
- Chest X-ray: Pericardial calcification, and pleural effusions are common findings.
- Echocardiography: The echographic finding is an exaggerated anterior motion of the septum with the atrial filling. Since the posterior ventricular wall is unable to expand, an increase in left ventricular volume with the atrial systole produces a marked displacement of the septum.
- CT and MRI: Useful in select cases.
- BNP blood test: Tests for the existence of the cardiac hormone, brain natriuretic peptide which is only present in RCMP but not in CP, and is particularly helpful in determining the specific CHF type.
- Pulmonary catheterization showed all four heart chambers having equal diastolic pressures.
Clinical Features
- Kussmaul's sign (raised JVP on inspiration)
- Increased JVP (almost universal), rapid descent (prominent diastolic collapse of JVP)
- Hepatomegaly and other signs of right heart failure; ascites; fatigue; peripheral edema
Treatment
Pericardial stripping: The definitive treatment for constrictive pericarditis is pericardial stripping, which is a surgical procedure where the entire pericardium is peeled away from the heart. This procedure has significant risk involved, with mortality rates of 6%. The high risk of the procedure is attributed to adherence of the thickened pericardium to the myocardium and coronary arteries. In patients who have undergone coronary artery bypass surgery with pericardial sparing, there is danger of tearing a bypass graft while removing the pericardium. Due to the significant risks involved with pericardial stripping, many patients are treated medically, with judicious use of diuretics.
Common Causes of Chronic Pericarditis
- Long-standing pyogenic infections
- Postviral infections
- Tuberculosis
- Hemopericardium.
Common Signs and Symptoms of Chronic Pericarditis
- Congestive heart failure
- Dyspnea
- Chronic atrial fibrillation
- Fatigue on exertion
- Leg edema
- Ascites
- Low pulse pressure
- Distended neck vein
- Delay in capillary refill time
Common Treatment of Chronic Pericarditis
- Medical treatment: Digitalis and diuretics
- Surgical treatment: Surgical removal of the tough encasing pericardium (pericardiectomy) is the only treatment of benefit. The objective of the operation is to release both ventricles from the constrictive and restrictive inflammation. Surgery may be considered if the pericardium is scarred and inflexible, or if pericarditis keeps recurring.
- The procedure begins when the surgeon makes an incision in the skin over the breastbone and divides it to expose the pericardium. During the surgery, the surgeon will grasp the pericardium, cut the top of this fibrous covering of the heart, drop it into the specimen bag, and re-cover the heart. The breastbone is then wired back together and the incision is closed, completing the procedure. When the portion of pericardium lying between the two phrenic nerves is excised, it is called total pericardiectomy. In cases where total pericardiectomy is not possible, subtotal pericardiectomy is performed or, in extreme cases, a cruciate incision on the pericardium is performed.
Nursing Management
Assessment
- Assess signs of pain.
- Assess association of pain with respiratory movements, cough, swallowing.
- Assess for pericardial friction rub (helps to distinguish between pericarditis and MI).
- Frequently check client for temperature (pericarditis can cause abrupt onset of fever in a previously afebrile patient).
Nursing Diagnosis
- Acute pain related to inflammation of layers of heart.
Goal: To relieve pain.
Interventions
- Check the intensity of pain.
- Assist the patient to sit upright or to lean forward to relieve pain.
- Restrict the activities of patient.
- Provide prescribed analgesics (morphine).
- Hyperthermia related to inflammatory process.
Goal: To maintain normal temperature.
Interventions
- Monitor temperature 2–4 hourly.
- Observe for basic principles of asepsis like handwashing.
- Provide cold compression if chills are not present along with fever.
- Administer prescribed antibiotics and antipyretics.
- Decreased cardiac output related to structural abnormality of valves.
Goal: To reduce risk of complications.
Interventions
- Monitor BP and pulse (pulsus alternans indicates left-sided heart failure).
- Evaluate jugular vein distension.
- Check laboratory findings (ECG, cardiac enzymes).
- Maintain intake.
- Output chart.
- Obtain daily weight.
- Administer prescribed drugs like digitalis.
- Risk for complications related to disease process.
Goal: To reduce risk of complications.
Interventions
- Assess vital signs of patient.
- Assess peripheral edema.
- Check the laboratory findings (ECG, cardiac enzymes).
- Administer digitalis and digoxin if signs of heart failure appear.
- Prepare for emergency pericardiocentesis.
RHEUMATIC HEART DISEASE
Rheumatic heart disease is a group of acute and long-term chronic heart disorders that can occur as a result of rheumatic fever. One common result of rheumatic fever is heart valve damage. This damage to the heart valves may lead to a valve disorder. It is caused by an autoimmune reaction to Group A β-hemolytic streptococci (GAS) that results in valvular damage characterized by fibrosis and scarring of valve leaflets, commissures and cusps leads to abnormalities that can result in valve stenosis or regurgitation.
Etiology
- Autoimmune reaction to Group A β-hemolytic streptococci (GAS)
- Rheumatic fever is a systemic disease affecting the periarteriolar connective tissue and can occur after an untreated Group A β-hemolytic streptococcal pharyngeal infection.
- It is believed to be caused by antibody cross-reactivity. This cross-reactivity is a Type II hypersensitivity reaction and is termed as molecular mimicry.
- Usually, self-reactive B-cells remain anergic in the periphery without T-cell co-stimulation. During a Streptococcus infection, mature antigen presenting cells such as B-cells present the bacterial antigen to CD4-T-cells which differentiate into helper T2 cells.
- Helper T2-cells subsequently activate the B-cells to become plasma cells and induce the production of antibodies against the cell wall of Streptococcus.
- The antibodies may also react against the myocardium and joints producing the symptoms of rheumatic fever.
Pathophysiology
Signs and Symptoms
- Polyarthritis
- Carditis
- Subcutaneous nodules: Painless, firm collections of collagen fibers over bones or tendons. They commonly appear on the back of the wrist, the outside elbow, and the front of the knees.
- Chorea: A characteristic series of rapid movements without purpose of the face and arms. This can occur very late in the disease for at least three months from the onset of infection.
- Fever of 38.2–38.9 °C (101–102 °F)
- Arthralgia: Joints pain without swelling.
- Raised erythrocyte sedimentation rate or C-reactive protein
- Leukocytosis
- ECG showing features of heart block, such as a prolonged PR interval.
Diagnostic Evaluation
- Signs and symptoms you report
- Evidence of recent group A streptococcal infection
- Physical examination:
- Checking the joints for signs of inflammation
- Examining the skin for nodules under the skin or a rash
- Listening to the heart for abnormal rhythms, murmurs or muffled sounds that may indicate inflammation of the heart
- Conducting a series of simple movement tests to detect indirect evidence of inflammation of the central nervous system
- CBC
- Electrocardiogram (ECG)
- Echocardiography
Management
- Antibiotics, penicillin, ampicillin and amoxicillin
- A single dose of intramuscular benzathine penicillin G or benzathine/procaine penicillin combination can also be used.
- Alternate drugs include narrow-spectrum cephalosporins, amoxicillin-clavulanate, dicloxacillin, erythromycin, or other macrolides.
- Place hospitalized patients with Group A β-hemolytic streptococci on droplet precautions, as well as standard precautions, until 24 hours after initiation of appropriate antibiotics.
- Salicylates and steroids can also be included in treatment regimen to decrease the inflammatory response.
- Include digoxin and diuretics, afterload reduction, supplemental oxygen, bed rest, and sodium and fluid restriction as an additional treatment.
- In patients with critical stenosis, mitral valvulotomy, percutaneous balloon valvuloplasty, or mitral valve replacement may be indicated.
Nursing management
Nursing assessment
- About heart function.
- Nutritional status of patients.
- Disturbances in sleep patterns.
- Level of discomfort felt by rheumatic fever patients.
- Ability of the patient in terms of troubleshooting.
- Knowledge of patients and families will be suffered by rheumatic heart disease.
- History of rheumatic heart disease.
- Monitor cardiac complications in the event.
- Auscultation of heart sounds.
- Assessment of the patient's vital signs.
- Assessment of pain.
- Assessment of the presence of markers of inflammation in the joints.
- Assessment of the presence of lesions on the skin.
- Decreased cardiac output related to valvular stenosis
Nursing interventions
- Monitor vital signs such as blood pressure, apical pulse and peripheral pulse.
- Monitor cardiac rhythm and frequency.
- Semi-Fowler bed rest in a position which is 45 degrees.
- Encourage the patient to stress management techniques (quiet environment, meditation).
- Medical collaboration in terms of oxygen delivery and therapy.
- Activity intolerance related to decreased cardiac output, oxygen supply and demand imbalance.
Nursing interventions
- Energy saving in case of acute patients.
- Maintain bed rest until the results of laboratory and clinical status of patients are improved.
- Monitor the gradual increase in the level of activity undertaken.
- Teach to participate in activities of daily necessities.
- Create a schedule of activities and also of the breaks.
VALVULAR HEART DISEASE
Heart valve disease occurs when heart's valves do not work properly. It includes narrowing of valve opening, stiff valve cusps and leaflets and contracture of valves. Valvular heart disease process involving one or more of the four valves of the heart affected by the functional alteration, stenosis and regurgitation.
Types
There are several types of valve diseases:
- Valvular stenosis: This occurs when a valve opening is smaller than normal due to stiff or fused leaflets. The narrowed opening may make the heart work very hard to pump blood through it. This can lead to heart failure and other symptoms. All four valves 64can be stenotic (hardened, restricting blood flow). The conditions are called tricuspid stenosis, pulmonic stenosis, mitral stenosis or aortic stenosis.
Pathophysiology
- Valvular insufficiency: Also called regurgitation, incompetence or ‘leaky valve’, this occurs when a valve does not close tightly. If the valves do not seal, some blood will leak backward across the valve. As the leak worsens, the heart has to work harder to make up for the leaky valve, and less blood may flow to the rest of the body. Depending on which valve is affected, the condition is called tricuspid regurgitation, pulmonary regurgitation, mitral regurgitation or aortic regurgitation.
Pathophysiology of Aortic Regurgitation
- Congenital valve disease: Most often affects the aortic or pulmonic valve. Valves may be the wrong size, have malformed leaflets, or have leaflets that are not attached to the annulus correctly.
- Acquired valve disease: This includes problems that develop with valves that were once normal. These may involve changes in the structure or valve due to a variety of diseases or infections, including rheumatic fever or endocarditis.
- Mitral valve prolapse (MVP): MVP causes the leaflets of the mitral valve to flop back into the left atrium during the heart's contraction. MVP also causes the tissues of the valve to become abnormal and stretchy, causing the valve to leak.
- Tricuspid regurgitation
- Most common cause is annular dilatation due to RV failure of any cause.
- The pansystolic murmur of TR is usually loudest at the left sternal edge and augmented by deep inspiration.
- Severe functional TR may be treated by annuloplasty or valve replacement. Severe TR due to intrinsic tricuspid valve disease requires valve replacement.
Etiology and Pathophysiology
There are many different types of valve disease; some types can be present at birth (congenital), while others may be acquired later in life.
- Heart valve tissue may degenerate with age.
- Rheumatic fever may cause valvular heart disease.
- Bacterial endocarditis.
- High blood pressure and atherosclerosis may damage the aortic valve.
- A heart attack may damage the muscles that control the heart valves.
- Other disorders, such as carcinoid tumors, rheumatoid arthritis, systemic lupus erythematosus, or syphilis may damage one or more heart valves.
Signs and Symptoms
- Mitral valve stenosis:
- Mitral valve regurgitation:
- Acute poorly tolerated with fulminating pulmonary edema
- Shock developing rapidly
- Systolic murmur
- Mitral valve prolapse:
- Palpitation
- Dyspnea
- Chest pain
- Activity intolerance
- Syncope
- Aortic valve stenosis:
- Abrupt onset of profound dyspnea
- Chest pain
- Left ventricular failure
- Shock
- Chronic:
- Fatigue
- Exertional dyspnea
- Orthopnea
- High-pitched murmur sound
- Absent S1 and S2 sound
- Tricuspid and pulmonic stenosis:
- Peripheral edema
- Ascitis
- Hepatomegaly
- Diastolic low-pitched
- Increased intensity during inspiration
- Other signs and symptoms:
- Low BP
- Dizziness
- Dysrythmia
Diagnosis
- An electrocardiogram: Also called an ECG or EKG, to measure the electrical activity of the heart, regularity of heartbeats, thickening of heart muscle (hypertrophy) and heart-muscle damage from coronary artery disease.
- Stress-testing: Also known as treadmill test, to measure blood pressure, heart rate, ECG changes and breathing rates during exercise. During this test, the heart's electrical activity is monitored through small metal sensors applied to your skin while you exercise on a treadmill.
- Echocardiogram: To evaluate heart function. During this test, sound waves bounced off the heart are recorded and translated into images. The pictures can reveal abnormal heart size, shape and movement. Echocardiography can also be used to calculate the ejection fraction, or volume of blood pumped out to the body when the heart contracts.
- Cardiac catheterization: Which is the threading of a catheter into the heart chambers to measure pressure irregularities across the valves (to detect stenosis) or to observe backflow of an injected dye on an X-ray (to detect incompetence).
Management
Medical management
- Medical therapy
- Antibiotic therapy: Clindamycin, ampicilline or cerazolin or ceftriaxone, clarithromycine
- Treatment for heart faliure
- Diuretics
- Vasodilators: Nitrates, sorbitrate
- ACE inhibitors: Captopril, enapril
- Positive isotope: Digoxin
- Anticoagulant therapy
- Beta blockers: Metoprolol
Surgical Management
For mitral stenosis:
- Closed mitral vulvotomy
- Open mitral valvotomy
- Mitral valve replacement
- Balloon valvuloplasty (percutaneously):
- For mitral insufficiency: (Mitral valve replacement or annuloplasty (the valve ring)
For aortic stenosis or insufficiency:
- Replacement of aortic valve with prosthetic or tissue valves
- Ballon valvuloplasty (aortic stenosis)
For tricuspid stenosis and insufficiency: Valvuloplasty replacement may be done at the time of surgical interventions for associated rheumatic mitral or aortic disease.
Complications
- Left-sided heart failure
- Possible, right-sided heart failure
- Dysrythmia
Nursing Management
- Mitral stenosis:
- Auscultate the first heart sound, usually accompanied by an ‘opening snap’
- Place patient in lateral recumbent position with bell to hear the low-pitched murmur (rumbling murmur)
- Duration
-
- Auscultate for diminished first heart sound
- Auscultate for systolic murmur
- Note radiation of heart sound to axilla and left intrascapular region
- Mild insufficiency may produce a systolic murmur
- Aortic stenosis:
- Auscultate for prominent fourth sound and possible splitting of second heart sound (LV dysfunction). First heart sound is normal.
- Auscultate for midsystolic murmur at the base of the heart
- Note harsh and rasping quality at base
- Higher pitch at apex
- Aortic insufficiency:
- Auscultate for soft first heart sound
- Place the stethoscope at the 3rd and 4th intercostal space
- Tricuspid stenosis:
- Auscultate for a blowing diastolic murmur at the lower left sternal border (increased with inspiration)
- Tricuspid insufficiency:
- Auscultate the third heart sound
- Murmur usually listened at 4th intercostal space
- Murmur usually high-pitched
Nursing Diagnosis
- Decreased cardiac output related to altered preload, afterload, or contractility.
- Activity intolerance related to reduced oxygen supply.
- Ineffective coping related to acute and chronic illness.
Nursing Interventions
- Maintaining adequate cardiac output:
- Assess frequently for change in existing murmur or new murmur
- Assess for right- and left-sided heart failure
- Monitor and treat the dysrythmia as ordered
- Prepare the patient for surgical interventions
- Improve activity intolerance:
- Maintain the bed rest while symptoms of heart failure are present.
- Allow patient to rest between interventions
- Allow chair-sitting activity
- Assist or perform the hygiene needs for patient to reserve strength for ambulation
- Strength-coping abilities:
- Instruct the patient about specific valvular dysfunction, possible etiology and therapies
- Include family members in any discussion
- Discuss with patient the surgical intervention as the treatment modality
- Refer the patient to appropriate counseling services (vocational, social work and cardiac rehabilitation)
Health Education
- Review activity restriction and schedule with the patient's family
- Instruct the patient to report signs of impending or worsening heart failure—dyspnea, cough, increased fatigue, and ankle swelling
- Review sodium and fluid restrictions
- Inform adverse effects of drugs.
- Keep regular appointments with the physician
- Increase walking activity and other activities gradually.
ARTERIOSCLEROTIC VASCULAR DISEASE
Atherosclerosis (or arteriosclerotic vascular disease) is a condition where the arteries become narrowed and hardened due to an excessive buildup of plaque around the artery wall. The disease disrupts the flow of blood around the body, posing serious cardiovascular complications.
Arteriosclerosis is characterized by irregularly distributed lipid deposits in the intima of large and medium-sized arteries, causing narrowing of arterial lumens and proceeding eventually to fibrosis and calcification. Lesions are usually focal and progress slowly and intermittently. Limitation of blood flow accounts for most clinical manifestations, which vary with the distribution and severity of lesions.
Risk Factors for Atherosclerosis
Any factor associated with a doubling in the incidence of ischemic heart disease has been defined as a ‘risk factor’. A major advance in the clinical assessment and treatment of atherosclerosis is a thorough screening for risk factors, followed by aggressive treatment to eliminate the risk factor. Risk factors can be categorized as genetic and environmental.
- Hypertension: An increase in blood pressure is consistently associated with an increased risk of myocardial infarction. In fact, men with systolic blood pressure over 160 mm Hg have almost three times the incidence of myocardial infarction as compared to those with blood pressure under 120 mm Hg. Treatment of hypertension, which is usually clinically silent, especially in the early stages of hypertension, has resulted in a significant reduction in the incidence of myocardial infarction and stroke.
- Serum cholesterol level: Numerous epidemiology and clinical studies have shown that the levels of serum cholesterol have been directly correlated with the incidence of ischemic heart disease. Indeed, of all the known risk factors, serum cholesterol seems to be the most important determinant of the geographical differences in the incidence of atherosclerotic coronary artery disease. In the absence of genetic disorders of lipid metabolism, such as familial hypercholesterolemia, the amount of cholesterol in the blood is related to the dietary intake of saturated fat. A number of studies have demonstrated a reduction in the incidence of myocardial infarction following treatment with cholesterol-lowering drugs.
- Cigarette smoking: Atherosclerosis of the coronary arteries and the aorta is more severe and extensive among cigarette smokers than among nonsmokers, and the effect is dose-related. Second-hand smoking is a risk factor. As a result, the incidence of myocardial infarction, ischemic stroke, and abdominal aortic aneurysms is markedly increased among smokers. Smoking is an environmental risk factor that is best addressed by eliminating smoking in preteens and teens and eliminating environment with second-hand smoking.
- Diabetes mellitus: Diabetics have a substantially greater risk of occlusive atherosclerotic vascular disease in many organs. The metabolic syndrome consisting of hypertension, glucose intolerance, truncal obesity and dyslipidemias has become an important target for early diagnosis and treatment.
- Increasing age and male gender: These factors are strong determinants of the risk for myocardial infarction.
- Physical inactivity and stressful life patterns: Both these factors have been correlated with an increased risk of ischemic heart disease.
- Homocysteine: Homocystinuria is a rare autosomal recessive disease caused by mutations in the gene encoding cystathionine synthase. The disorder results in premature and severe atherosclerosis. Mild elevations of plasma homocysteine are common and represent an independent risk factor for atherosclerosis of the coronary arteries and other large vessels. Homocysteine is toxic to endothelial cells and inhibits several anticoagulant mechanisms in endothelial cells. It inhibits thrombomodulin on the endothelial cell surface, the antithrombin III-binding activity of heparan sulfate proteoglycan, the binding of tissue plasminogen activator, and the ecto-ADPase activity on the endothelial cell surface, which promotes the aggregation of platelets. A low dietary intake of folic acid may aggravate an underlying genetic predisposition to hyperhomocysteinemia, 71but it has not been established that treatment with folic acid actually protects against atherosclerotic vascular disease.
- C-Reactive Protein and Inflammation Biomarkers: Elevated concentrations of C-reactive protein (CRP), an acute phase reactant produced mainly by hepatocytes, is a marker for systemic inflammation, and has been linked to an increased risk of myocardial infarction and ischemic stroke.
Pathophysiology
Natural history of atherosclerosis: Stable angina → unstable angina → MI → complications → death
Pathology of Atherosclerosis
- Fatty streak (yellow streak of lipid-filled macrophage foam cells). Lipid gets deposited first, then macrophages infiltrate and ingest it.
- Fibrous plaque (whitish yellow lump occluding lumen of coronary arteries, aorta, and carotids) leads to stable angina.
- Thrombus (plaque rupture, platelet aggregation and thrombus) leads to unstable angina or MI.
Pathophysiology of Atherosclerosis
- The intimal endothelium becomes dysfunctional, losing its ability to produce NO, and starting to express selectins for leukocyte recruitment.
- Endothelial cells normally provide a permeability barrier, reduce clotting, and regulate vascular tone.
- NO is a vasoprotective gas released by endothelium. NO is vasodilatory, anti-thrombotic, and anti-inflammatory.
- NO activates guanylate cyclase to generate cGMP, which causes smooth muscle relaxation/dilation.
- NO blocks vascular inflammation by inhibiting endothelial release of inflammatory granules. It also blocks platelet aggregation.
- Endothelial cells lose ability to produce NO due to inflammation, toxins, atherosclerosis, or oxidized LDL.
- Endothelial dysfunction leads to monocyte recruitment and atherosclerosis.
- ACh stimulates NO release and dilation.
- Initial inflammation → endothelial dysfunction → monocyte recruitment/differentiation to intima → growth factors stimulate smooth muscle proliferation → platelet activation/aggregation → atheroma formation.
Signs and Symptoms
Carotid arteries: These arteries provide blood to the brain. When the blood supply is limited, patients can suffer stroke and may experience:
- Weakness
- Difficulty breathing
- Headache
- Facial numbness
- Paralysis
Coronary arteries: These arteries provide blood to the heart. When the blood supply to the heart is limited, it can cause angina and heart attack. Symptoms include:
- Vomiting
- Extreme anxiety
- Chest pain
- Coughing
- Feeling faint
Renal arteries: These supply blood to the kidneys. If the blood supply becomes limited, there is a serious risk of developing chronic kidney disease, and the patient may experience:
- Loss of appetite
- Swelling of the hands and feet
- Difficulty concentrating
Peripheral arterial disease: The arteries to the limbs, usually the legs, are blocked. The most common symptom is leg pain, either in one or both the legs, usually in the calves, thighs or hips. The pain may be described as one of heaviness, cramp, or dullness in the leg muscles. Other symptoms may include:
- Hair loss on legs or feet
- Male impotence (erectile dysfunction)
- Numbness in the legs
- The color of the skin on the legs changes
- Weakness in the legs
Diagnostic Evaluation
- Those who are at risk of developing atherosclerosis should be tested, as the symptoms do not show up until cardiovascular disease develops. A diagnosis will be based on the medical history of a patient, test results and a physical examination.
- Blood tests: These measure how much sugar, fat and protein there is in your blood. If there are high levels of fat and sugar, it can be an indicator that you are at risk of developing the condition.
Physical Examination
- The doctor will listen to the arteries using a stethoscope to see if there is an unusual ‘whooshing’ sound reflecting turbulence of flow, called a bruit. If a bruit is heard, then it can mean there is a plaque obstructing blood flow.
- There may also be a very weak pulse below the area of the artery that has narrowed. Sometimes there is no detectable pulse.
- An affected limb may have abnormally low blood pressure
- There may be signs of an aneurysm (pulsating bulge) behind the patient's knee or in their abdomen
- Where blood flow is restricted, wounds may not heal properly
- Ultrasound: An ultrasound scanner is able to create a picture of the inside of your body using sound waves. It can check your blood pressure at distinct parts of the body; changes in pressure indicate where arteries may have obstruction of blood flow.
- Computed tomography (CT) scan: A CT scan uses X-ray images to create detailed pictures of the inside parts of the body. It can be used to find arteries that are hardened and narrowed.
Complications of Atherosclerosis
- Complicated atherosclerotic plaques: A complicated plaque is characterized by erosion, ulceration or fissuring of the surface of the plaque; plaque hemorrhage; mural thrombosis; calcification; and/or aneurysm formation.
- Aneurysm formation: A complicated plaque extends into the media of an elastic artery and so weakens the wall to result in the formation of an aneurysm, typically in the abdominal aorta. At a certain size, these aneurysms may suddenly rupture and cause a vascular catastrophe.
- Calcification occurs in areas of necrosis and elsewhere in the plaque. Calcification in the artery is thought to depend on focal mineral deposition and resorption, which is regulated by osteoblast-like and osteoclast-like cells in the vessel wall.
- Mural thrombosis results from abnormal laminar and/or turbulent blood flow around the plaque that protrudes into the lumen. The disturbance in flow causes damage to the endothelial lining, which may become dysfunctional or locally denuded and is no longer an effective thrombus-resistant surface. Thrombi often form at sites of erosion and 74fissuring on the surface of the fibrous cap of the plaque. Mural thrombi may embolize to more distal sites. A thrombus formed over an atherosclerotic plaque may detach, become an embolism and lodge in a distal vessel. Ulceration of an atherosclerotic plaque may also dislodge atheromatous debris and produce so-called cholesterol crystal emboli, which appear as needle-shaped spaces in affected tissues, most commonly in the kidney.
- Vulnerable atheroma has structural and functional alterations that predispose the fibroinflammatory lipid plaque to plaque destabilization.
- Atheroma destabilization, resulting often in acute coronary syndromes, may occur at any time when the dynamic balance of opposing biological and physical processes is disrupted, leading to occlusive thrombosis, fibrous cap rupture, and occlusive thrombosis, or intraplaque hemorrhage. Plaque rupture has been associated with:
- Areas of inflammation
- Large lipid core size
- Thin fibrous cap
- Reduced number of smooth muscle cells owing to apoptosis, and
- Imbalance of proteolytic enzymes and their inhibitors in the fibrous cap
- Calcification in the plaque
- Intraplaque hemorrhage leading to inside-out rupture of the fibrous cap.
- Acute coronary syndromes: Coronary artery disease is the leading cause of mortality in the western world. Acute coronary syndromes (ACS) are characterized clinically into three groups, patients with unstable angina, those with non-ST elevation myocardial infarction and those with ST elevation myocardial infarction.
Treatment
- Those who are at risk of developing atherosclerosis will likely be told by their doctors to change their lifestyle and maintain a healthy weight. In some cases, treatment may include medication or surgery.
- Lifestyle changes: The changes will focus on weight management, physical activity and a healthy diet. Your doctor may recommend eating foods high in soluble fiber and limiting your intake of saturated fats, sodium and alcohol.
- Medication: The doctor may prescribe medications to prevent the buildup of plaque or to help prevent blood clots (anteplatelets). Other medications such as statins may be prescribed to lower cholesterol, and angiotensin-converting enzyme (ACE) inhibitors to lower blood pressure.
- Surgery: Severe cases of atherosclerosis may be treated by surgical procedures, such as angioplasty or coronary artery bypass grafting (CABG) and PTCA.
- Percutaneous transluminal coronary angioplasty: It is a technique in which a balloon-tipped catheter is usually inserted into the femoral artery (although brachial or radial artery can be used) and threaded under guidance into a blocked coronary artery. The balloon is inflated several times to reshape the lumen by stretching the arterial wall.
- Directional coronary atherectomy: It reduces the stenosis of coronary artery by excising and removing atheromatus plaque. The DCA cutter consists of a catheter that contain 75a rigid cylinder housing with a central rotating blade. The blade shaves the plaque and deposits it in the nose cone housing for later histopathologic study.
- Intracoronary stents: Nowadays these are used instead of PTCA to eliminate the risk of acute closure and to improve long-term patency. Several different designs are available, but most are balloon-expandable or self-expandable tubes that, when placed in coronary artery, act as mechanical scaffold to reopen the blocked artery.
- Laser ablation: In this the lasers are used with balloon angioplasty to vaporize the plaque. After the initial balloon angioplasty, a brief burst of laser radiation is administered and the additional remaining plaque is removed.
- Transmyocardial revascularization: A new type of laser catheter is used called transmyocardial revascularization (TMR), which may be able to help clients who are not candidates for surgery or angioplasty because of ill health or degree of disease. The higher power laser is guided into left ventricle between heartbeat when the ventricle is filling with blood. The laser creates from 15 to 40 1-mm channels through the myocardium.
Prevention
The best way to prevent atherosclerosis is to eliminate any risk factors you might have. The best way to do this is by living a healthy lifestyle.
Diet: Try to avoid saturated fats. They increase your levels of bad cholesterol. The following foods are high in unsaturated fats and can help keep bad cholesterol levels down:
- Olive oil
- Avocados
- Walnuts
- Oily fish
- Nuts
- Seeds
- Exercise: Exercise will improve your fitness level and lower your blood pressure. If you are overweight, then exercise can help you lose weight through activities, such as walking, swimming, and cycling.
- Smoking: This is one of the major risk factors for atherosclerosis. It also raises your blood pressure. If you are a smoker, you should quit as soon as possible.
- Flu vaccine: Researchers from the School of Public Health and Community Medicine in Australia say that the flu vaccination may reduce the risk of heart attack by 50% in middle-aged individuals with narrowed arteries.
Nursing Management
Acute pain related to an impaired ability of blood vessels to supply oxygen to the tissues.
Intervention and Rational
- Monitor characteristics of pain through verbal and hemodynamic responses (crying, pain, grimacing, cannot rest, respiratory rhythm, blood pressure and changes in heat rate).
- Assess the description of pain experienced by patients which include: place, intensity, duration, quality, and distribution.
- Rationale: Pain is a subjective feeling that is experienced and is described by the patient and should be compared with other symptoms to obtain accurate data.
- Provide a comfortable environment, reduce the activity, limit visitors.
- Rationale: Helps reduce external stimuli that can add to the tranquility so patients can rest in peace and the power of the heart is not too hard.
- Teach relaxation techniques with a sigh.
- Rationale: Helps relieve pain experienced by patients psychologically, which can distract the patient who is not focused on the pain experienced.
- Observation of vital signs before and after drug administration.
- Rationale: Knowing the patient's progress after being given the drug.
- Knowledge deficit, ineffective management of therapeutic regimen, or altered health maintenance.
Inform the client that certain modifiable factors, such as elevated serum lipids, a sedentary lifestyle, cigarette smoking, and hypertension have been shown to increase the risk of atherosclerosis.
- Assist client to identify changes in lifestyle that could reduce the risk for atherosclerosis (e.g. dietary modifications, smoking cessation, physical exercise on a regular basis).
- Provide instructions on ways the client can reduce intake of saturated fat and cholesterol:
- Reduce intake of meat fat (e.g. trim visible fat off meat; replace fatty meats such as fatty cuts of steak, hamburger, and processed meats with leaner products)
- Reduce intake of milk fat (avoid dairy products containing more than 1% fat)
- Reduce intake of trans-fatty acids (e.g. avoid stick margarine and shortening and foods such as commercially baked goods that are prepared with these products)
- Use vegetable oil rather than coconut or palm oil in cooking and food preparation
- Use cooking methods, such as steaming, baking, broiling, poaching, microwaving, and grilling rather than frying
- Restrict intake of eggs (recommendations about the number of whole eggs allowed per week vary depending on the client's lipid levels).
Instruct client to take lipid-lowering agents (e.g. HMG-CoA reductase inhibitors [statins], gemfibrozil, niacin) as prescribed.
ARRHYTHMIA
The term ‘arrhythmia’ refers to any change from the normal sequence of electrical impulses. The electrical impulses may happen too fast, too slowly, or erratically – causing the heart to beat too fast, too slowly, or erratically. When the heart does not beat properly, it can not pump blood effectively. When the heart does not pump blood effectively, the lungs, brain and all other organs can not work properly and may shut down or be damaged.
Signs and Symptoms: Signs and symptoms depend on the type of heart block you have. First-degree heart block rarely causes symptoms.
Symptoms of second- and third-degree heart block include:
- Fainting
- Slow heartbeat
- Dizziness or light-headedness
- Irregular heartbeat
- Fatigue (tiredness)
- Arrhythmias
- Shortness of breath
- Blackouts (Stokes-Adams syndrome)
- Chest pain
Symptoms of severe cases include:
- Breathlessness
- Breathlessness with exertion
- Breathlessness caused by fever
- Dizziness
- Weakness
- Fainting
- Fatigue
Classification
Arrhythmia may be classified by rate (normal sinus rhythm, tachycardia, bradycardia) or mechanism (automaticity, re-entry, junctional, fibrillation). It is also appropriate to classify by site of origin:
Sinus Node
- Sinus bradycardia
- Sinus tachycardia
- Sinus arrhythmias
Atrial
- Premature atrial contractions
- Atrial flutter
- Atrial fibrillation
Junctional Arrhythmias
- Supraventricular tachycardia (SVT)
- AV nodal re-entrant tachycardia is the most common cause of paroxysmal supra-ventricular tachycardia (PSVT)
- Junctional rhythm
- Junctional tachycardia
- Premature junctional contraction
Ventricular
- Premature ventricular contractions (PVC)
- Premature ventricular fibrillation
- Three premature ventricular grouped together is termed as ‘run of PVCs’. Runs lasting longer than three beats are generally referred to as ventricular tachycardia
- Accelerated idioventricular rhythm
- Monomorphic ventricular tachycardia
- Polymorphic ventricular tachycardia
HEART BLOCK
- First-degree heart block, which manifests as PR prolongation
- Second-degree heart block:
- Type 1 Second-degree heart block, also known as Mobitz I or Wenckebach
- Type 2 Second-degree heart block, also known as Mobitz II
- Third-degree heart block, also known as complete heart block.
- SADS (sudden arrhythmic death syndrome)
- Atrial fibrillation: In atrial fibrillation, the electrical activity of the heart is uncoordinated, with electricity travelling about the upper chambers in a chaotic fashion, causing the upper chambers to quiver (like a ‘bag of worms’) and contract inefficiently or not at all. Atrial fibrillation is common particularly in the elderly and those with heart disease. It 79is also common in patients with heart valve disease who may require surgery to repair or replace the mitral valve. There are a variety of treatment options for atrial fibrillation, including drugs, an ablation—a nonsurgical technique which eliminates the abnormal heart tissue with a catheter, or surgery in some cases.
- Atrial flutter: Atrial flutter causes a rapid but coordinated electrical stimulation of the upper chamber of the heart, often leading to a rapid pulse. The atria are stimulated so quickly that they cannot contract or squeeze. This arrhythmia is due to a loop of electricity in the upper chambers of the heart. It is often curable with ablation.
- Supraventricular tachycardia (PSVT): This is a fast heart rhythm from the top part of the heart. In this condition, repeated periods of very fast heartbeats begin and end suddenly. These arrhythmias are usually due to extra connections between the upper and lower chambers of the heart. They are often difficult to control with medication and are often curable with an ablation.
- Wolff-Parkinson-White syndrome: This is a special type of PSVT. This syndrome involves episodes of a rapid heart rate (tachycardia) caused by abnormal electrical connection in the heart. In people with Wolff-Parkinson-White syndrome, there is an extra (accessory) 80connection between the top and bottom chambers of the heart. Wolff-Parkinson-White syndrome occurs in approximately 4 out of 100,000 people, and is one of the most common causes of fast heart rate disorders (tachyarrhythmia) in infants and children.
- Premature supraventricular contraction or premature atrial contraction (PAC): Premature beats or extra beats frequently cause irregular heart rhythms. Those that start in the upper chambers are called premature atrial contractions (PACs). Usually no cause can be found and no special treatment is needed.
- Sick sinus syndrome: The sinus node (heart's pacemaker) does not fire its signals properly, so that the heart rate slows down. Sometimes the rate changes back and forth between a slow (bradycardia) and fast (tachycardia) rate. This most often occurs in the elderly as a result of degenerative changes to the conduction pathways of the heart.
- Sinus arrhythmia: Cyclic changes in the heart rate during breathing. Common in children and often found in normal, healthy adults. A pacemaker may be required for treatment.
- Sinus tachycardia: The sinus node sends out electrical signals faster than usual, speeding up the heart rate. This is a normal response to exercise.
- Multifocal atrial tachycardia: In multifocal atrial tachycardia (MAT), multiple locations within the atria ‘fire’ and initiate an electrical impulse. Most of these impulses are conducted to the ventricles, leading to a rapid heart rate, anywhere from 100 to 250 beats per minute. MAT is most common in people 50 years old and over and it is often seen in patients with lung disease.
- Premature ventricular contraction (PVC): An electrical signal from the ventricles causes an early heartbeat that generally goes unnoticed. The heart then seems to pause until the next beat of the ventricle occurs in a regular fashion. These are commonly detected in normal, healthy adults.
- Ventricular fibrillation is where electrical signals in the ventricles fire in a very fast and uncontrolled manner. This causes the lower chambers to quiver, and not pump blood. 81If the person does not receive immediate medical attention and a normal rhythm is not restored quickly, the patient will suffer brain and heart damage and die. Patients who survive this should have a defibrillator (ICD) implanted.
- Ventricular tachycardia is a rapid, regular heartbeat arising in the ventricles, and the bottom chamber of the heart. When it is occurs, it is usually fatal. About 400,000 people a year die from it. The treatment of choice for this invariably includes an implantable defibrillator and/or medication and/or interventions like ablation to try to minimize or limit the number of shocks.
- Paroxysmal atrial tachycardia (PAT): PAT is a very rapid supraventricular rhythm that comes from the atria – usually it runs at a very rapid rate, about 250-300 bpm.
HEART BLOCKS
Introduction
An atrioventricular block (AV block) involves the impairment of the conduction between the atria and ventricles of the heart. Under normal conditions, SA node in the atria sets the pace for the heart, and these impulses travel down to the ventricles. In an AV block, this message does not reach the ventricles or is impaired along the way. The ventricles of the heart have their own pacing mechanisms, which can maintain a lowered heart rate in the absence of SA stimulation.
Conduction block: Any obstruction of the normal pathways of electrical conduction is called as conduction block
Definition
A heart block is a disease of electrical system of the heart. This is opposed to coronary artery disease, which is disease of the blood vessels of the heart.
Etiology
The causes of pathological AV block are varied. The blocks may be complete or may only impair the signaling between the SA and AV nodes. Certain AV blocks can also be found as normal variants, such as in athletes or children, and are benign.
- Ischemia
- Infarction
- Fibrosis
- Drugs
- Strong vagal stimulation may also produce AV block.
Types
- SA nodal block:
- SA node arrest
- SA node asystole
- SA node exit block
- AV nodal block: There are three types:
- First-degree AV block: PR interval greater than 0.20 sec.
- Second-degree AV block:
- Type 1: Progressive prolongation of PR interval with dropped beats (the PR interval gets longer and longer; finally one beat drops).
- Type 2: PR interval remains unchanged prior to the P wave which suddenly fails to conduct to the ventricles.
- Third-degree AV blocks: No association between P waves and QRS complexes.
- Infra-Hisian bundle block: Infra-Hisian block describes block of the distal conduction system. Types of infra-Hisian block include:
Type 2 second-degree heart block (Mobitz II):
- Left bundle branch block:
- Left anterior fascicular block
- Left posterior fascicular block
- Right bundle branch block
SA NODAL BLOCK
This is sinus exit block. The sinus node fires normally, but wave of depolarization is immediately blocked and is not transmitted into the arterial tissue. ECG shows just pause in normal cardiac cycle.
|
|
| |
|
|
|
The sinus node continues to fire but the wave of depolarization is blocked immediately as it exits the node. Again, ECG shows no electrical activity.
Atrioventricular Block
Definition
Conduction block between sinus node and up to and including AV node.
First-degree Atrioventricular Block
Introduction
- The electrical impulses are slowed as they pass through the conduction system, but they all successfully reach the ventricles.
- First-degree AV block occurs when all the impulses are conducted through AV node to ventricles at a rate slower than normal. It has a prevalence in the normal (young adult) population of 0.65–1.1% and the incidence is 0.13 per 1000 persons.
- PR prolongation is a disease of the electrical conduction system of the heart in which the PR interval is lengthened beyond 0.20 seconds.
Characteristics
- Ventricular or arterial rate: Depends upon underlying rhythm
- Ventricular or arterial rhythm: Depends upon underlying rhythm
- QRS shape and duration: Usually normal, but may be abnormal
- P-wave: In front of the QRS complex; shows sinus rhythm, regular rhythm
- PR interval: Greater than 0.20 seconds
- P : QRS ratio: 1:1
Interpretation: 1st-degree AV block
--PR interval:- > 0.20 s
- Rate: 60 bpm
- Regularity: regular
- P-wave: normal
- PR interval: > 0.20 sec. (0.36 sec.)
- QRS duration: Normal 0.08 sec.
- Causes: The most common cause of first-degree heart block in an AV nodal disease is prolonged conduction delay in the AV node or bundle of His.
- Enhanced vagal tone (for example, in athletes)
- Myocarditis
- Acute inferior wall myocardial infarction
- Electrolyte disturbances
- Medication
- Drugs: Calcium channel blockers, beta-blockers, cardiac glycosides, and anything that increases cholinergic activity, such as cholinesterase inhibitors.
- Hypothyroidism
- Digitalis toxicity
Diagnostic Test
ECG: PR interval measurement
- The normal PR interval is from 120 ms to 200 ms in length. This is measured from the initial deflection of the P-wave to the beginning of the QRS complex.
- In first-degree heart block, the diseased AV node conducts the electrical activity more slowly. This is seen as a PR interval greater than 200 ms in length on the surface ECG. It is usually an incidental finding on a routine ECG.
- Electrolyte and drug screens: First-degree heart block does not require any particular investigations except for electrolyte and drug screens, especially if an overdose is suspected.
Treatment
- Cautious use of digitalis glycosides.
- Atropine is effective if PR interval exceeds 0.26 sec. or bradycardia develops.
- Identifying and correcting electrolyte imbalances and withholding any offending medications.
- This condition does not require admission unless there is an associated myocardial infarction.
- It may require outpatient follow-up
- Regular monitoring of the ECG.
Prognosis
Isolated first-degree heart block has no direct clinical consequences. There are no symptoms or signs associated with it. It was originally thought of as having a benign prognosis. The presence of a prolonged PR interval or first-degree AV block doubled the risk of developing atrial fibrillation (irregular heartbeat), tripled the risk of requiring an artificial pacemaker, and was associated with a small increase in mortality. This risk was proportional to the degree of PR prolongation.
SECOND-DEGREE ATRIOVENTRICULAR BLOCK
Introduction
Second-degree AV block is a disease of the electrical conduction system of the heart. It refers to a conduction block between the atria and ventricles.
The presence of second-degree AV block is diagnosed when one or more (but not all) of the atrial impulses fail to conduct to the ventricles due to impaired conduction.
The electrical impulses are delayed further and further with each heartbeat until a beat fails to reach to the ventricles entirely. It sometimes causes dizziness and/or other symptoms. People with normal conduction systems may sometimes have type 1 second-degree heart block when they sleep.
Types
There are two nondistinct types of second-degree AV called Type 1 and Type 2. In both types, a P-wave is blocked from initiating a QRS complex; but, in Type 1, there are increasing delays in each cycle before the omission, whereas in Type 2, there is no such pattern.
- Type 1 second-degree heart block is considered a more benign entity than type 2 second-degree heart block. Both types are named after Woldemar Mobitz. Type I is also named for Karel Frederik Wenckebach and type II is also named for John Hay.
Second-degree AV block, Type 1/Mobitz I (Wenckebach)
- Type 1 second-degree AV block, also known as Mobitz I or Wenckebach periodicity, is always a disease of the AV node. It occurs when there is repeating pattern in which all but one of a series of atrial impulses conducted through AV node into the ventricles (e.g. every 4 or 5 impulses are conducted). Each impulse takes a longer time for conduction than one before, until one impulse is fully blocked. Because AV node was not depolarized by the blocked atrial impulse, the AV node has a time to fully repolarize, so that next atrial impulse can be conducted within the shortest period of time.
Characteristics
- Ventricular or arterial rate: Depends upon underlying rhythm
- Ventricular or arterial rhythm: PP interval is regular if the patient has an underlying normal sinus rhythm; the RR interval characteristically reflects a pattern of change. Starting from the RR interval which is longest, the RR interval is gradually shorter until there is another long RR interval.
- QRS shape and duration: Usually normal, but may be abnormal
- P-wave: In front of the QRS complex, shape depends upon underlying rhythm
- PR interval: PR interval becomes longer with each succeeding ECG complex until there is a P-wave not followed by a QRS. The changes in the PR interval are repeated between each dropped ‘QRS’, creating a pattern in the regular PR interval measurements.
- P : QRS ratio: 3:2, 4:3, 5:4 and so on...
- Interpretation: Second degree AV block Type I
- PR interval progressively lengthens, then the impulse is completely blocked (P-wave not followed by QRS).
- Rate: 50 bpm
- Regularity: Irregular (atrial rhythm regular and ventricular rhythm irregular)
- P wave: normal
- PR interval: lengthens
- QRS duration: 0.08 sec.
Causes
- Inferior wall MI
- Cardiac surgery
- Acute rheumatic fever
- Vagal stimulation
- Digitalis toxicity; propranolol, quinidine, procainamide.
Treatment
- This is almost always a benign condition for which no specific treatment is needed.
- Atropine is effective for treating second degree type 1
- Temporary pacemaker implantation for symptomatic bradycardia
- Discontinuation of digitalis glycosides.
- In symptomatic cases, intravenous atropine or proterenol may transiently improve conduction.
Second-degree AV block, Type II/Mobitz II
Introduction
Type 2 second-degree AV block, also known as ‘Mobitz II’, is almost always a disease of the distal conduction system (His-Purkinje System).
Type 2 second-degree AV block occurs when only some of the atrial impulses are conducted through the AV node into the ventricles. Mobitz II heart block is characterized on a surface ECG by intermittently nonconducted P-waves not preceded by PR prolongation and not followed by PR shortening. The medical significance of this type of AV block is that it may progress rapidly to complete heart block, in which no escape rhythm may emerge. In this case, the person may experience a Stokes-Adams attack, cardiac arrest, or sudden cardiac death.
With this condition, some of the electrical impulses are unable to reach the ventricles. This condition is less common than Type I, and is more serious.
Characteristics
- Ventricular or arterial rate: Depends upon underlying rhythm
- Ventricular or arterial rhythm: PP interval is regular if the patient has an underlying normal sinus rhythm; the RR interval is regular but may be irregular, depending on the ratio P : QRS.
- QRS shape and duration: Usually abnormal, but may be normal.
- P-wave: In front of the QRS complex; shape depends upon underlying rhythm.
- PR interval: PR interval is constant for those P-waves just before QRS complexes.
- P : QRS ratio: 2:1, 3:1, 4:1, 5:1 and so on...
Occasional P-waves are completely blocked (P-wave not followed by QRS).
- Rate: 40 bpm
- Regularity: Regular (both atrial or ventricular)
- P-wave: Normal
- PR interval: 0.14 sec.
- PP interval: Constant
- QRS duration: Periodically absent
Etiology
- Conduction is all or nothing (no prolongation of PR interval)
- Typically block occurs in the bundle of His
- Severe coronary artery disease
- Anterior wall MI
- Acute myocarditis
- Digitalis toxicity
Treatment
- The definitive treatment for this form of AV block is an implanted temporary and permanent pacemaker
- Isoproterenol for symptomatic bradycardia
- Discontinuation of digitalis glycosides
Difference between Mobitz type-I and Mobitz type-II
Mobitz type-I | Mobitz type-II |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Third-degree Atrioventricular Block (Complete Heart Block)
Introduction
- Third-degree AV block, also known as complete heart block, is a medical condition in which the impulse generated in the SA node in the atrium does not propagate to the ventricles.
- Lead I and II demonstrating complete AV block. Note that the P-waves are not related to the QRS complexes (PP interval and QRS interval, both are constant), demonstrating 88that the atria are electrically disconnected from the ventricles. The QRS complexes represent an escape rhythm arising from the ventricle.
- The QRS complexes with a regular R-to-R interval represent the second rhythm. The PR interval will be variable, as the hallmark of complete heart block is no apparent relationship between P waves and QRS complexes.
- One of the pathognomonic characteristics of this block is the absolute absence of the opportunity for atrial impulses to enter and capture the ventricles (unlike AV dissociation with functional block) due to the organic nature of block (e.g. fibrosis, calcification, or infiltration of the node). In the presence of complete heart block, fusion or capture beats will never be seen. Patients with third-degree AV block typically experience bradycardia (an abnormally low measured heart rate), hypotension, and, at times, hemodynamic instability. In some cases, exercising may be difficult, as the heart cannot react quickly enough to sudden changes in demand or sustain the higher heart rates required for prolonged activity.
Characteristics
- Ventricular or arterial rate: Depends upon escape rhythm and also upon underlying atrial rhythm.
- Ventricular or arterial rhythm: PP interval is regular, RR interval is regular. PP interval is not equal to RR interval.
- QRS shape and duration: Depends upon escape rhythm; with junctional rhythm, QRS shape and duration are usually normal; with idioventricular rhythm, QRS shape and duration are usually abnormal.
- P-wave: Depends upon underlying rhythm
- PR interval: Very irregular
- P : QRS ratio: More P wave than QRS complexes
- Interpretation: 3rd-degree AV block
- The P-waves are completely blocked in the AV junction; QRS complexes originate independently from below the junction.
- Rate: 40 bpm
- Regularity: Regular (atrial regular and ventricular rate slow and regular)
- P-wave: No relation to QRS
- PR interval: None
- QRS duration: Wide (> 0.12 sec.)
Etiology
- There is complete block of conduction in the AV junction. So, the atria and ventricles form impulses independently of each other. Without impulses from the atria, the ventricles own intrinsic pacemaker kicks in at around 30–45 beats/minute.
- Many conditions can cause third-degree heart block, but the most common cause is coronary ischemia. Progressive degeneration of the electrical conduction system of the heart can lead to third-degree heart block. This may be preceded by first-degree AV block, second-degree AV block, bundle branch block, or bifascicular block. In addition, acute myocardial infarction may present with third-degree AV block.
- An inferior wall myocardial infarction: It may cause damage to the AV node, causing third-degree heart block. In this case, the damage is usually transitory. Studies have shown that third-degree heart block in the setting of an inferior wall myocardial infarction typically resolves within 2 weeks. The escape rhythm typically originates in the AV junction, producing a narrow complex escape rhythm.
- An anterior wall myocardial infarction: It may damage the distal conduction system of the heart, causing third-degree heart block. This is typically extensive, permanent damage to the conduction system, necessitating a permanent pacemaker to be placed. The escape rhythm typically originates in the ventricles, producing a wide complex escape rhythm.
- Third-degree heart block may also be congenital and has been linked to the presence of lupus in the mother. It is thought that maternal antibodies may cross the placenta and attack the heart tissue during gestation. The cause of congenital third-degree heart block in many patients is unknown.
PROGNOSIS
The prognosis of patients with complete heart block is generally poor without artificial pacemaker therapy.
Treatment
- Pacemaker: Third-degree AV block can be treated by use of a dual-chamber artificial pacemaker. This type of device typically listens for a pulse from the SA node and sends a pulse to the AV node at an appropriate interval, essentially completing the connection between the two nodes. Pacemakers in this role are usually programmed to enforce a minimum heart rate and to record instances of atrial flutter and atrial fibrillation, two common secondary conditions that can accompany third-degree AV block.
- Treatment may also include medicines to control blood pressure and atrial fibrillation, as well as lifestyle and dietary changes to reduce risk factors associated with heart attack and stroke.
- Treatment in emergency situations ultimately involves electrical pacing. However, the American Heart Association states that giving a trial of atropine while waiting for the pacer to be set up is acceptable.
- Atropine is effective for treating early heart blocks (1st-degree and 2nd-degree type 1) but generally thought to have no effect on 3rd-degree blocks.
Difference Between 1, 2, 3-degree Block
BUNDLE BRANCH BLOCK
Anatomy and Physiology
The heart's electrical activity begins in the sinoatrial node (the heart's natural pacemaker), which is situated on the upper right atrium. The impulse travels next through the left and right atria and summates at the atrioventricular node. From the AV node, the electrical impulse travels down the Bundle of His and divides into the right and left bundle branches. The right bundle branch contains one fascicle. The left bundle branch subdivides into two fascicles: the left anterior fascicle and the left posterior fascicle.
Other sources divide the left bundle branch into three fascicles:
- The left anterior
- The left posterior
- The left septal fascicle.
Normal Conduction System
Sinoatrial node (SA node)→AV node→Bundle of His→Bundle branches→Purkinje fibers
Introduction
Bundle branch block is a condition in which there is a delay or obstruction along the pathway that electrical impulses travel to make the heart beat. The delay or blockage may occur on the pathway that sends electrical impulses to the left or the right side of the heart. Bundle branch block sometimes makes it harder for heart to pump blood efficiently through circulatory system.
When a bundle branch or fascicle becomes injured (due to underlying heart disease, myocardial infarction, or cardiac surgery), it may cease to conduct electrical impulses appropriately. This results in altered pathways for ventricular depolarization. Since the electrical impulse can no longer use the preferred pathway across the bundle branch, it may move instead through the muscle fibers in a way that both slow down the electrical movement and change the directional propagation of the impulses.
Definition
When a bundle branch or fascicle becomes injured, it may cease to conduct electrical impulses appropriately, resulting in altered pathways for ventricular depolarization called bundle block.
Types
- Right bundle block
- Left bundle block:
- Left anterior fascicular block: In this case, only the anterior half of the left bundle branch (fascicle) is involved.
- Left posterior fascicular block: In this case, only the posterior half of the left bundle branch (fascicle) is involved.
NOTE: A mnemonic to remember the ECG change is—in left bundle branch block, there is a W in V1 and an M in V6 and with an RBBB, there is an M in lead V1 and a W in lead V6.
RIGHT BUNDLE BRANCH BLOCK
Characteristics of QRS Complex
For RBBB, the wide QRS complex assumes a unique, virtually diagnostic shape in those leads overlying the right ventricle (V1 and V2).
- The heart rhythm must originate above the ventricles (i.e. sinoatrial node, atria or atrioventricular node) to activate the conduction system at the correct point.
- The QRS duration must be more than 100 ms (incomplete block) or more than 120 ms (complete block)
- There should be a terminal R-wave in lead V1
- There should be a slurred S wave in leads V1 and V6.
- ST depression and T-wave inversion seen leads V1, V2
Causes
- Congenital abnormalities: Such as atrial septal defect, a hole in the wall separating the upper chambers of the heart.
- A heart attack (myocardial infarction)
- A viral or bacterial infection of the heart muscle (myocarditis)
- High blood pressure (hypertension)
- Scar tissue that develops after heart surgery
- A blood clot in the lungs (pulmonary embolism)
LEFT BUNDLE BRANCH BLOCK
Left bundle branch block (LBBB) is a cardiac conduction abnormality seen on the electrocardiogram (ECG). In this condition, activation of the left ventricle is delayed, which causes the left ventricle to contract later than the right ventricle.
Characteristics of QRS Complex
For LBBB, the wide QRS complex assumes a characteristic change in shape in those leads opposite the left ventricle (right ventricular leads—V1 and V2).
- The heart rhythm must be supraventricular in origin
- The QRS duration must be ≥ 120 ms
- There should be a QS or rS complex in lead V1
- There should be an RSR' wave in lead V6.
- ST depression and T-wave inversion seen in leads V1, V2
Causes
- Aortic stenosis
- Dilated cardiomyopathy
- Extensive coronary artery disease
- Primary disease of the cardiac electrical conduction system
- Long-standing hypertension leading to aortic root dilatation and subsequent aortic regurgitation
Classification
Left Anterior Fascicular Block
Definition: It is caused by only the anterior half of the left bundle branch being defective. It is manifest on the ECG by left axis deviation. It is much more common than left posterior fascicular block.
- In left anterior fascicular block, impulses are conducted to the left ventricle via the left posterior fascicle, which inserts into the inferoseptal wall of the left ventricle along its endocardial surface.
- On reaching the left ventricle, the initial electrical vector is, therefore, directed downwards and rightwards (as excitation spreads outwards from endocardium to epicardium), producing small R-waves in the inferior leads (II, III, aVF) and small Q-waves in the left-sided leads (I, aVL).
- The major wave of depolarization then spreads in an upward and leftward direction, producing large positive voltages (tall R-waves) in the left-sided leads and large negative voltages (deep S-waves) in the inferior leads.
- This process takes about 20 milliseconds longer than simultaneous conduction via both fascicles, resulting in a slight widening of the QRS.
- The impulse reaches the left-sided leads later than normal, resulting in an increased R-wave peak time (the time from onset of the QRS to the peak of the R-wave) in aVL.
Characteristics
- Left axis deviation (usually between –45 and –90 degrees)
- Small Q-waves with tall R-waves (= ‘QR complexes’) in leads I and aVL
- Small R-waves with deep S-waves (= ‘RS complexes’) in leads II, III, aVF
- QRS duration normal or slightly prolonged (80–110 ms)
- Prolonged R-wave peak time in aVL > 45 ms
Left Posterior Fascicular Block
It is a condition where the left posterior fascicle, i.e. the backside of the left cardiac bundle, does not conduct electrical impulses from AV node. The electricity then has to go through the other fascicle and thus is a frontal right axis deviation seen on ECG.
Characteristics
- Right axis deviation (> 120 degrees)
- Small Q-waves with Tall R-waves: In leads II, III, aVF
- Small R-waves with deep S-waves (= ‘RS complexes’) in leads I and aVL
- QRS: Slightly widened or slightly prolonged (100–120 ms)
- Prolonged R-wave peak time in aVF
- Increased QRS voltage in limb leads
- No evidence of right ventricular hypertrophy
Left anterior fascicular block | Left posterior fascicular block |
|---|---|
|
|
|
|
|
|
|
|
Clinical Manifestations
- Fainting (syncope)
- Presyncope
- Bradycardia
- Light-headedness
- Dizziness
DIAGNOSTIC TESTS
ECG
Depolarization of the Bundle Branches and Purkinje fibers are seen as the QRS complex on the ECG. Therefore, a conduction block of the bundle branches would be reflected as a change in the QRS complex. With bundle branch blocks, you will see two changes on the ECG.
- QRS morphology changes (varies depending on ECG lead, and if it is a right vs. left bundle branch block).
- A bundle branch block can be diagnosed when the duration of the QRS complex on the ECG exceeds 120 ms.
- A right bundle branch block typically causes prolongation of the last part of the QRS complex, and may shift the heart's electrical axis slightly to the right.
- The ECG will show a terminal R-wave in lead V1 and a slurred S-wave in lead I.
- Left bundle branch block widens the entire QRS, and in most cases, shifts the heart's electrical axis to the left.
- The ECG will show a QS or RS complex in lead V1 and a monophasic R-wave in lead I.
Treatment
- Patients with LBBB require complete cardiac evaluation, and those with LBBB and syncope or near-syncope may require a pacemaker.
- Some patients with LBBB, a markedly prolonged QRS (usually > 150 ms), and systolic heart failure may benefit from a biventricular pacemaker, which allows for better synchrony of heart contraction.
Combination of Bundle Branch Block and Hemiblock
It is also known as bi fascicular block refers to combination of either left anterior or left posterior fascicular or hemi block with right bundle branch block. Only one fascicle of left bundle branch supplying the bulk of both ventricle
Right bundle branch block | Left anterior hemiblock |
|---|---|
|
|
|
|
Right bundle branch block | Right anterior hemiblock |
|---|---|
|
|
|
|
Combination of AV block, bundle branch blocks and hemiblock:
- Is there any AV block?: Relationship between P- and QRS-wave complex
- Is there any bundle branch block?: Check pericardial leads for wide QRS complex and ST segment and T-wave changes
- Is there any hemi block?: Check for axis deviation.
SUDDEN ARRHYTHMIC DEATH SYNDROME (SADS)
Introduction
SADS, or sudden arrhythmic death syndrome, is a term (sudden unexpected death syndrome) used to describe sudden death due to cardiac arrest brought on by an arrhythmia in the absence of any structural heart disease on autopsy. The most common cause of sudden death in the US is coronary artery disease.
Etiology
- Viral myocarditis
- Long QT syndrome
- Brugada syndrome
- Catecholaminergic polymorphic ventricular tachycardia
- Hypertrophic cardiomyopathy
- Arrhythmogenic right ventricular dysplasia.
Signs and Symptoms
- Some arrhythmias do not cause symptoms, and are not associated with increased mortality. However, some asymptomatic arrhythmias are associated with adverse events.
- Palpitations: The most common symptom of arrhythmia is an abnormal awareness of heartbeat, called palpitations. These may be infrequent, frequent, or continuous.
Others include:
- A higher risk of blood clotting within the heart
- A higher risk of insufficient blood being transported to the heart because of weak heartbeat.
- Increased risks of embolization and stroke
- Heart failure
- Sudden cardiac death
- Lower blood pressure
- Lightheadedness or dizziness, or syncope (fainting).
DIFFERENTIAL DIAGNOSIS
Normal Electrical Activity
- Bradycardias: A slow rhythm (less than 60 beats/min) is bradycardia. This may be caused by a slowed signal from the sinus node (sinus bradycardia) or by blocking of the electrical impulse on its way from the atria to the ventricles (AV block or heart block). Heart block comes in varying degrees and severity. It may be caused by reversible poisoning of the AV node (with drugs that impair conduction) or by irreversible damage to the node. Bradycardias may also be present in the normally functioning heart of endurance athletes or other well-conditioned persons.
- Tachycardias: In adults and children over 15, resting heart rate faster than 100 beats/minute is tachycardia. Tachycardia may result in palpitation; tachycardia is not an 97arrhythmia. Increased heart rate is a normal response to physical exercise or emotional stress. This is mediated by the sympathetic nervous system on the sinus node and is called sinus tachycardia. Other things that increase sympathetic nervous system activity in the heart include ingested or injected substances, such as caffeine or amphetamines, and an overactive thyroid gland (hyperthyroidism).
- Abnormal impulses can begin by one of the three mechanisms: Automaticity, reentry or triggered activity:
- Automaticity: Any part of the heart that initiates an impulse without waiting for the sinoatrial node is called an ectopic focus and is, by definition, a pathological phenomenon. This may cause a single premature beat now and then, or, if the ectopic focus fires more often than the sinoatrial node, it can produce a sustained abnormal rhythm. Rhythms produced by an ectopic focus in the atria, or by the atrioventricular node, are the least dangerous dysrhythmias; but they can still produce a decrease in the heart's pumping efficiency, because the signal reaches the various parts of the heart muscle with different timing than usual and can be responsible for poorly coordinated contraction.
- Re-entry: Excitation in the myocardium is considered to be the main mechanism of life-threatening cardiac arrhythmias. In particular, the autowave reverberator is typical in thin walls of the atria, with the atrial flutter producing. Re-entry is also responsible for most paroxysmal supraventricular tachycardia, and dangerous ventricular tachycardia. These types of re-entry circuits are different from WPW syndromes in which the real pathways existed.
- Triggered beats: Triggered beats occur when problems at the level of the ion channels in individual heart cells result in abnormal propagation of electrical activity and can lead to sustained abnormal rhythm. They are relatively rare and can result from the action of antiarrhythmic drugs.
Diagnostic Evaluation
- Electrocardiogram (ECG or EKG): A picture of the electrical impulses travelling through the heart muscle. An ECG is recorded on graph paper, through the use of electrodes (small, sticky patches) that are attached to your skin on the chest, arms and legs.
- Ambulatory monitors, such as:
- Holter monitor: A small portable recorder that is attached to electrodes on your chest. It continuously records your heart's rhythm for 24 hours.
- Transtelephonic monitor: A small monitor is attached to electrode leads, usually on your finger or wrist. With the help of this device, your heart's rhythm is transmitted over the phone line to your doctor's office.
- Transtelephonic monitor with a memory loop: A small, portable recorder that is worn continuously for an extended period of time to record and save information about your heart's rhythm around the time you experience an arrhythmia. The recording is triggered by pushing a button (event button). The rhythm is recorded, saved and transmitted over the phone line.
- Stress test: A test used to record arrhythmias that start or are worsened with exercise. This test may also be helpful in determining if there is underlying heart disease or coronary artery disease associated with an arrhythmia.
- Cardiac catheterization: Using a local anesthetic, a catheter (small, hollow, flexible tube) is inserted into a blood vessel and guided to the heart with the help of an X-ray machine. A contrast dye is injected through the catheter so X-ray movies of your coronary arteries, heart chambers and valves may be taken. This test helps your doctor determine if the cause of an arrhythmia is coronary artery disease. This test also provides information about how well your heart muscle and valves are working.
- Electrophysiology study (EPS): A special heart catheterization that evaluates your heart's electrical system. Catheters are inserted into your heart to record the electrical activity. The EPS is used to find the cause of the abnormal rhythm and determine the best treatment for you. During the test, the arrhythmia can be safely reproduced and terminated.
- Tilt table test (also called a passive head-up tilt test or head upright tilt test): Records your blood pressure and heart rate on a minute-by-minute basis while the table is tilted in a head-up position at different levels. The test results may be used to evaluate heart rhythm, blood pressure and sometimes other measurements as you change position.
Management
Physical Maneuvers
A number of physical acts can increase parasympathetic nervous supply to the heart, resulting in blocking of electrical conduction through the AV node. This can slow down or stop a number of arrhythmias that originate above or at the AV node. Parasympathetic nervous supply to the heart is via the vagus nerve, and these maneuvers are collectively known as vagal maneuvers.
Antiarrhythmic Drugs
There are many classes of antiarrhythmic medications, with different mechanisms of action and many different individual drugs within these classes. Although the goal of drug therapy is to prevent arrhythmia, nearly every antiarrhythmic drug has the potential to act as a proarrhythmic, and so must be carefully selected and used under medical supervision.
Other Drugs
Arrhythmias promote blood-clotting within the heart, and increase risk of embolus and stroke. Anticoagulant medications such as warfarin and heparins, and anti-platelet drugs such as aspirin can reduce the risk of clotting.
Cardioversion
It is either achieved pharmacologically or via the application of a shock synchronized to the underlying heartbeat. It is used for treatment of supraventricular tachycardias. In elective cardioversion, the recipient is usually sedated or lightly anesthetized for the procedure.
Defibrillation
It differs in that the shock is not synchronized. It is needed for the chaotic rhythm of ventricular fibrillation and is also used for pulseless ventricular tachycardia. Often, more 99electricity is required for defibrillation than for cardioversion. In most defibrillation cases, the recipient has lost consciousness; so there is no need for sedation.
Electrical cautery
In specialized catheter laboratories, they use fine probes inserted through the blood vessels to map electrical activity from within the heart. This allows abnormal areas of conduction to be located very accurately, and subsequently destroyed with heat, cold, electrical or laser probes.
Nursing Management
Nursing Assessment
- General complaints: Palpitations, dizziness, light-headedness, chest pain, syncope
- Physical examination:
- Skin: Pallor, Diaphoresis
- Arterial pulse: Normal, Tachycardia, Bradycardia
- Rhythm: Normal, Irregular
- Hypotension
- Mental status: Confusion, Agitation, Anxiety
Drug History
- Names, dosages of current antidysrhythmic drugs
- Laboratory values: Electrolyte imbalance
- Use of artificial stimulants: Narcotics, amphetamine
Nursing diagnosis: Decreased cardiac output related to electrical and mechanical dysfunction
Goal: To monitor and maintain cardiac output
Interventions
- Assess the patient continuously for rate, rhythm and level of consciousness.
- Monitor vital signs frequently.
- Monitor and record ECG changes.
- Notify the physician for if any change can occur in normal parameters.
- Administer antidysrhythmic drugs as order or monitor serum blood level.
- Administer oxygenation therapy.
Nursing diagnosis: Decreased tissue perfusion related to decreased cardiac output
Goal: To maintain tissue perfusion.
Interventions
- Monitor pulse rate.
- Monitor central venous pressure.
- Provide sterile dressing on wound.
- Give IV fluid to the patient.
- Give oxygenation therapy.
- Epinephrine drugs are first line drug used for its alpha adrenergic effect to increase perfusion pressure, e.g. adrenaline
- Norepinephrine drugs are used for its alpha adrenergic effect to increase perfusion pressure.
- Vagal maneuver induces vagal stimulation of cardiac conduction system.
- Massaging the carotid sinus causes vagal stimulation.
- Sodium bicarbonate is used for those who are having hyperkalemia.
- Pacemaker should be implanted.
Nursing diagnosis: Anxiety related to death secondary to altered heart rate.
Goal: To reduce anxiety level.
Interventions
- Assess the anxiety level and level of understanding.
- Provide continuous explanation about the health status and management.
- Offer reassurance to the patient and their family members.
- Administer sedation to reduce anxiety level.
- Provide referral for continued supportive counseling to deal with fear and anxiety.
Nursing diagnosis: Chest pain related to electrical and mechanical dysfunction.
Goal: To reduce pain.
Interventions
- Assess for the presence of pain, the scale and intensity of pain.
- Teach the client about pain management and relaxation with distraction.
- Secure the chest tube to restrict movement and avoid irritation.
- Assess pain reduction measures.
Nursing diagnosis: Ineffective breathing pattern related to disease condition as evidenced by breathlessness.
Goal: To maintain breathing pattern.
Interventions
- Open the airway with head tilt, chin lift, jaw thrust
- Set the position to maximize ventilation
- Use tools airway
- Perform chest physiotherapy
- Teach breathing deeply and coughing effectively
- Perform suction
- Auscultation of breath sounds
- Give bronchodilators (collaboration)
- Oxygenation therapy
Nursing diagnosis: Activity intolerance related to conduction problem as evidenced by tachycardia.
Goal: To maintain activity status of the patient.
- Check the vital signs of the patient.
- Check for the activity level of the patient.
- Provide small activity.
- Involve in activities of daily living.
- Avoid staining activity.
Nursing diagnosis: Knowledge deficit about dysrhythmia and its treatment.
Goal: To provide knowledge about disease and its treatment.
Interventions
- Explain the dysrhythmias and its side effects.
- Describe the medications regimen and its rationale.
- Explain the need for therapeutic serum level of the medication.
- Describe the plan to eradicate or limit the factors that contribute to the dysrhythmias.
- State the actions to take in the event of an emergency.
Management
The goal of management is to:
- Prevent blood clots from forming to reduce stroke risk.
- Control your heart rate within a relatively normal range.
- Restore a normal heart rhythm, if possible.
- Treat heart disease/condition that may be causing arrhythmia.
- Reduce other risk factors for heart disease and stroke.
Medications: Common drugs used to treat arrhythmias are –
- Antiarrhythmic drugs:
- Amiodarone
- Bepridil hydrochloride
- Disopyramide
- Dofetilide
- Dronedarone
- Flecainide
- Ibutilide
- Lidocaine (Xylocaine)
- Procainamide
- Propafenone
- Propranolol
- Quinidine
- Sotalol
- Tocainide
- Beta-blockers decrease the heart rate and cardiac output, which lowers blood pressure by blocking the effects of adrenalin. They are also used with therapy for cardiac arrhythmias and in treating angina pectoris.
- Anticoagulants
- Anticoagulants (blood thinners) work by making it harder for the blood to clot, or coagulate, warfarin.
Avoid
Certain substances can contribute to an irregular heartbeat, including:
- Caffeine
- Tobacco
- Alcohol
- Cold and cough medications
- Appetite suppressants
- Psychotropic drugs
- Beta-blockers for high blood pressure
- Street drugs such as cocaine, marijuana and ‘speed’ or methamphetamines
Manage Your Risk Factor
Just having an arrhythmia increases your risk of heart attack, cardiac arrest and stroke. Work with your health care team and follow their instructions to control other risk factors:
- Reduce high blood pressure
- Control cholesterol levels
- Lose excess weight
- Eat a heart-healthy diet
- Avoid tobacco smoke
- Enjoy regular physical activity
ECG—ELECTRICAL CONDUCTION, BASIC ECG, 12-LEAD ECG, AXIS DETERMINATION
Normal Electrical Conduction of the Heart
The normal electrical conduction in the heart allows the impulse that is generated by the sinoatrial node (SA node) of the heart to be propagated to (and stimulate) the myocardium (cardiac muscle). The myocardium contracts after stimulation. It is the ordered stimulation 103of the myocardium that allows efficient contraction of the heart, thereby allowing blood to be pumped throughout the body.
Electrochemical Mechanism
Cardiac muscle has some similarities to neurons and skeletal muscle, as well as important unique properties. Like a neuron, a given myocardial cell has a negative membrane potential when at rest. Stimulation above a threshold value induces the opening of voltage-gated ion channels and a flood of cations into the cell. The positively charged ions entering the cell cause the depolarization characteristic of an action potential. Like skeletal muscle, depolarization causes the opening of voltage-gated calcium channels and release of Ca2+ from the t-tubules. This influx of calcium causes calcium-induced calcium release from the sarcoplasmic reticulum, and free Ca2+ causes muscle contraction. After a delay, potassium channels reopen and the resulting flow of K+ out of the cell causes repolarization to the resting state.
- S-A node (sinoatrial node)—known as the heart's natural pacemaker, the S-A node has special cells that create the electricity that makes your heart beat.
- A-V node (atrioventricular node)—the A-V node is the bridge between the atria and ventricles. Electrical signals pass from the atria down to the ventricles through the A-V node.
- His-Purkinje system—the His-Purkinje system carries the electrical signals throughout the ventricles to make them contract. The parts of the His-Purkinje system include:
- Right bundle branch
- Left bundle branch
- Purkinje fibers (the end of the system)
Conduction Pathway
Signals arising in the SA node stimulate the atria to contract and travel to the AV node. After a delay, the stimulus is conducted through the bundle of His to the Purkinje fibers and the endocardium at the apex of the heart, then finally to the ventricular epicardium.
Depolarization and the ECG
- 104SA node: P-wave: Under normal conditions, electrical activity is spontaneously generated by the SA node, the physiological pacemaker. This electrical impulse is propagated throughout the right atrium, and through Bachmann's bundle to the left atrium, stimulating the myocardium of the atria to contract. The conduction of the electrical impulse throughout the atria is seen on the ECG as the P-wave.
- As the electrical activity is spreading throughout the atria, it travels via specialized pathways, known as internodal tracts, from the SA node to the AV node.
AV Node/Bundles: PR Interval
The AV node functions as a critical delay in the conduction system. Without this delay, the atria and ventricles would contract at the same time, and blood would not flow effectively from the atria to the ventricles. The delay in the AV node forms much of the PR segment on the ECG. And part of atrial repolarization can be represented by PR segment.
The distal portion of the AV node is known as the bundle of His. The bundle of His splits into two branches in the interventricular septum, the left bundle branch and the right bundle branch. The left bundle branch activates the left ventricle, while the right bundle branch activates the right ventricle. The left bundle branch is short, splitting into the left anterior fascicle and the left posterior fascicle. The left posterior fascicle is relatively short and broad, with dual blood supply, making it particularly resistant to ischemic damage. The left posterior fascicle transmits impulses to the papillary muscles, leading to mitral valve closure. As the left posterior fascicle is shorter and broader than the right, impulses reach the papillary muscles just prior to depolarization, and therefore contraction, of the left ventricle myocardium. This allows pre-tensioning of the chordae tendinea, increasing the resistance to flow through the mitral valve during left ventricular contraction. This mechanism works in the same manner as pretensioning of car seatbelts.
Purkinje Fibers/Ventricular Myocardium: QRS Complex
The two bundle branches taper out to produce numerous Purkinje fibers, which stimulate individual groups of myocardial cells to contract. The spread of electrical activity through the ventricular myocardium produces the QRS complex on the ECG.
Ventricular Repolarization
The last event of the cycle is the repolarization of the ventricles. It is the restoring of the resting state. In the ECG, repolarization includes the J-wave, ST-segment, and T- and U-waves.
Electrical Signals and Blood Flow
The S-A node normally produces 60–100 electrical signals per minute —heart rate or pulse. With each pulse, signals from the S-A node follow a natural electrical pathway through heart walls.
(ECG) ELECTROCARDIOGRAPHY
Introduction
More than two electrodes are used, and they can be combined into a number of pairs for example: left arm (LA), right arm (RA) and left leg (LL) electrodes form the three pairs LA + RA, LA + LL, and RA + LL. The output from each pair is known as a lead. Each lead looks at the heart from a different angle. Different types of ECGs can be referred to by the 105number of leads that are recorded; for example, 3-lead, lead or 12-lead ECGs (sometimes simply ‘a 12-lead’). A 12-lead ECG is one in which 12 different electrical signals are recorded at approximately the same time and will often be used as a one-off recording of an ECG, traditionally printed out as a paper copy. Three- and 5-lead ECGs tend to be monitored continuously and viewed only on the screen of an appropriate monitoring device; for example, during an operation or whilst being transported in an ambulance. There may or may not be any permanent record of a 3- or 5-lead ECG, depending on the equipment used.
History
Alexander Muirhead is reported to have attached wires to a feverish patient's wrist to obtain a record of the patient's heartbeat while studying for his Doctor of Science (in electricity) in 1872 at St Bartholomew's Hospital. Einthoven assigned the letters P, Q, R, S and T to the various deflections, and described the electrocardiographic features of a number of cardiovascular disorders. In 1924, he was awarded the Nobel Prize in Medicine for his discovery.
Meaning
The word is derived from the Greek electro, because it is related to electrical activity, kardio, Greek for heart, and graph, a Greek root meaning ‘to write’.
Definition
Electrocardiography is a transthoracic interpretation of the electrical activity of the heart over a period of time, as detected by electrodes attached to the surface of the skin and recorded by a device external to the body. The recording produced by this noninvasive procedure is termed as an electrocardiogram and also ECG.
ECG: A galvanometer and electrodes with six limb leads and six chest leads. A graphic recording of the electric forces generated by the heart during depolarization and repolarization. The electrocardiogram is recorded on graph paper with divisions.
Other Definitions
- Depolarization: Electrical activation of the myocardium.
- Repolarization: Restoration of the electrical potential of the myocardial cell.
- Sequence: Depolarization occurs in the sinoatrial (SA) node; current travels through internodal tracts of the atria to the atrioventricular (AV) node; then through bundle of His, which divides into right and left bundle branches; left bundle branch divides into left anterior and posterior fascicles.
Functions
- To measure and diagnose abnormal rhythms of the heart.
- Helps to diagnose properly, particularly abnormal rhythms caused by damage to the conductive tissue that carries electrical signals, or abnormal rhythms caused by electrolyte imbalances.
Characteristics of the Normal Basic ECG
It is important to remember that there is a wide range of normal variability in the 12-lead ECG. The following ‘normal’ ECG characteristics, therefore, are not absolute. It takes considerable ECG reading experience to discover all the normal variants. Only by following a structured ‘Method of ECG Interpretation’ (Lesson II) and correlating the various ECG findings with the particular patient's clinical status will the ECG become a valuable clinical tool.
- Rhythm
- Conduction
- Measurements:
- Heart Rate: 60–90 bpm
- PR Interval: 0.12–0.20 sec
- QRS Duration: 0.06–0.10 sec
- QT Interval (QTc ≤ 0.40 sec):
- Bazett's Formula: QTc = (QT)/Sq Root RR (in seconds)
- Poor Man's Guide to upper limits of QT: For HR = 70 bpm, QT ≤ 0.40 sec; for every 10 bpm increase above 70, subtract 0.02 sec, and for every 10 bpm decrease below 70, add 0.02 sec. For example:
- QT ≤ 0.38 @ 80 bpm
- QT ≤ 0.42 @ 60 bpm
- Frontal Plane QRS Axis: +90° to –30° (in the adult)
- Rhythm: Normal sinus rhythm
- The P-waves in leads I and II must be upright (positive) if the rhythm is coming from the sinus node.
- Conduction: Normal Sinoatrial (SA), Atrioventricular (AV), and Intraventricular (IV) conductions
Both the PR interval and QRS duration should be within the limits specified above.
ECG PAPER
Time is measured from the L to the R—one large box = 0.20 sec and one small one = 0.04 sec.
The rate of the ECG machine is 25 mm/sec. Marks on the upper or lower border of paper fall every 3 sec or 3 inches.
Voltage or current strength is determined from the magnitude or height of the various waveforms and is measured in mV or mm—one small box normally = 0.1 mV or 1 mm and one large box = 0.5 mV or 5 mm.
Voltage strength can be adjusted when recording the ECG. Thus, if the waveform is especially large, as in the precordial leads of a patient with ventricular hypertrophy, or especially small, as in a patient with severe lung disease, the size of the waveforms can be adjusted to fit the paper. A calibration mark is thus made at the beginning of the recording to denote whether it is at full-, half-, or, occasionally, double-amplitude. The normal calibration mark should be a full 10 mm for a 0.1 mV calibration. At half-amplitude, each vertical block equals 0.2 mV; at double-amplitude, each vertical block equals 0.05 mV.
If an electrical impulse is moving toward the sensing electrode, a positive (upright) deflection is recorded; if the impulse is moving away from the sensing electrode, a negative (downward) deflection is recorded; when an impulse travels perpendicular to (90° away from) the sensing electrode, a straight line (isoelectric deflection) or an equiphasic (small amplitude complex with approximately equal height of upward and downward deflections) deflection is recorded.
- Monophasic waveform = complex (e.g. P or T) peaks in one direction, either all positive or all negative.
- Biphasic = complex has a positive peak and a negative peak (nadir).
- Triphasic = three points to the complex, e.g., RSR'.
- Equiphasic = negative part of the waveform is equal in size to the positive portion.
Dimensions of grids on ECG paper: Horizontal axis represents time. Large blocks are 0.2 seconds in duration, while small blocks are 0.04 seconds in duration. Vertical axis represents voltage. Large blocks are 5 mm, while small blocks represent 1 mm.
Estimation of Heart Rate
- Heart rates of 50 to 300 beats/min.: These can be estimated from the number of large squares in an R-R interval. Because there are 300 large blocks in one minute, the number of blocks between R-R intervals can be divided into 300 to approximate the rate. For example, one large block between R-R intervals would be determined thus:
- Heart rates of < 50 beats/minute: These can be estimated with the aid of markings at 3-second intervals along the graph paper. To calculate the rate, the cycles on a 6-second interval (two 3-second markings) are multiplied by 10 (to give the rate per 60 seconds; i.e. per minute).
Layout
By definition, a 12-lead ECG will show a short segment of the recording of each of the 12-leads. This is often arranged in a grid of four columns by three rows, the first column being the limb leads (I, II and III), the second column being the augmented limb leads (aVR, aVL and aVF) and the last two columns being the chest leads (V1-V6). It is usually possible to change this layout, so it is vital to check the labels to see which lead is represented. Each column will usually record the same moment in time for the three leads and then the recording will switch to the next column, which will record the heartbeats after that point. It is possible for the heart rhythm to change between the columns of leads.
Leads
The term ‘lead’ in electrocardiography causes much confusion because it is used to refer to two different things. In accordance with common parlance, the word ‘lead’ may be used to refer to the electrical cable attaching the electrodes to the ECG recorder. As such, it may be 108acceptable to refer to the ‘left arm lead’ as the electrode (and its cable) that should be attached at or near the left arm. Usually, 10 of these electrodes are standard in a ‘12-lead’ ECG.
Placement of Electrodes
Ten electrodes are used for a 12-lead ECG. The electrodes usually consist of a conducting gel, embedded in the middle of a self-adhesive pad onto which cables clip. Proper placement of the limb electrodes, color-coded recommended by the American Heart Association (a different colour scheme).
109
ELECTRODE LABEL (USA) | ELECTRODE PLACEMENT |
|---|---|
RA | On the right arm, avoiding thick muscle |
LA | In the same location where RA was placed, but on the left arm |
RL | On the right leg, lateral calf muscle |
LL | In the same location where RL was placed, but on the left leg |
V1 | In the fourth intercostal space (between ribs 4 and 5) just to the right of the sternum (breastbone) |
V2 | In the fourth intercostal space (between ribs 4 and 5) just to the left of the sternum |
V3 | Between leads V2 and V4 |
V4 | In the fifth intercostal space (between ribs 5 and 6) in the midclavicular line |
V5 | Horizontally even with V4 in the left anterior axillary line |
V6 | Horizontally even with V4 and V5 in the midaxillary line |
Additional Electrodes
The classical 12-lead ECG can be extended in a number of ways in an attempt to improve its sensitivity in detecting myocardial infarction involving territories not normally ‘seen’ well. This includes an rV4 lead, which uses the equivalent landmarks to the V4 but on the right side of the chest wall and extending the chest leads onto the back with a V7, V8 and V9.
The Lewis lead or S5 has the LA electrode placed in the second intercostal space to the right of the sternum with the RA at the fourth intercostal space. It is read as lead I and is supposed to demonstrate atrial activity much better to aid in identification of atrial flutter or broad-complex tachycardia.
A posterior ECG can aid in the diagnosis of a posterior myocardial infarction. This is performed by the addition of leads V7, V8 and V9 extending around the left chest wall toward the back.
Limb Leads
In both the 5- and 12-lead configurations, leads I, II and III are called limb leads. The electrodes that form these signals are located on the limbs—one on each arm and one on the left leg. The limb leads form the points of what is known as Einthoven's triangle.
- Lead I is the voltage between the (positive) left arm (LA) electrode and right arm (RA) electrode:I = LA–RA
- Lead II is the voltage between the (positive) left leg (LL) electrode and the right arm (RA) electrode:II = LL–RA
- Lead III is the voltage between the (positive) left leg (LL) electrode and the left arm (LA) electrode:III = LL–LA
Simplified electrocardiograph sensors designed for teaching purposes, e.g. at high school level, are generally limited to three arm electrodes serving similar purposes.
- Limb leads are usually labeled but also occasionally color coded so that:
- Right arm—‘White is on the right’.
- Right leg—‘Green is for go’. (Right leg is gas pedal.)
- Left leg—‘Red is for stop’. (Some brake with left leg.)
- Left arm—black lead.
- Unipolar vs. bipolar leads
- The two types of leads are unipolar and bipolar.
- Bipolar leads have one positive and one negative pole. In a 12-lead ECG, the limb leads (I, II and III) are bipolar leads.
- Unipolar leads also have two poles, as a voltage is measured; however, the negative pole is a composite pole (Wilson's central terminal or WCT) made up of signals from multiple other electrodes. In a 12-lead ECG, all leads except the limb leads are unipolar (aVR, aVL, aVF, V1, V2, V3, V4, V5, and V6).
- Wilson's central terminal VW is produced by connecting the electrodes RA, LA, and LL together, via a simple resistive network, to give an average potential across the body, which approximates the potential at infinity (i.e. zero):
Augmented Limb Leads
Leads aVR, aVL, and aVF are augmented limb leads (after their inventor Dr Emanuel Goldberger known collectively as the Goldberger's leads). They are derived from the same three electrodes as leads I, II, and III. However, they view the heart from different angles (or vectors) because the negative electrode for these leads is a modification of Wilson's central terminal. This zeroes out the negative electrode and allows the positive electrode to become the ‘exploring electrode’. This is possible because Einthoven's Law states that I + (−II) + III = 0. The equation can also be written as I + III = II. It is written this way (instead of I - II + III = 0) because Einthoven reversed the polarity of lead II in Einthoven's triangle, possibly because he liked to view upright QRS complexes. Wilson's central terminal paved the way for the development of the augmented limb leads aVR, aVL, aVF and the precordial leads V1, V2, V3, V4, V5 and V6.
- Lead augmented vector left (aVL) has the positive (black) electrode on the left arm. The negative electrode is a combination of the right arm (white) electrode and the left leg (red) electrode, which ‘augments’ the signal strength of the positive electrode on the left arm:
- Lead augmented vector foot (aVF) has the positive (red) electrode on the left leg. The negative electrode is a combination of the right arm (white) electrode and the left arm (black) electrode, which ‘augments’ the signal of the positive electrode on the left leg:
The augmented limb leads aVR, aVL, and aVF are amplified in this way because the signal is too small to be useful when the negative electrode is Wilson's central terminal. Together with leads I, II, and III, augmented limb leads aVR, aVL, and aVF form the basis of the hexaxial reference system, which is used to calculate the heart's electrical axis in the frontal plane. The aVR, aVL, and aVF leads can also be represented using the I and II limb leads:
Precordial Leads
- The electrodes for the precordial leads (V1, V2, V3, V4, V5 and V6) are placed directly on the chest. Because of their close proximity to the heart, they do not require augmentation. Wilson's central terminal is used for the negative electrode, and these leads are considered to be unipolar (recall that Wilson's central terminal is the average of the three limb leads. This approximates common, or average, potential over the body). The precordial leads view the heart's electrical activity in the so-called horizontal plane. The heart's electrical axis in the horizontal plane is referred to as the Z axis.
- Unipolar leads (all have ‘V’ in their names) — aVR, aVL, aVF, and the precordial leads V1-V6.
- Require more electrodes on the patient (a minimum of 4-5).
- All have an ‘exploring’ electrode which ‘looks’ directly at the heart from its site of placement.
- All also require three ‘indifferent’ electrodes (RA, LA, and LL) but which do not contribute toward the tracing.
Precordial Placement of Unipolar Electrodes for 12-lead ECGs
*Addendum: V3 is placed halfway between V2 and V4.
All precordial leads bisect at AV node [point toward the AV node in a horizontal plane]:
- Right chest (or anterior) leads — V1, V2; also aVR.
- Septal leads—V3 and V4—located over the interventricular septum.
- Left chest (or lateral) leads—V5, V6; also I and aVL.
- V1 and V2 mirror changes occurring from the posterior side of the heart. None of the usual electrodes are directly adjacent to the posterior surface of the heart.
- If additional posterior leads need to be seen (e.g. to diagnose a true posterior infarction) do another 12-lead ECG but move 3 electrodes to these positions:
- V7 = same horizontal plane as V4–V6; PAL (posterior axillary line).
- V8 = same horizontal plane as V4–V6; mid-scapula.
- V9 = same horizontal plane as V4–V6; over spine.
- If additional right chest leads need to be seen (e.g. to diagnose a right ventricular infarction), do another 12-lead ECG but move 4 electrodes to these positions:
- V3R = halfway between V1 and V4R.
- V4R = 5 RICS at MCL.
- V5R = same horizontal plane as V4R at AAL (anterior axillary line).
- V6R = same horizontal plane as V4R at MAL (mid-axillary line).
- PLACEMENT OF BIPOLAR LEADS (I, II, III, MCL1, MCL6) FOR MONITORING
- RA = right arm; LA = left arm
- RL = right leg; LL = left leg
- Lead I — LA is +, RA is–, RL is ground.
- Lead II — LL is +, RA is–, RL is ground.
- Lead III — LL is +, LA is–, RL is ground.
* NOTE: LL electrode should always be placed below the umbilicus in order to avoid problems in patients with ventricular hypertrophy.
* NOTE: In placing electrodes on the chest, always find the angle of Louis (palpable junction of the manubrium and body of the sternum). Slide to the side and you are on the second rib with the 2nd intercostal space just below it. Count down to the correct interspace from there.
- Bipolar leads look from a positive pole toward a negative pole. The positive electrode ‘looks’ directly at the heart from the site where it is placed. Ground electrodes do not contribute to the tracing. ‘Quick look’ defibrillator paddles are also bipolar.
- Lead MCL1— use lead I selection on monitor; positive electrode placed in 4ICS (4th intercostal space) at the RSB (right sternal border); negative electrode placed on L shoulder; ground may be placed anywhere.
- V1 is the single best lead for diagnosing dysrhythmias; MCL1 is a substitute for V1 which can be recorded from 3 lead-wire patient cables.
- MCL1 is very similar to, but not identical to, pattern seen in V1 precordial lead.
- Rhythms which can be distinguished in V1 or MCL1 but not in other leads include those with a widened QRS complex, such as right versus left ventricular rhythms, right and left bundle branch blocks, and differentiation of supraventricular rhythms with aberration from ventricular rhythms. Also, P-waves are often visible in V1 and MCL1 when they are invisible in other leads because the exploring electrode is the one closest to the atria.
- Lead MCL6 — almost the same electrode placement as MCL1, but the positive electrode is placed in the 5ICS in the left MAL (midaxillary line). MCL6 is very similar to, but not identical to, pattern seen in V6 precordial lead.
Clinical Lead Groups
Of the 12 leads in total, each records the electrical activity of the heart from a different perspective, which also correlates to different anatomical areas of the heart for the purpose of identifying acute coronary ischemia or injury. Two leads that look at neighboring anatomical areas of the heart are said to be contiguous. The relevance of this is in determining whether an abnormality on the ECG is likely to represent true disease or a spurious finding.
I Lateral | aVR | V1 Septal | V4 Anterior |
II Inferior | aVL Lateral | V2 Septal | V5 Lateral |
III | aVF Inferior | V3 Anterior | V6 Lateral |
Category | Color on chart | Leads | Activity |
|---|---|---|---|
Inferior leads | Yellow | Leads II, III and aVF | Look at electrical activity from the vantage point of the inferior surface (diaphragmatic surface of heart) |
Lateral leads | Green | I, aVL, V5 and V6 | Look at the electrical activity from the vantage point of the lateral wall of left ventricle
|
Septal leads | Orange | V1 and V2 | Look at electrical activity from the vantage point of the septal surface of the heart (interventricular septum) |
Anterior leads | Blue | V3 and V4 | Look at electrical activity from the vantage point of the anterior wall of the right and left ventricles (sternocostal surface of the heart) |
Waves and Intervals
Upper limit of normal QT interval, corrected for heart rate according to Bazett's formula, Fridericia's formula and subtracting 0.02 s from QT for every 10 bpm increase in heart rate. Up to 0.42 s (≤ 420 ms) is chosen as normal QTc of QTB and QTF in this diagram.
Feature | Description | Duration |
|---|---|---|
| The interval between an R-wave and the next R-wave; normal resting heart rate is between 60 and 100 bpm. | 0.6 to 1.2 s |
| During normal atrial depolarization, the main electrical vector is directed from the SA node toward the AV node and spreads from the right atrium to the left atrium. This turns into the P-wave on the ECG. | 80 ms |
| The PR interval is measured from the beginning of the P-wave to the beginning of the QRS complex. The PR interval reflects the time the electrical impulse takes to travel from the sinus node through the AV node and entering the ventricles. The PR interval is, therefore, a good estimate of AV node function. | 120 to 200 ms |
| The PR segment connects the P-wave and the QRS complex. The impulse vector is from the AV node to the bundle of His to the bundle branches and then to the Purkinje fibers. This electrical activity does not produce a contraction directly and is merely traveling down toward the ventricles, and this shows up flat on the ECG. The PR interval is more clinically relevant. | 50 to 120 ms |
| The QRS complex reflects the rapid depolarization of the right and left ventricles. The ventricles have a large muscle mass compared to the atria, so the QRS complex usually has a much larger amplitude than the P-wave. | 80 to 120 ms |
| The point at which the QRS complex finishes and the ST segment begins. It is used to measure the degree of ST elevation or depression present. | N/A |
| The ST segment connects the QRS complex and the T-wave. The ST segment represents the period when the ventricles are depolarized. It is isoelectric. | 80 to 120 ms |
| The T-wave represents the repolarization (or recovery) of the ventricles. The interval from the beginning of the QRS complex to the apex of the T-wave is referred to as the absolute refractory period. The last half of the T-wave is referred to as the relative refractory period (or vulnerable period). | 160 ms |
| The ST interval is measured from the J point to the end of the T wave. | 320 ms |
| The QT interval is measured from the beginning of the QRS complex to the end of the T-wave. A prolonged QT interval is a risk factor for ventricular tachyarrhythmias and sudden death. It varies with heart rate and, for clinical relevance, requires a correction for this, giving the QTc. | Up to 420 ms in heart rate of 60 bpm |
| The U-wave is hypothesized to be caused by the repolarization of the interventricular septum. It normally has a low amplitude, and even more often is completely absent. It always follows the T-wave, and also follows the same direction in amplitude. If it is too prominent, suspect hypokalemia, hypercalcemia or hyperthyroidism. | |
| The J-wave, elevated J-point or Osborn wave appears as a late delta wave following the QRS or as a small secondary R-wave. It is considered pathognomonic of hypothermia or hypercalcemia. | |
Normal ECG Waveforms and Intervals
P-waves — represent depolarization of the atrial myocardium (Sinus node depolarization is too small in amplitude to be recorded from the body surface, so it is not seen.)
The normal P-wave is:
- Not wider than 0.11 sec (under 3 little boxes on the ECG paper).
- Not taller than 3 mm.
- Not notched or peaked; does not have an excessive trough if biphasic.
- Positive and rounded in leads I, II, and aVF in 94% of normals; usually upright in V4–V6.
Inverted P-waves in these leads are either abnormal or due to improper lead placement.
- Negative in aVR.
- Positive, negative, or biphasic in lead III, aVL, and V1–V3.
- P-wave axis = + 60°.
- Normally has 1:1 ratio with the QRS and should be regular.
- Initial portion of P is largely a reflection of R atrial depolarization and the terminal portion reflects depolarization of the L atrium. The P-waves should all look alike.
ECG Waveforms
PR Interval—represents atrial depolarization plus the normal delay at the AV node.
- Normally = 0.12–0.20 sec. (No longer than one large box.)
- Increased in length if AV conduction is prolonged (first-degree AV block).
PR Segment—begins at the end of the P-wave and ends with the onset of the QRS complex.
- Should be isoelectric (flat).
- Can be elevated with atrial infarction or pericarditis.
- Can be depressed if there is a large repolarization wave (Tp) following the P-wave.
QRS Complex—represents depolarization of the ventricular myocardium. (Depolarization of the AV node, His bundle, bundle branches, and Purkinje fibers are too small in amplitude to be detected by electrodes on the body surface.)
- All positive waves of the QRS complex are labeled R-waves. If there are more than one, the second one is labeled R'. An upper case capital letter describes a sizable R-wave (> 5 mm); a lower case letter describes a tiny R-wave (< 4 mm).
- Negative waves of the QRS are labeled with Q-waves (preceding the R-wave) or S-waves (following the R-wave). Subsequent negative waves are labeled S' waves.
Relative size is denoted by upper- or lower-case letters.
- Although termed the ‘QRS’ complex, many complexes do not contain all three waves.
- Monomorphic = one shape; refers to a cardiac rhythm in which each QRS complex has a consistent pattern as, for example, monomorphic ventricular tachycardia which arises from one specific location. Older term, ‘unifocal’ means the same thing.
- Polymorphic = multiple shapes such as polymorphic ventricular tachycardia which arise from multiple sites in the ventricles. Older term, ‘multifocal’ means the same thing.
- 0.07–0.11 sec in width. QRS widths often vary in different leads. The widest QRS measurement on the 12-lead ECG is the correct one. Best leads to look at are usually leads I and V1.
- Should neither be smaller than 6 mm in leads I, II, and III and nor should it be taller than 25–30 mm in the precordial leads.
- R-wave Progression—in the precordial leads, the QRS starts off primarily negative (rS) in V1 and gradually becomes primarily positive (qRs) with the tallest R-wave in V5 or V6.
- Early R-waves—R-waves in leads V1 and V2 as large as those in the next several leads can reflect posterior infarction, lateral MI, right ventricular hypertrophy (RVH), or septal hypertrophy.
- Tall R-wave in V1—consider RVH, posterior MI, or Wolff-Parkinson-White (W-P-W).
- ‘Low’ R-waves in the right precordial leads — most likely due to left ventricular hypertrophy (LVH) but also consider left anterior fascicular block (LAFB), COPD, or MI. R-wave < 2–3 mm in V3 is abnormal unless there is LVH. LVH causes loss of R height from V1–V3 without MI. Loss of R height between V1-2 or V2-3 in the absence of LVH suggests anterior MI.
- Poor R-wave Progression—R-waves do not begin to dominate QRS until V5 or V6. This may represent infarction or injury of the anterior LV and carries almost as much significance as Q-waves.
- Q-Wave — a negative wave preceding the R-wave. Not all leads normally record a Q-wave. Normal Q-waves represent septal depolarization and they must be distinguished from pathologic Q-waves which indicate myocardial infarction.
Normal Q-wave
- Present only in leads I, aVL, V5, and V6 (left lateral leads).
- Small in aVF and V5—normal variant.
- If there is no Q where there should be one — septal fibrosis is present.
- If large — myocardial damage. Large, diagnostic Q-waves represent altered electrical activity in the myocardium due to transmural myocardial damage.
- Less than 0.04 sec.
- ‘Diagnostic’ Q wave in V1, aVL, and III may be present without indicating myocardial damage.
- ST segment — represents the time when ventricular cells are in the plateau phase (phase 2) of the action potential in which there is no current flow and thus little, if any transmembrane gradient (transmembrane potential hovers around zero). QRS and ST segment also represent a time when the ventricles are in their absolute refractory period and will not respond to stimulation.
- ST segment starts at the J point (junction of the end of the QRS complex with the ST segment) and ends at the beginning of the T-wave.
- ST segment (as well as the PR and TP segments) should be isoelectric (flat).
- ST segment always has a smooth contour unless something else is added to it.
- Clinical importance is related to its level relative to the isoelectric line rather than to its duration.
- QT interval—measurement of the refractory period or the time during which the myocardium would not respond to a second impulse; measured from the beginning of the QRS complex to the end of the T-wave.
- Best leads to measure the QT are V2 or V3.
- If there is a U-wave visible, the measurement is made to the end of the U-wave and is called the Q-TU interval.
- Q-T interval should be roughly less than half the preceding R-R interval.
- It is longer with slower rates and shorter with faster rates. Normals also vary with age and gender.
- If a Q-T table is not available, the Q-T interval can be corrected for heart rate using Basset's formula:
- If a patient develops a wide QRS complex (a problem with depolarization) such as a bundle branch block, the QT interval will be increased. Thus, a long QT interval is not thought of as abnormal in patients with a wide QRS complex unless you have subtracted the extra width of the QRS from the QT interval and still found it prolonged.
- If the rhythm is irregular, measure the QT relative to the rate of the prior R-R interval.
QT dispersion—QT is measured on the same beat in all 12 ECG leads and the shortest QT interval is subtracted from the longest QT interval. Recent evidence indicates that if there is much of a difference, heterogenous refractoriness exists in the heart muscle and the patient may be at higher risk of cardiac death from development of ventricular tachycardia/fibrillation, especially from any proarrhythmic effects of antiarrhythmic drugs.
JT Interval—JT interval reflects repolarization alone, not both depolarization and repolarization.
Sometimes used to measure the refractory period in patients who have been started on a Na+ channel blocker antiarrhythmic drugs (e.g. Quinidine, Pronestyl, and other class I agents). This is because such drugs slow depolarization, slightly prolonging the QRS complex.
- Earliest time ventricles can respond to another stimulus usually coinciding with the apex of the T-wave.
- T-wave should have the same polarity as the QRS complex. Thus, if the QRS complex is primarily negative, the T-wave should be negative.
- There are literally dozens of conditions that cause abnormal-looking T-wave in leads with positive QRS waveforms.
- T-waves are very fickle; not as reliable as ST depression or elevation in diagnosis of ischemia.
- Myocardial ischemia/non-Q-waves.
- Normal variants (juvenile T-wave pattern; early repolarization).
- Cerebrovascular accidents (especially intracranial bleeds) and related neurogenic patterns (e.g. radical neck dissection, Stokes-Adams syndrome).
- Post-tachycardia or post-pacemaker T-wave pattern.
- Intermittent left bundle branch block (LBBB).
- Left or right ventricular overload (e.g. classic ‘strain’ patterns or apical hypertrophic cardiomyopathy.
- Secondary T-wave alterations due to bundle branch blocks or Wolff-Parkinson-White patterns.
- Respiratory alkalosis.
- It is no longer believed that the first sign of infarction is T-wave inversion.
- U-wave—A shallow, gently curved wave (in the same direction as the T-wave but smaller) following the T-wave. May not be visible at all.
- It is not clear what the U-wave represents. May represent repolarization of intramural Purkinje conduction system.
- Conditions which may cause a pronounced U-wave are antiarrhythmic drug effects, especially when the patient is prone to proarrhythmia (drug-induced arrhythmias such as polymorphic ventricular tachycardia or ‘torsades de pointes’).
- Prominent U-wave—usually suggests digitalis toxicity or hypokalemia. Also seen in bradycardias.
Axis Determination
Definition: Direction of depolarization (vector) of the QRS complex.
- The left ventricle is thicker, so the mean QRS vector is down and to the left (The origin of the vector is the AV node with the left ventricle being down and to the left of this).
- The vector will point toward hypertrophy (thickened wall) and away from the infarct (electrically dead area).
- The mean direction of electrical forces in the frontal plane (limb leads) as measured from the zero reference point (lead 1)
- Normal values
- P-wave: 0 to 75 degrees
- QRS complex: 30 to 90 degrees
- T-wave: QRS-T angle < 45 degrees frontal or < 60 degrees precordial
- It is usually oriented in a right shoulder to left leg direction, which corresponds to the left inferior quadrant of the hex axial reference system, although -30° to +90° is considered to be normal.
- If the left ventricle increases its activity or bulk, then there is said to be ‘left axis deviation’ as the axis swings round to the left beyond -30°; alternatively, in conditions where the right ventricle is strained or hypertrophied, then the axis swings round beyond +90° and ‘right axis deviation’ is said to exist. Disorders of the conduction system of the heart can disturb the electrical axis without necessarily reflecting changes in muscle bulk.
Normal | -30° to 90° | Normal | Normal |
Left axis deviation | -30° to -90° | May indicate left anterior fascicular block or Q-waves from inferior MI. | Left axis deviation is considered normal in pregnant women and those with emphysema. |
Right axis deviation | +90° to +180° | May indicate left posterior fascicular block, Q-waves from high lateral MI, or a right ventricular strain pattern | Right deviation is considered normal in children and is a standard effect of dextrocardia. |
Extreme right axis deviation | +180° to -90° | Is rare, and considered an ‘electrical no-man's land’ | |
Measurement
Quick look tests
- The simplest method of identifying gross deviations in axis is to look at the QRS complexes in leads I and aVF. Lead I is a left-sided lead, and as aVF is perpendicular to lead I, it can be considered a right-sided lead.
- Both leads I and aVF have mainly positive QRS complexes.
- Lead I is positive and aVF is negative left axis deviation (LAD).
- Lead I is negative and aVF is positive right axis deviation (RAD).
- Both leads negative extreme RAD or ‘North-West’ axis
Interpretation of QRS Axis
- Normal: 0 to 90 degrees
- Both leads I and aVF have mainly positive QRS complexes.
Normal Axis Deviation
Right Axis Deviation (RAD)
- > 90 degrees (+90° to +180°)
- Moderate RAD: 90 to 120 degrees
- Marked RAD: 120 to 180 degrees
- Differential diagnosis:
- Right Ventricular Hypertrophy (RVH) — most common
- Left Posterior Fascicular Block (LPFB) — diagnosis of exclusion
- Lateral and apical MI
- Acute Right Heart Strain, e.g. acute lung disease such as pulmonary embolus
- Chronic lung disease, e.g. COPD
- Hyperkalemia
- Sodium-channel blockade, e.g. tricyclic toxicity
- Secundum ASD
- Normal in infants and children
Right Axis Deviation (RAD)
Left Axis Deviation (LAD)
- < –30 degrees (-30° to -90°)
- Moderate LAD: –30 to –45 degrees
- Marked LAD: –45 to –90 degrees
- Differential diagnosis
Left Axis Deviation (LAD)
Extreme Axis Deviation
- 180 to –90 degrees (rare)
- Differential diagnosis:
- Right Ventricular Hypertrophy (RVH)
- Apical MI
- VT
- Hyperkalemia
Rotation
- Can be thought of as the axis of the heart in the transverse axis (the precordial leads)
- Normal:
- Isoelectric QRS in V3 and V4, indicating the transition point between the right and left ventricular electric forces
- Clockwise rotation
- Isoelectric QRS in V5, V6
- Anticlockwise rotation:
- Isoelectric QRS in V1, V2
AORTIC ANEURYSM
Definition
- Aneurysm is a localized sac or dilation formed at a weak point in the wall of the aorta.
- An aneurysm is an abnormal bulge in the wall of a blood vessel. A larger bulge, more than 1.5 times the size of normal aorta, is called an aneurysm.
Incidence
- 30–60/1000
- Increasing incidence over past 3 decades
- Carotid Artery Stenosis –10%
- Smoker: Nonsmoker –8:1
- Male: Female –4:1
- HTN: 40% of pts
Shapes: Aneurysm may be classified by its shape and form:
- True aneurysms: One, two and all three layers of artery may be involved. It is classified into different types:
- Fusiform aneurysms: Symmetric, spindle-shaped expansion of entire circumference of involved vessel. It appears as symmetrical bulges around the circumference of the aorta. They are the most common shape of aneurysm.
- Dissecting aneurysms: A bilateral out pouching in which layers of the vessels wall separate, creating a cavity. This is usually is a haematoma that split the layer of arterial wall.
- False aneurysms: The wall rupture and a blood clot is retained in an out pouching of tissue or there connection between and artery that does not close.
Types: The two types of aortic aneurysms are:
- Thoracic aortic aneurysms: Develop in the part of the aorta that runs through the chest. This includes the ascending aorta (the short stem of the cane); the aortic arch (the cane handle); and the descending thoracic aorta (the longer stem of the cane).
- Abdominal aortic aneurysms: Develop in the part of the aorta that runs through the abdomen. Most abdominal aortic aneurysms develop below the renal arteries (the area where the aorta branches out to the kidneys). Sometimes aortic aneurysms extend beyond the aorta into the iliac arteries (the blood vessels that go to the pelvis and legs).
Causes: The exact cause is unknown. But recent evidence includes:
- Atherosclerosis
- Hypertension
Congenital: | Inflammatory (Noninfectious) |
|
|
Mechanical disorder: | Infectious: |
|
|
Traumatic (Psedoaneurysm): | Pregnancy related degenerative: |
|
|
Risk Factors
- CAD
- Hypertension
- Hypercholesterolemia
- Hyperhomocysteinemia
- Elevated C-reactive protein
- Tobacco use
- Peripheral vascular disease
- Marfan's syndrome
- Ehlers-Danlos type IV
- Bicuspid aortic valve
Pathophysiology
Clinical Manifestations
- Asymptomatic: 70–75%
- Symptoms:
- Early satiety, N, V
- Abdominal, flank, or back pain
- 1/3 of patients experience abdominal and flank pain
- Abrupt onset of pain –> Rupture or expansion of aneurysm
Diagnostic Evaluation
Physical Examination
- If < 5 cm in diameter, then cannot be detected by routine physical examination
Radiographs
- Calcified wall. Can determine size in 2/3
- Cannot rule out and AAA
Arteriography
- Cannot determine aneurysm size because of mural thrombus
- Indications for obtaining arteriography
- Suspicion of visceral ischemia
- Occlusive disease of iliac and femoral arteries
- Severe HTN, or impair renal function
- Horseshoe kidney
- Suprarenal of TAAA component
- Femoropopliteal aneurysms
Ultrasound
- Establishes diagnosis easily
- Accurately measures infrarenal diameter
- Difficult to visualize thoracic or suprarenal aneurysms
- Difficult to establish relationship to renal arteries
- Techniciandependent
- Widely available, quick, no risk, cheap
CT Scan
- Very reliable and reproducible
- Can image entire aorta
- Can visualize relationship to visceral vessels
- Longer to obtain and is more costly than U/S
- Most useful
- Requires contrast agent—renal toxicity
Complications
- Thrombosis
- Distal embolization
- Rupture
THORACIC AORTIC ANEURYSM
Introduction
Approximately 85% of all cases of thoracic aortic aneurysm are caused by atherosclerosis. They occur most frequently in men between ages 40 and 70 years. The thoracic area is the 129most common site for a dissecting aneurysm. About one-third of patients with thoracic aortic aneurysm die of rupture of aneurysm.
Clinical Manifestations
Symptoms are variable and depend on how rapidly the aneurysm dilates and how the pulsating mass affects the surrounding intrathoracic structures. Some of the patients are asymptomatic. But some are having:
- Pain occurring in supine position
- Dyspnea
- Hoarseness
- Stridor
- Weakness
- Aphonia
- Dysphasia
Assessment and Diagnostic Tests
- Physical Examination: superficial veins of neck, chest or arm dilated
- Chest X-ray
- Transesophageal echocardiography
- CT scan
Treatment (Medical Management)
- Antihypertensive: Hydralazine hydrochloride
- Beta blocker: Atenolol, Timolol maleate
Surgical Management
Abdominal Aortic Aneurysm
Introduction
The most common cause of abdominal aortic aneurysm is arteriosclerosis. The condition which is more common among Caucasians population affects men 4 times more often than 130women and it is most prevalent in elderly patients. Most of this aneurysm occurs below renal arteries. Untreated, the eventual outcome may be rupture and death.
Causes
- Congenital weakness
- Trauma or disease
Risk Factors
- Genetic predisposition
- Smoking
- Hypertension (50% cases)
Clinical Manifestations
- Patient feels his heart beating in abdomen
- Abdominal mass
- Abdominal throbbing
Assessment and Diagnostic Tests
- Physical Examination: Superficial veins of neck, chest or arm dilated
- Duplex ultrasonography
- CT scan: Determine size, length and location of aneurysm
Treatment (Medical Management)
- Medical therapy of aortic aneurysms involves strict blood pressure control. This does not treat the aortic aneurysm per se, but control of hypertension within tight blood pressure parameters may decrease the rate of expansion of the aneurysm.
- The tetracycline antibiotic doxycycline is currently being investigated for use as a potential drug in the prevention of aortic aneurysm due to its metalloproteinase inhibitor and collagen-stabilizing properties.
Prevention
Attention to patient's general blood pressure, smoking and cholesterol risks helps reduce the risk on an individual basis. There have been proposals to introduce ultrasound scans as a screening tool for those most at risk: men over the age of 65.
Surgical Management
For abdominal aortic aneurysms, suggest elective surgical repair when the diameter of the aneurysm is greater than 5 cm (2 in). However, suggest medical management for abdominal aneurysms with a diameter of less than 5.5 cm (2 in).
Open Surgery
Open surgery typically involves dissection of the dilated portion of the aorta and insertion of a synthetic (Dacron or Gore-Tex) patch tube. Once the tube is sewn into the proximal and distal portions of the aorta, the aneurysmal sac is closed around the artificial tube. Instead of sewing, the tube ends, made rigid and expandable by nitinol wireframe, can be much more 131simply, quickly and effectively inserted into the vascular stumps and there permanently fixed by external ligature.
Endovascular Surgery
The endovascular treatment of aortic aneurysms involves the placement of an endo-vascular stent via a percutaneous technique (usually through the femoral arteries) into the diseased portion of the aorta. This technique has been reported to have a lower mortality rate compared to open surgical repair, and is now being widely used in individuals with comorbid conditions that make them high-risk patients for open surgery.
DISSECTION/DISSECTING AORTA
Introduction
Occasionally, in an aorta diseased by atherosclerosis, a tear develops in the intima or the media degenerate, resulting in a dissection.
Definition
An aortic dissection is a serious condition in which a tear develops in the inner layer of the aorta, the large blood vessel branching off the heart. Blood surges through this tear into the middle layer of the aorta, causing the inner and middle layers to separate (dissect). If the blood-filled channel ruptures through the outside aortic wall, aortic dissection is often fatal.
Incidence
Arterial dissection is commonly associated with poorly controlled hypertension. It is 3 times more common in men than in women and occur most commonly in 50- to 70-year-old age group. Dissection is caused by rupture in the intima layer. A rupture may occur through adventitia or into the lumen through intima, allowing blood to re-enter the main channel and resulting in chronic dissection or occlusion of branches of the aorta.
Classification
Stanford Classification
The Stanford classification divides dissections into 2 types, type A and type B. Type A involves the ascending aorta (DeBakey types I and II); type B does not involve (DeBakey type III).
- Type A dissections involve the ascending aorta and arch.
- Type B involves the descending aorta.
- A patient can have a type A dissection, type B dissection, or a combination of both.
DeBakey Classification
The DeBakey system, named after surgeon and aortic dissection sufferer Michael E. DeBakey, is an anatomical description of the aortic dissection. It categorizes the dissection based on where the original intimal tear is located and the extent of the dissection (localized to either the ascending aorta or descending aorta, or involves both the ascending and descending aorta. The DeBakey classification divides dissections into 3 types as follows:
- Type I: Originates in ascending aorta, propagates at least to the aortic arch and often beyond it distally. It is most often seen in patients less than 65 years of age and is the most lethal form of the disease.
- Type III: Originates in descending aorta, rarely extends proximally but will extend distally. It most often occurs in elderly patients with atherosclerosis and hypertension.
Etiology
- High blood pressure: Most cases (over 70%) are associated with high blood pressure (hypertension).
- Bicuspid aortic valve (a congenital abnormality of the aortic valve)
- Marfan's syndrome
- Ehlers-Danlos syndrome
- Turner syndrome
- Syphilis
- Cocaine use
- Pregnancy: Pregnancy is a rare associated risk factor, especially in the third trimester and early in the postpartum period.
- Trauma: Blunt trauma is known to cause aortic dissection, which is often seen after car wrecks in which the patient's chest hits the steering wheel.
- Surgical complications: Operations including coronary artery bypass grafting and aortic and mitral valve repairs. It can also be a complication of heart catheterization.
Risk Factors
The exact cause is unknown, but more common risks include:
- Aging
- Atherosclerosis
- Blunt trauma to the chest, such as hitting the steering wheel of a car during an accident
- High blood pressure
Pathophysiology
Clinical Manifestations
- Onset of symptoms is sudden
- Severe and persistent pain—anterior chest or back extend to shoulder, epigastric region and abdomen
- Sweating
- Tachycardia
- Appear pale
- Increased blood pressure
The symptoms usually begin suddenly, and include severe chest pain. The pain may feel like a heart attack, and can:
- Be described as sharp, stabbing, tearing, or ripping
- Be felt below the chest bone, then move under the shoulder blades or to the back
- Move to the shoulder, neck, arm, jaw, abdomen, or hips
- Change position—pain typically moves to the arms and legs as the aortic dissection gets worse
The symptoms are caused by a decrease of blood flowing to the rest of the body, and can include:
- Anxiety and a feeling of doom
- Fainting or dizziness
- Heavy sweating (clammy skin)
- Nausea and vomiting
- Shortness of breath—trouble breathing when lying flat (orthopnea)
Other symptoms may include:
- Pain in the abdomen
- Stroke symptoms
- Swallowing difficulties from pressure on the esophagus
Assessment and Diagnostic Tests
- Physical examination: Superficial veins of neck, chest or arm dilated.
- D-dimer: A blood D-dimer level less than 500 ng/ml may be able to rule out the diagnosis of aortic dissection alleviating the need for further imaging.
- Chest X-ray: Widening of the mediastinum on an X-ray of the chest has moderate sensitivity in the setting of an ascending aortic dissection. Pleural effusions may be seen on chest X-ray. They are more commonly seen in descending aortic dissections. Depression of the left main stem bronchus and tracheal deviation.
Computed Tomography
Computed tomography angiography is a fast noninvasive test that will give an accurate three-dimensional view of the aorta. These images are produced by taking rapid thin-cut slices of the chest and abdomen, and combining them in the computer to create cross-sectional slices. In order to delineate the aorta to the accuracy necessary to make the proper diagnosis, an iodinated contrast material is injected into a peripheral vein. Contrast is injected and the scan performed using a bolus tracking method. This is a type of scan timed to an injection 134to capture the contrast as it enters the aorta. The scan will then follow the contrast as it flows through the vessel.
It has a sensitivity of 96 to 100% and a specificity of 96 to 100%. Disadvantages include the need for iodinated contrast material and the inability to diagnose the site of the intimal tear.
Magnetic Resource Imaging
Magnetic resonance imaging (MRI) is currently the gold standard test for the detection and assessment of aortic dissection, with a sensitivity of 98% and a specificity of 98%. An MRI examination of the aorta will produce a three-dimensional reconstruction of the aorta, allowing the physician to determine the location of the intimal tear, the involvement of branch vessels, and locate any secondary tears. It is a noninvasive test, does not require the use of iodinated contrast material, and can detect and quantitate the degree of aortic insufficiency. The disadvantage of the MRI scan in the face of aortic dissection is that it has limited availability and is often located only in the larger hospitals, and the scan is relatively time-consuming. Due to the high-intensity magnetic fields used during MRI, an MRI scan is contraindicated in individuals with metallic implants. In addition, many individuals experience claustrophobia while in the MRI scanning tube.
Transesophageal Echocardiography
It is an echocardiogram displaying the true lumen and false lumen of an aortic dissection. In the image to the left, the intimal flap can be seen separating the two lumens. In the image to the right, color flow during ventricular systole suggests that the upper lumen is the true lumen.
The transesophageal echocardiogram (TEE) is a relatively good test in the diagnosis of aortic dissection, with a sensitivity of up to 98% and a specificity of up to 97%. It has become the preferred imaging modality for suspected aortic dissection. It is a relatively noninvasive test, requiring the individual to swallow the echocardiography probe. It is especially good in the evaluation of AI in the setting of ascending aortic dissection, and to determine whether the ostia (origins) of the coronary arteries are involved. While many institutions give sedation during transesophageal echocardiography for added patient comfort, it can be performed in cooperative individuals without the use of sedation. Disadvantages of the TEE include the inability to visualize the distal ascending aorta (the beginning of the aortic arch), and the descending abdominal aorta that lies below the stomach. A TEE may be technically difficult to perform in individuals with esophageal strictures or varices.
Aortogram
An aortogram involves placement of a catheter in the aorta and injection of contrast material while taking X-rays of the aorta. The procedure is known as aortography. Previously thought to be the diagnostic ‘gold standard’, it has been supplanted by other less–invasive imaging modalities.
Medical Management
- Antibiotic: The tetracycline antibiotic Doxycycline is currently being investigated for use as a potential drug in the prevention of aortic aneurysm due to its metalloproteinase inhibitor and collagen stabilizing properties.
- Beta blocker: Atenolol, Timolol maleate
- Vasodilators: Sodium nitroprusside
- Calcium channel blockers: Verapamil and diltiazem
Surgical Management
Replacement of the damaged section with a tube graft (often made of dacron) when there is no damage to the aortic valve.
- Bentall procedure: Replacement of the damaged section of aorta and replacement of the aortic valve.
- David procedure: Replacement of the damaged section of aorta and reimplantation of the aortic valve.
- Tevar: Insertion of a stent graft (covered stent), e.g. in TEVAR (thoracic endovascular aortic repair). It is usually combined with ongoing medical management.
- Vascular ring connector (VRC): Replacement of the damaged section of aorta with a sutureless vascular ring connector-reinforced dacron graft. Vascular ring connector (VRC) is a titanic ring used as a stent in the vascular graft to achieve a quick, blood-sealed, and sutureless anastomosis. There are two furrows on the surface of the ring for fixation of the vascular graft and the aorta. The tapes used to tie against the ring provide a larger contact surface area than the traditional stitches, thus providing stronger anastomosis and better surgical results.
RAYNAUD'S PHENOMENON
Definition
Raynaud's phenomenon is a vasospastic disorder causing discoloration of the fingers, toes, and occasionally other areas. This condition may also cause nails to become brittle with longitudinal ridges. Due to vasospasm that decrease blood supply to the respective regions. Stress and cold are classic triggers of the phenomenon.
Types of Raynaud's Disease
There are two types of Raynaud's disease – Primary and Secondary:
- Primary Raynaud's disease: In these cases, the cause of the condition is unknown. It does run in families, however, so a genetic cause is suspected. Primary Raynaud's disease is five times more common in women than it is in men, and usually starts between the ages of 20 and 45 years.
- Secondary Raynaud's disease: Secondary Raynaud's disease is so-called because it occurs secondary to another condition or factor, such as:
- Medications that narrow the blood vessels, e.g. beta blockers.
- Hormone imbalances, e.g. hypothyroidism.
- Injury, e.g. frostbite.
- Occupational exposure to constant vibration (e.g. chainsaws) or repetitive movement (e.g. typing).
Causes
- Primary Raynaud's (disease): Raynaud's disease, or ‘Primary Raynaud's’, is diagnosed if the symptoms are idiopathic. It often develops in young women in their teens and early adulthood. Primary Raynaud's is hereditary.
- Secondary Raynaud's (syndrome): Raynaud's syndrome, or ‘Secondary Raynaud's,’ occurs secondary to a wide variety of other conditions. Secondary Raynaud's has a number of associations:
- Connective tissue disorders:
- Scleroderma
- Systemic lupus erythematosus
- Rheumatoid arthritis
- Polymyositis
- Mixed connective tissue disease
- Ehlers-Danlos Syndrome
- Eating disorders
- Anorexia nervosa
- Obstructive disorders
- Atherosclerosis
- Buerger's disease
- Takayasu's arteritis
- Subclavian aneurysms
- Thoracic outlet syndrome
- Drugs
- Betablockers
- Cytotoxic drugs—particularly chemotherapeutics and most especially bleomycin
- Cyclosporin
- Bromocriptine
- Ergotamine
- Sulfasalazine
- Anthrax vaccines whose primary ingredient is the anthrax protective antigen
- Occupation
- Jobs involving vibration, particularly drilling, suffer from vibration white finger
- Exposure to vinyl chloride, mercury
- Exposure to cold
- Others
Risk Factors
Smoking worsens frequency and intensity of attacks, and there is a hormonal component. Caffeine also worsens the attacks. Sufferers are more likely to have migraine and angina than controls.
Pathophysiology
Signs and Symptoms
- Pain
- Discoloration (paleness)
- Sensations of cold and/or numbness
- Swelling
- Tingling
- Raynaud's also has occurred in breastfeeding mothers, causing nipples to turn white and become extremely painful.
Diagnostic Tests
- Digital artery pressure: Pressures are measured in the arteries of the fingers before and after the hands have been cooled. A decrease of at least 15 mm Hg is diagnostic (positive).
- Doppler ultrasound: To assess blood flow.
- Full blood count: This may reveal a normocytic anemia suggesting the anemia of chronic disease or renal failure.
- Thyroid function tests: this may reveal hypothyroidism.
- An autoantibody screen, tests for rheumatoid factor, erythrocyte sedimentation rate, and C-reactive protein, which may reveal specific causative illnesses or a generalized inflammatory process.
- Nailfold vasculature: This can be examined under the microscope.
Treatment (General Care)
- Environmental triggers should be avoided, e.g. cold, vibration, etc.
- Emotional stress is another recognized trigger.
- Extremities should be kept warm.
- Consumption of caffeine and other stimulants and vasoconstrictors must be prevented.
Emergency Measures
- Keeping warm and maintaining a constant body temperature.
- Wearing gloves and warm socks when out in the cold.
- Not smoking—nicotine can narrow the blood vessels.
- Not directly handling cold things, e.g. bottles of milk, items just out of the freezer.
- Keeping the skin supple by using moisturizers.
- Learning how to manage stress and emotional situations.
- Avoiding medications that can aggravate blood vessel spasm, e.g. some cold and flu medications.
- Warming the hands and feet with clothing or in warm water.
- Medications to widen the blood vessels and promote circulation (calcium channel blockers).
- Medications to thin the blood, e.g. aspirin.
- Treatment of underlying conditions in cases of secondary Raynaud's disease.
- Alternative therapies, e.g. massage, acupuncture.
Drug Therapy
- Calcium channel blockers: (Nifedipine) or diltiazem.
- Side effects: Headache, flushing, and ankle edema; but these are not typically of sufficient severity to require cessation of treatment.
- Angiotensin II receptor antagonists: (Losartan) reduce frequency and severity of attacks
- Vasodilator therapy: Sildenafil (Viagra) improves both microcirculation and symptoms in patients with secondary Raynaud's phenomenon.
- Selective serotonin reuptake inhibitor: Fluoxetine, a selective serotonin reuptake inhibitor.
- Antidepressant medications: May reduce the frequency and severity of episodes if caused mainly by psychological stress.
Surgical Intervention
- Sympathectomy: Procedure can be performed. The nerves that signal the blood vessels of the fingertips to constrict are surgically cut.
Nursing Management
Nursing diagnosis: Risk for hemorrhage related to graft procedure
Goal: To reduce risk of bleeding
Interventions
- Monitor pulse rate.
- Monitor central venous pressure.
- Provide sterile dressing on wound.
- Give vitamin K as per doctor's advice.
Nursing Diagnosis: Pain related to disease condition as evidences by verbal communication.
Goal: Pain is reduced or lost.
Intervention
- Assess for the presence of pain, the scale and intensity of pain.
- Teach the client about pain management and relaxation with distraction.
- Secure the chest tube to restrict movement and avoid irritation.
- Assess pain reduction measures.
- Provide analgesics as indicated.
Nursing Diagnosis: Risk for impaired gas exchange related to cough and pain from incision.
Goal: To clear secretions from airway.
Interventions
- Airway Management:
- Open the airway with headtilt, chinlift, jaw thrust
- Set the position to maximize ventilation
- Use tools airway
- Perform chest physiotherapy
- Teach breathing deeply and coughing effectively
- Perform suction
- Auscultation of breath sounds
- Give bronchodilators (collaboration)
- Oxygenation Therapy:
- Provide humidification system of oxygen equipment
- Monitor the flow of oxygen and the amount given
- Monitor signs of oxygen toxicity
BIBLIOGRAPHY
- Ahmad EN. Cardiovascular diseases, conduction disturbances 2010. Aliabad University Hospital, Kabul Medical University, Kabul, Afghanistan.
- Basvanthappa BT. A textbook of medical surgical nursing, 2nd ed. Jaypee Brothers Medical publishers 1106–9.
- Black M. A textbook of medical surgical nursing. Elsevier. Noida. 8th ed. 1307–40.
- Black M Joyce ‘Medical surgical nursing’, 8th ed., Elsevier 809–11.
- Brunner S. A Textbook of medical surgical nursing. Lippincott Company. Philadelphia. 10th ed. 2007: 1052–60.
- ECG Conduction Abnormalities. Retrieved 2009-01-07.
- Edhag O, Swahn A. ‘Prognosis of patients with complete heart block or arrhythmic syncope who were not treated with artificial pacemakers. A long-term follow-up study of 101 patients’. Acta medica Scandinavica 1976. 200 (6): 457–63.
- Hopper P, William L. Understanding of medical surgical nursing. Devis. ed. 2nd. 2003:286–97
- Lemone P, Burke K. A Textbook of medical surgical nursing. South Asia Dorling Kindersely 4th ed. 2007.
- Suddarth Brunner. A textbook of medical surgical nursing 10th ed., Lippincott Williams and Wilkins 713–24.