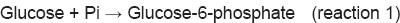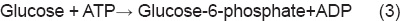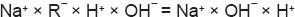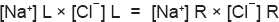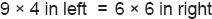- Chapter 1 Biochemical Perspective to Medicine
- Chapter 2 Subcellular Organelles and Cell Membranes
- Chapter 3 Amino Acids: Structure and Properties
- Chapter 4 Proteins: Structure and Function
- Chapter 5 Enzymology: General Concepts and Enzyme Kinetics
- Chapter 6 Clinical Enzymology
- Chapter 7 Chemistry of Carbohydrates
- Chapter 8 Chemistry of Lipids
Chapter at a Glance
- The learner will be able to answer questions on the following topics:
- ❒ History of biochemistry
- ❒ Ionic bonds
- ❒ Hydrogen bonding
- ❒ Hydrophobic interactions
- ❒ Principles of thermodynamics
- ❒ Donnan membrane equilibrium
Biochemistry is the language of biology. The tools for research in all the branches of medical science are mainly biochemical in nature. The study of biochemistry is essential to understand basic functions of the body. This study will give information regarding the functioning of cells at the molecular level. How the food that we eat is digested, absorbed, and used to make ingredients of the body? How does the body derive energy for the normal day-to-day work? How are the various metabolic processes interrelated? What is the function of genes? What is the molecular basis for immunological resistance against invading organisms? Answer for such basic questions can only be derived by a systematic study of biochemistry.
It is estimated that about 70% of clinical diagnosis is based on the laboratory analysis of body fluids, especially the blood. The disease manifestations are reflected in the composition of blood and other tissues. Hence, the demarcation of abnormal from normal constituents of the body is another aim of the study of biochemistry.
The word chemistry is derived from the Greek word “chemi” (the black land), the ancient name of Egypt. Indian medical science, even from ancient times, had identified the metabolic and genetic basis of diseases. Charaka, the great master of Indian Medicine, in his treatise (circa 400 BC) observed that madhumeha (diabetes mellitus) is produced by the alterations in the metabolism of carbohydrates and fats; the statement still holds good.
Biochemistry has developed as an offshoot of organic chemistry, and this branch was often referred as “physiological chemistry”. The term “Biochemistry” was coined by Neuberg in 1903 from Greek words, bios (= life) and chymos (= juice). One of the earliest treatises in biochemistry was the “Book of Organic Chemistry and its Applications to Physiology and Pathology”, published in 1842 by Justus von Liebig (1803–73), who introduced the concept of metabolism. The “Textbook of Physiological Chemistry” was published in 1877 by Felix Hoppe-Seyler (1825–95), who was Professor of Physiological 4Chemistry at Strasbourg University, France. Some of the milestones in the development of the science of biochemistry are given in Table 1.1.
The practice of medicine is both an art and a science. The word “doctor” is derived from the Latin root, “docere”, which means “to teach”. Knowledge devoid of ethical background may sometimes be disastrous! Hippocrates (460 BC to 377 BC), the father of modern medicine articulated “the Oath”. About one century earlier, Sushruta (?500 BC), the great Indian surgeon, enunciated a code of conduct for the medical practitioners, which is still valid. He proclaims: “You must speak only truth; care for the good of all living beings; devote yourself to the healing of the sick even if your life be lost by your work; be simply clothed and drink no intoxicant; always seek to grow in knowledge; in face of God, you can take upon yourself these vows.”
Biochemistry is perhaps the most rapidly developing discipline in medicine. No wonder, the major share of Nobel Prizes in medicine has gone to research workers engaged in biochemistry. Thanks to the advent of DNA recombinant technology, genes can now be transferred from one person to another, so that many of the genetically determined diseases are now amenable to gene therapy. Many genes, (e.g. human insulin gene) have already been transferred to microorganisms for largescale production of human insulin. Advances in genomics like RNA interference for silencing of genes and creation of transgenic animals by gene targeting of embryonic stem cells are opening up new vistas in therapy of diseases like cancer and AIDS. It is hoped that in future, the physician will be able to treat the patient, after understanding his genetic basis, so that very efficient “designer medicine” could cure the diseases. The large amount of data, especially with regard to single nucleotide polymorphisms (SNPs) that are available, could be harnessed by “Bioinformatics”. Computers are already helping in drug designing process. Studies on oncogenes have identified molecular mechanisms of control of normal and abnormal cells. Medical practice is now depending more on the science of Medical Biochemistry. With the help of Human genome project (HGP) the sequences of whole human genes are now available; it has already made great impact on medicine and related health sciences.
BIOMOLECULES
More than 99% of the human body is composed of 6 elements, i.e. oxygen, carbon, hydrogen, nitrogen, calcium and phosphorus. Human body is composed of about 60% water, 15% proteins, 15% lipids, 2% carbohydrates and 8% minerals. Molecular structures in organisms are built from 30 small precursors, sometimes called the alphabets of biochemistry. These are 20 amino acids, 2 purines, 3 pyrimidines, sugars (glucose and ribose), palmitate, glycerol and choline.
|
In living organisms, biomolecules are ordered into a hierarchy of increasing molecular complexity. These biomolecules are covalently linked to each other to form macromolecules of the cell, e.g. glucose to glycogen, amino acids to proteins, etc. Major complex biomolecules are proteins, polysaccharides, lipids and nucleic acids. The macromolecules associate with each other by noncovalent forces to form supramolecular systems, e.g. ribosomes, lipoproteins.
Finally at the highest level of organization in the hierarchy of cell structure, various supramolecular complexes are further assembled into cell organelle. In prokaryotes (e.g. bacteria; Greek word “pro” = before; karyon = nucleus), these macromolecules are seen in a homogeneous matrix; but in eukaryotic cells (e.g. higher organisms; Greek word “eu” = true), the cytoplasm contains various subcellular organelles. Comparison of prokaryotes and eukaryotes are shown in Table 1.2.
STUDY OF METABOLIC PROCESSES
Our food contains carbohydrates, fats and proteins as principal ingredients. These macromolecules are to be first broken down to small units; carbohydrates to monosaccharides and proteins to amino acids. This process is taking place in the gastrointestinal tract and is called digestion or primary metabolism. After absorption, the small molecules are further broken down and oxidized to carbon dioxide. In this process, NADH or FADH2 are generated. This is named as secondary or intermediary metabolism. Finally, these reducing equivalents enter the electron transport chain in the mitochondria, where they are oxidized to water; in this process energy is trapped as ATP. This is termed tertiary metabolism. Metabolism is the sum of all chemical changes of a compound inside the body, which includes synthesis (anabolism) and breakdown (catabolism). (Greek word, kata = down; ballein = change).
STABILIZING FORCES IN MOLECULES
Covalent Bonds
Molecules are formed by sharing of electrons between atoms (Fig. 1.1).
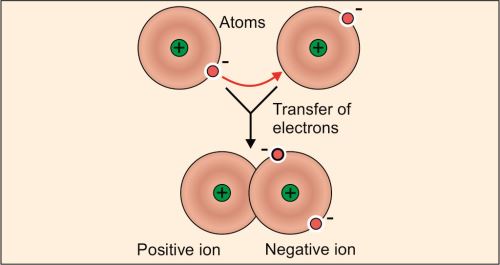
Ionic Bonds or Electrostatic Bonds
Ionic bonds result from the electrostatic attraction between two ionized groups of opposite charges (Fig. 1.2). They are formed by transfer of one or more electrons from the outermost orbit of an electropositive atom to the outermost orbit of an electronegative atom. This transfer results in the formation of a ‘cation’ and an ‘anion’, which get consequently bound by an ionic bond. Common examples of such compounds include NaCl, KBr and NaF.
With regard to protein chemistry, positive charges are produced by epsilon amino group of lysine, guanidinium group of arginine and imidazolium group of histidine. Negative charges are provided by beta and gamma carboxyl groups of aspartic acid and glutamic acid (Fig. 1.3).
Hydrogen Bonds
These are formed by sharing of a hydrogen between two electron donors. Hydrogen bonds result from electrostatic attraction between an electronegative atom and a hydrogen atom that is bonded covalently to a second electronegative atom. Normally, a hydrogen atom forms a covalent bond with only one other atom. However, a hydrogen atom covalently bonded to a donor atom, may form an additional weak association, the hydrogen bond with an acceptor atom. In biological systems, both donors and acceptors are usually nitrogen or oxygen atoms, especially those atoms in amino (NH2) and hydroxyl (OH) groups.
With regard to protein chemistry, hydrogen releasing groups are –NH (imidazole, in dole, peptide); –OH (serine, threonine) and –NH2 (arginine, lysine). Hydrogen accepting groups are COO—(aspartic, glutamic) C=O(peptide); and S–S (disulphide). The DNA structure is maintained by hydrogen bonding between the purine and pyrimidine residues.
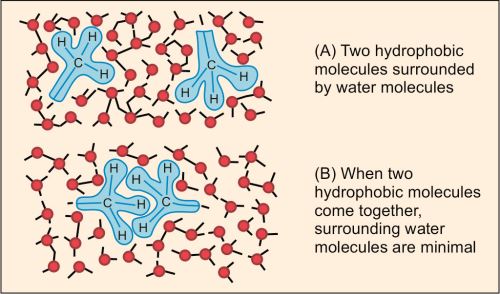
Hydrophobic Interactions
Nonpolar groups have a tendency to associate with each other in an aqueous environment; this is referred to as hydrophobic interaction. These are formed by interactions between nonpolar hydrophobic side chains by eliminating water molecules. The force that causes hydrophobic molecules or nonpolar portions of molecules to aggregate together rather than to dissolve in water is called the ‘hydrophobic bond’ (Fig. 1.4). This serves to hold lipophilic side chains of amino acids together. Thus nonpolar molecules will have minimum exposure to water molecules.
Van der Waals Forces
These are very weak forces of attraction between all atoms, due to oscillating dipoles, described by the Dutch physicist Johannes van der Waals (1837–1923). He was awarded Nobel Prize in 1910. These are short range attractive forces between chemical groups in contact. This force will drastically reduce, when the distance between atoms is increased. Although very weak, van der Waals forces collectively contribute maximum towards the stability of protein structure, especially in preserving the nonpolar interior structure of proteins.
WATER: THE UNIVERSAL SOLVENT
Water constitutes about 70 to 80 percent of the weight of most cells. The hydrogen atom in one water molecule is attracted to a pair of electrons in the outer shell of an oxygen atom in an adjacent molecule. The structure of liquid water contains hydrogen-bonded networks (Fig. 1.5).
Water is a polar molecule. Molecules with polar bonds that can easily form hydrogen bonds with water and can dissolve in water are termed “hydrophilic”.It has immense hydrogen bonding capacity both with other molecules and also the adjacent water molecules. This contributes to cohesiveness of water. Water favors hydrophobic interactions and provides a basis for metabolism of insoluble substances.
PRINCIPLES OF THERMODYNAMICS
Thermodynamics is concerned with the flow of heat and it deals with the relationship between heat and work. Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biological systems use chemical energy to drive processes within living cells.
First Law of Thermodynamics
The total energy of a system, including its surroundings, remains constant. Or, ∆E = Q – W, where Q is the 7heat absorbed by the system and W is the work done. This is also called the law of conservation of energy. If heat is transformed into work, there is proportionality between the work obtained and the heat dissipated. A system is an object or a quantity of matter, chosen for observation. All other parts of the universe, outside the boundary of the system, are called the surrounding.
Second Law of Thermodynamics
The total entropy of a system must increase if a process is to occur spontaneously. A reaction occurs spontaneously if ∆E is negative, or if the entropy of the system increases. Entropy (S) is a measure of the degree of randomness or disorder of a system. Entropy becomes maximum in a system as it approaches true equilibrium. Enthalpy is the heat content of a system and entropy is that fraction of enthalpy which is not available to do useful work.
A closed system approaches a state of equilibrium. Any system can spontaneously proceed from a state of low probability (ordered state) to a state of high probability (disordered state). The entropy of a system may decrease with an increase in that of the surroundings. The second law may be expressed in simple terms as Q = T × ∆S, where Q is the heat absorbed, T is the absolute temperature and ∆S is the change in entropy.
Gibbs Free Energy Concept
The term free energy is used to get an equation combining the first and second laws of thermodynamics. Thus, ∆G = ∆H – T∆S, where ∆G is the change in free energy, ∆H is the change in enthalpy or heat content of the system and ∆S is the change in entropy. The term free energy denotes a portion of the total energy change in a system that is available for doing work.
For most biochemical reactions, it is seen that ∆H is nearly equal to ∆E. So, ∆G = ∆E – T∆S. Hence, ∆G or free energy of a system depends on the change in internal energy and change in entropy of a system.
Standard Free Energy Change
It is the free energy change under standard conditions. It is designated as ∆G0. The standard conditions are defined for biochemical reactions at a pH of 7 and 1 M concentration, and differentiated by a priming sign ∆G0´. It is directly related to the equilibrium constant. Actual free energy changes depend on reactant and product.
Most of the reversible metabolic reactions are near-equilibrium reactions and therefore their ∆G is nearly zero. The net rate of near-equilibrium reactions are effectively regulated by the relative concentration of substrates and products. The metabolic reactions that function far from equilibrium are irreversible. The velocities of these reactions are altered by changes in enzyme activity. A highly exergonic reaction is irreversible and goes to completion. Such a reaction that is part of a metabolic pathway, confers direction to the pathway and makes the entire pathway irreversible.
Laws of thermodynamics have many applications in biology and biochemistry, such as study of ATP hydrolysis, membrane diffusion, enzyme catalysis as well as DNA binding and protein stability. These laws have been used to explain hypothesis of origin of life.
Three Types of Reactions
-
A reaction can occur spontaneously when ∆G is negative. Then the reaction is exergonic. If ∆G is of great magnitude, the reaction goes to completion and is essentially irreversible.
-
When ∆G is zero, the system is at equilibrium.
-
For reactions where ∆G is positive, an input of energy is required to drive the reaction. The reaction is termed as endergonic. (Examples are given in Chapter 5).
Similarly a reaction may be exothermic (∆H is negative), isothermic (∆H is zero) or endothermic (∆H is positive).
Energetically unfavorable reaction may be driven forward by coupling it with a favorable reaction.
8Reaction 1 cannot proceed spontaneously. But the 2nd reaction is coupled in the body, so that the reaction becomes possible. For the first reaction, ∆G0 is +13.8 kJ/mole; for the second reaction, ∆G0 is –30.5 kJ/mole. When the two reactions are coupled in the reaction 3, the ∆G0 becomes –16.7 kJ/mole, and hence the reaction becomes possible. Details on ATP and other high-energy phosphate bonds are described in Chapter 21.
Reactions of catabolic pathways (degradation or oxidation of fuel molecules) are usually exergonic. On the other hand, anabolic pathways (synthetic reactions or building up of compounds) are endergonic. Metabolism constitutes anabolic and catabolic processes that are well co-ordinated.
DONNAN MEMBRANE EQUILIBRIUM
When two solutions are separated by a membrane permeable to both water and small ions, but when one of the compartments contains impermeable ions like proteins, distribution of permeable ions occurs according to the calculations of Donnan.
In Figures 1.6A and B, the left compartment contains NaR, which will dissociate into Na+ and R¯. Then Na+ can diffuse freely, but R¯ having high molecular weight cannot diffuse. The right compartment contains NaCl, which dissociates into Na+ and Cl¯, in which case, both ions can diffuse freely.
Thus, if a salt of NaR is placed in one side of a membrane, at equilibrium
To convey the meaning of the mathematical values, a hypothetical quantity of each of the ion is also incorporated in brackets. Initially 5 molecules of NaR are added to the left compartment and 10 molecules of NaCl in the right compartment and both of them are ionized (Fig. 1.6A). When equilibrium is reached, the distributions of ions are shown in Figure 1.6B. According to Donnan's equilibrium, the products of diffusible electrolytes in both the compartments will be equal, so that
If we substitute the actual numbers of ions, the formula becomes
Donnan's equation also states that the electrical neutrality in each compartment should be maintained. In other words the number of cations should be equal to the number of anions, such that
In left: Na+= R¯+ Cl¯; substituting: 9 = 5 + 4
In right: Na+ = Cl¯; substituting: 6 = 6
The equation should also satisfy that the number of sodium ions before and after the equilibrium are the same; in our example, initial Na+ in the two compartments together is 5 + 10 = 15; after equilibrium also, the value is 9 + 6 = 15. In the case of chloride ions, initial value is 10 and final value is also 4 + 6 = 10.
In summary, Donnan's equations satisfy the following results:
-
The products of diffusible electrolytes in both compartments are equal.
-
The electrical neutrality of each compartment is maintained.
-
The total number of a particular type of ions before and after the equilibrium is the same.
-
As a result, when there is nondiffusible anion on one side of a membrane, the diffusible cations are more, and diffusible anions are less, on that side.
Clinical Applications of the Equation
-
The total concentration of solutes in plasma will be more than that of a solution of same ionic strength containing only diffusible ions; this provides the net osmotic gradient (see under Albumin, in Chapter 26).
-
The lower pH values within tissue cells than in the surrounding fluids are partly due to the concentrations of negative protein ions within the cells being higher than in surrounding fluids.
-
The pH within red cells is lower than that of the surrounding plasma is due, in part, to the very high concentration of negative nondiffusible hemoglobin ions. This will cause unequal distribution of H+ ions with a higher concentration within the cell.
-
The chloride shift in erythrocytes as well as higher concentration of chloride in CSF are also due to Donnan's effect (see under Hemoglobin, in Chapter 23).
-
Osmolarity of body fluid compartments and sodium concentration will follow Donnan equation.
LEARNING POINTS, CHAPTER 1
-
Almost 99% of biomolecules of human tissues are made up of only 6 elements; Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), Calcium (Ca) and Phosphorus (P).
-
Living organisms are ordered into a hierarchy of increasing molecular complexity; Elements > Compounds > Macromolecules > Supramolecular systems.
-
Bonds responsible for stabilizing these molecules are covalent bonds and noncovalent bonds. The non-covalent bonds are hydrogen bonds, ionic or electrostatic bonds, hydrogen bonds, hydrophobic interactions and van der Waal's forces.
-
70-80% of the cell is composed of water. Water soluble compounds remain in solution, others are solubilized by binding to water soluble compounds like proteins.
-
Energy changes in cells are governed by the laws of thermodynamics and the term free energy denotes a portion of the total energy change in a system available for doing work (ΔG).
-
All reactions can be classified into two; a) exergonic or energy releasing reactions (degradative reactions) and b) endergonic reactions requiring energy input (synthetic reactions).