INTRODUCTION
Advances in the field of radiology and in turn modern neurosurgery have been so rapid that, it is almost impossible to recall the older techniques and still be abreast with the latest modalities at the same time. The obsolete modalities have become a part of theoretical textbooks only. The neurosurgical residents of this generation might not even know about the older techniques such as pneumoencephalography. These advances are not just useful in making the work of a neurosurgeon easier, but at the same time, it helps in reducing the mortality and morbidity in patients or aid in better outcomes.1
GENEALOGY OF NEUROIMAGING
X-ray
Sir Victor Horsley was the first neurosurgeon to use X-rays in neurosurgery way back in the late 19th century after their discovery.2 Soon after the discovery of plain radiograph and its utility in clinical medicine, some specialists tried to X-ray of skull of patients in order to diagnose suspected lesions in the brain.3–6 One of the most famous publications of its time was “Röntgen-diagnostik der Erkrankungen des Kopfes” by Arthur Schüller.7 The same was later translated into English under the name “Radiology of Diseases of the Head”. “Neuroradiology” was first used by Schüller describing pathologies of the brain using skull radiographs.8 The modality gained such popularity in those times that Walter Dandy and George Heuer published a paper proving changes seen in 45 patients with brain tumors out of their enlisted 100 patients (Figure 1.1).9
However, this modality had its own limitations namely long period of immobility to acquire image, soft tissue pathologies could not be detected, neoplasms without bony involvement or calcification were literally invisible. In reality, by the time actual pathologies were identified on skull radiographs, clinically, it was too late for the patient to undergo any intervention.10 Despite of the advances over the centuries, spine radiograph is still an essential part of orthopedics and neurosurgery.
Ventriculography and Pneumoencephalography
Walter Dandy came up with an idea of somehow analyzing the changes in the morphology of the ventricles by injecting some contrast material, in order to localize lesions in the brain and diagnosing pathologies in a timely manner.10 He failed with his experiments using different radiopaque contrast materials, until he accidentally noticed that air in the ventricles in head injury patients made the ventricles partially radiopaque.10,11 This discovery resulted in further increase in detection capacity of brain tumors in his patients (Figure 1.2).12
Pneumoencephalography was also discovered by him, when he further noticed that the air from the ventricles escaped into the subarachnoid space.13 The procedure was then popularized in the form of drawing small amount of CSF during lumbar puncture and injecting air instead. This discovery paved the path for contrast studies of the brain.
However, these techniques proved to be associated with significant morbidity and mortality, especially with neurosurgeons other than Dandy.8 Through the following years of discovery, refinement of the procedure led to the discovery of a skull table by Lysholm which helped with the movement of patient in some positions to enable the air to move to different parts of the brain as needed.14
Myelography with Contrast
Myelography was first done using air as contrast medium as was the case with ventriculography in the early 20th century.15,16 By the same time, iodine was discovered to be a safe radiopaque contrast.2
Although initially iodine was used with an oily medium, which led to cases of arachnoiditis and was later replaced by water soluble material over the next 50 years.17 Lumbar discography came to be popular in the mid-20th century where injection of radiopaque material was done directly into the intervertebral disks.18,19
Angiography of the Intracranial and Spinal Vessels
Egas Moniz dreamt of making either the brain or the cerebral vessels opaque on imaging. His efforts led to the discovery of angiography of cerebral vessels.20 In the early 20th century, he performed multiple experiments on animals and cadavers. Once confident of his method, he performed a “cutdown” to reach the carotid artery and injected contrast material directly into it making angiography possible. Loman and Myerson further refined the technique by allowing percutaneous access to the carotid artery and injecting contrast material without “‘cutdown” procedure.21 Angiography of the vertebral arteries was discovered approaching it through the brachial, subclavian, and vertebral arteries over the next few years.22 The most famous Seldinger technique, using a catheter instead of a needle, was devised in 1953, but it took almost 10 years to be used in cerebral angiography.23,243
Figure 1.2: Roentgenography of the brain after the injection of air into the spinal canalSource: Adapted from Dandy WE. Röntgenography of brain after the injection of air into the spinal canal. Ann Surg. 1919;70:397-403.
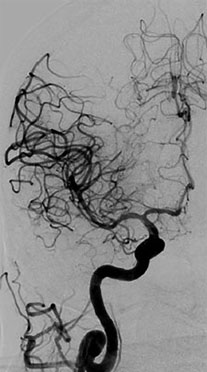
The transfemoral approach was also developed along the same time and was accepted quite late. The technique kept improving with the help of technological advances such as digital subtraction, safer contrast agents, magnification technology, and multiple planes of imaging. Glue, particles, coils, and Onyx were discovered for intravascular procedures (Figure 1.3).
The procedure has withstood the early complications25 and the modern day cerebral angiography does not require any general anesthesia and takes much shorter time, thus reducing the incidence of the known complications.
Spinal angiography was developed almost four decades after cerebral angiography.26,27 It is still considered the gold standard for diagnosing spinal vascular lesions.28
Computed Tomography
The development of computed tomography (CT) started with the ideas of William Oldendorf29,30 and mathematically developed by physicist Allan Cormack.31,32 A few years later, Geoffrey Hounsfield developed a cross-sectional scanner based on the same concept. The first patient to be scanned using CT was a woman with a suspected tumor in her brain in 1972.33 This was followed by multiple patients and the first paper on CT imaging of the head was published in 1973.34 This proved to be a ground-breaking discovery of its time. Hounsfield and Cormack were the most famous names in the medical community worldwide and even received the Nobel prize for their contribution in 1979. The initial versions of a CT scanner allowed only imaging of the head. Multiple improvements and advancements of the initial idea of CT scanner led to bigger and better machines which could scan the entire human body. Eventually, advancements were made in decreasing the total radiation dosage, time taken for scans and quality of images. Earlier scans used 8–13 mm slice thickness, thus their quality was poor based on current scans. It took a few hours for the first machines to process each scan. The market politics estimated that not many CT scanners would be sold worldwide at the time of its inception due to its high cost and fewer advantages.35,36 This modality changed neurosurgery as we speak. It was beyond anyone's imagination that without cutting into the brain we would be able to see the contents of the brain. The initial advantages of a CT scan of brain helped in identifying bleeds, calcification, edema, and necrosis. With the introduction of intravenous contrast, CT angiography of cerebral vessels was also made possible. Once all this became a part of the routine practice, the age-old procedure of pneumoencephalography became obsolete (Figure 1.4).37,38
The biggest advantage of CT scan in diagnosing many intracranial pathologies with one single scan turned down all concerns regarding scanners using high doses of ionizing radiation, high costs, etc. It started being called a “doctor's toy” and was leading to less skilled doctors.39 All such skepticism has been proven wrong even decades after its discovery when it continues to be an indispensable tool even today.
Magnetic Resonance Imaging
Felix Bloch and Edward Purcell were the ones who developed the magnetic resonance imaging (MRI) based on nuclear magnetic resonance.40,41 It was the first time that an imaging modality did not involve any nuclear radiation.42 Raymond Damadian, a physician from the US, came up with the idea almost 25 years later that this modality could differentiate diseased tissue, especially tumors from normal tissue.434
Herman Carr published his thesis based on MRI at Harvard University in the same year as Bloch and Purcell.44 Paul Lauterbur was inspired by Carr's thesis and used it to devise a way of generating two-dimensional and three-dimensional MR images. The credit for constructing the first MRI machine goes to Damadian (1972). Lauterbur took out the first scan using MRI of a mouse a year later.45,46 Peter Mansfield developed a new algorithm for MRI machines which greatly reduced the time required to acquire images and improve their quality too. In 1977, Damadian, Minkoff and Goldsmith were able to acquire the first human MRI scan.47
Publications started coming in during the 1980s using MRI for neuroimaging, especially in comparison to CT images.48,49 The first MRI machine to be used in hospital set-ups commercially and contrast for MRI based on Gadolinium were also developed during the same time.16,50 MRI ever since its inception has revolutionized neuroimaging.51 It was earlier considered to be “extremely difficult, time-consuming, and often disappointing”.52 Its challenges still remain, especially in unstable patients and the ones who have a fear of closed spaces.53
Image-guided Neurosurgery and Stereotaxy
The ever-improving radiological modalities and technologies over the past few decades have led to improved diagnosis of neurosurgical diseases and also better treatment outcomes. All machines developed with the intention of accurately localizing intracranial structures based on landmarks on the surface were first devised using Broca's craniograph54 and Kocher's craniometer.55 They proved to be less than optimal and were not of much use clinically. Then came the “encephalometer” developed by Russian anatomist Zernov. He developed a device which was based on an arc-shaped instrument attached to the skull.56 Three-dimensional Cartesian coordinate system was used in experimental monkeys through orthogonal stereotactic frame by Victor Horsley and Robert Henry Clarke.57
In the early 20th century, Kirschner first used frame-based stereotaxy while injecting into the foramen ovale to treat trigeminal neuralgia.58 Ernest Spiegel and Henry Wycis developed the “stereoencephalotome”,59 and Lars Leksell was the legendary neurosurgeon who first developed the most popular stereotactic device to be used in humans. Spiegel and Wycis were the first surgeons to perform stereotactic thalamotomy in humans using radiography in stereotaxy based on landmarks inside the brain such as calcified pineal gland and ventricles. Leksell based his system on the radius of the arc which was later used by the popular frame developed by Edwin Todd and Trent Wells. This led to the eventual development of Brown-Robert-Wells frame which used data from the computed tomographic scans into the surgical setting.60 Jean Talairach devised a special frame used in epilepsy surgery for temporal lobe lesions and used the anterior and posterior commissure as basis of intracranial landmarks.61
Stereotactic neurosurgery for psychiatric and functional disorders lost their popularity soon after levodopa started being used as a treatment for Parkinson's disease.62 Over the years, CT and MRI scans got integrated with stereotaxy and more accurate targeting of regions and lesions in the brain were made possible. This revived stereotactic neurosurgery as developed by Leksell in the form of deep brain stimulation, vascular neurosurgery, epilepsy, neuro-oncology, and radiation therapy.63
Rapid advances in technology have facilitated the development of frameless neuronavigation systems, which began with Roberts and colleagues in 1986.64 Stealth Station and Vector Vision navigation systems have introduced technology and instruments that allow frameless stereotaxy, and have become popular with many neurosurgeons.65 Intraoperative MRI (iMRI) has been proven to be more effective in achieving better resections and in turn better survival in patients with gliomas. Multiple papers have been published in international journals acknowledging the fact as at least a level 2 evidence of the same.66 A recent study showed the use of iMRI for placement of ventricular catheters to prevent complications.67
During endovascular surgery, angiography can be used to provide regular updates. Plain X-ray image intensifiers are widely used in spinal surgery, for example, to ensure correct level localization and confirm screw position. Intraoperative ultrasound is also used in some centers, and may be particularly useful in the emergency setting to exclude hematoma collection.1 The acquisition of more than just standard MRI intraoperatively, particularly, functional MRI (fMRI) and diffusion tensor imaging (DTI), may offer 5additional safety for complex tumor resections68 and management of vascular lesions69 but robust evidence in this regard is awaited. However, the equipment used to obtain this data is expensive and thus not widely available, and there are some important considerations, including that iMRI prolongs surgery and requires reconfiguration of the operating room. Certainly, wherever available, preoperative functional imaging and particularly fMRI can assist neurosurgical operation planning.70
REFERENCES
- Kirkman M. The role of imaging in the development of neurosurgery. J Clin Neurosci. 2015;22:55–61.
- Gunderman RB. X-ray vision: the evolution of medical imaging and its human significance. Oxford: Oxford University Press; 2013.
- van Dijck J. X-ray vision in Thomas Mann's the magic mountain. In: van Dijck J (Ed). The Transparent Body. A Cultural Analysis of Medical Imaging. Seattle: University Of Washington Press; 2005. p. 84.
- Proust M. Swann's way. Remembrance of things past, vol. 1. New York: Henry Holt and Company; 1922.
- Church A. Cerebellar tumor: recognized clinically, demonstrated by X-ray—proved at autopsy. Am J Med Sci. 1899;177:125–30.
- Pfahler GE. Cerebral skiagraphy: transactions of the American Roentgen Ray Society. In: Proceedings of the 5th Annual Meeting of the American Roentgen Ray Society, September 9, 10, 12, 13. Missouri: St. Louis; 1904;4:175–83.
- Schüller A. Röntgen-diagnostik der erkrankungen des kopfes. Vienna: Holder; 1912.
- Bull JW. The history of neuroradiology. Proc R Soc Med. 1970;63:637–43.
- Heuer GJ, Dandy WE. Roentgenography in the localization of brain tumor, based on a series of one hundred consecutive cases. Bull Johns Hopkins Hosp. 1916;27:311–22.
- Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. 1918;68:5–11.
- Haschek E, Lindenthal O. A contribution to the practical use of photography according to Roentgen. Wien Chir Wochenschr. 1896;9:63.
- Alper MG. Three pioneers in the early history of neuroradiology: the Snyder lecture. Doc Ophthalmol. 1999;98:29–49.
- Dandy WE. Röntgenography of brain after the injection of air into the spinal canal. Ann Surg. 1919;70:397–403.
- Lysholm E. Apparatus and technique for roentgen examination of the skull. Stockholm: PA Norstedt & Söner; 1931.
- Wilderoe S. Uber die diagnostische bedeutung der interspinalen luftinjektioned bei Ruckenmarksleiden, besonders bei geschwulsten. Z Chir. 1921;48:394–7.
- Jacobaeus HC. On insufflation of air into the spinal canal for diagnostic purposes in cases of tumors of the spinal canal. Acta Med Scand. 1921;55:555–64.
- Taveras JM. Diamond jubilee lecture. Neuroradiology: past, present, future. Radiology. 1990;175:593–602.
- Lindblom K. Diagnostic puncture of intervertebral disks in sciatica. Acta Orthop Scand. 1948;17:231–9.
- Collis JS Jr, Gardner WJ. Lumbar discography: an analysis of one thousand cases. J Neurosurg. 1962;19:452–61.
- Doby T. Cerebral angiography and Egas Moniz. Am J Roentgenol. 1992;159:364.
- Loman J, Myerson A. Visualization of the cerebral vessels by direct intracarotid injection of thorium dioxide (Thoratrast). AJR Am J Roentgenol. 1936;35:188–93.
- Schechter MM, de Gutiérrez-Mahoney CG. The evolution of vertebral angiography. Neuroradiology. 1973;5:157–64.
- Greitz T. The history of Swedish neuroradiology. Acta Radiol. 1996;37:455–71.
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 1953;39:368–76.
- Moniz E. L'encephalographie arterielle, son importance dans la localization des tumeurs cerebrales. Rev Neurol (Paris). 1927;2:72–90.
- Di Chiro G, Doppman J, Ommaya AK. Selective arteriography of arteriovenous aneurysms of spinal cord. Radiology. 1967;88:1065–77.
- Baker HL Jr, Love JG, Layton DD Jr. Angiographic and surgical aspects of spinal cord vascular anomalies. Radiology. 1967;88:1078–85.
- Chen J, Gailloud P. Safety of spinal angiography: complication rate analysis in 302 diagnostic angiograms. Neurology. 2011;77:1235–40.
- Goldman LW. Principles of CT and CT technology. J Nucl Med Technol. 2007;35:115–28; quiz 129-30.
- Oldendorf WH. Isolated flying spot detection of radiodensity discontinuities: displaying the internal structural patterns of a complex object. Biomed Electron Ire Trans. 1961;8:68–72.
- Cormack AM. Representation of a function by its line integrals, with some radiological applications. J Appl Phys. 1963;34:2722–7.
- Cormack AM. Representation of a function by its line integrals, with some radiological applications. II. J Appl Phys. 1964;35:2908–13.
- Bull J. History of computed tomography. In: Newton TH, Potts GD (Eds). Radiology of the Skull and Brain. St. Louis: CV Mosby Company; 1981.
- Ambrose J, Hounsfield G. Computerized transverse axial tomography. Br J Radiol. 1973;46:148–9.
- Hoeffner EG, Mukherji SK, Srinivasan A, et al. Neuroradiology back to the future: brain imaging. Am J Neuroradiol. 2012;33:5–11.
- Oldendorf WH. The quest for an image of the brain. New York: Raven Press; 1980.
- Huckman MS. Neuroradiology without benefit of computers: a memoir. Am J Neuroradiol. 2010;31:783–6.
- Amundson P, Dugstad G, Noyes W. Cerebral angiography via the femoral artery with reference to cerebrovascular disease. Acta Neurol Scand. 1967;43:115.
- Alker G. A great leap from tiny chips: computerization, 1960s and 1970s. In: Doby T, Alker G (Eds). Origins and Development of Medical Imaging. Springfield, IL: Southern Illinois University Press; 1997. p. 111.
- Bloch F, Hanson WW, Packard M. Nuclear Induction. Phys Rev. 1946;69:127.
- Purcell EM, Torrey HC, Pound RV. Resonance absorption by nuclear magnetic moments in a solid. Phys Rev. 1946;69:37–8.
- Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171:1151–3.
- Carr H. Free precession techniques in nuclear magnetic resonance [Ph.D. thesis]. Harvard University; 1952.
- Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–1.
- Lauterbur PC. Magnetic resonance zeugmatography. Pure Appl Chem. 1974;40:149–57.
- Damadian R, Goldsmith M, Minkoff L. NMR in cancer: XVI. FONAR image of the live human body. Physiol Chem Phys. 1977;9:97–100, 108.
- Holland GN, Moore WS, Hawkes RC. Nuclear magnetic resonance tomography of the brain. J Comput Assist Tomogr. 1980;4:1–3.
- Runge VM, Carollo BR, Wolf CR, et al. Gd DTPA: a review of clinical indications in central nervous system magnetic resonance imaging. Radiographics. 1989;9:929–58.
- Teasdale GM, Hadley DM, Lawrence A, et al. Comparison of magnetic resonance imaging and computed tomography in suspected lesions in the posterior cranial fossa. BMJ. 1989;299:349–55.
- Hoeffner EG, Mukherji SK, Srinivasan A, et al. Neuroradiology back to the future: spine imaging. Am J Neuroradiol. 2012;33:999–1006.
- Geva T. Magnetic resonance imaging: historical perspective. J Cardiovasc Magn Reson. 2006;8:573–80.
- Singer OC, Sitzer M, du Mesnil de Rochemont R, et al. Practical limitations of acute stroke MRI due to patient-related problems. Neurology. 2004;62:1848–9.
- Broca P. Memoir on the craniograph and some of its applications. Mémoires De La Société d'Anthropologie; 1863.
- Schültke E. Theodor Kocher's craniometer. J Neurosurg. 2009;64:1001–4; discussion 1004-5.
- Zernov DN. L'encéphalometrie. Rev Gen Clin Ther. 1890;19:302.
- Horsley V, Clarke RH. The structure and function of the cerebellum examined by a new method. Brain. 1908;31:45–124.
- Kirschner M. Die Punktionstechnik und die Elektrokoagulation des Ganglion Gasseri. Arch Klin Chir. 1933;176:581–620.
- Spiegel EA, Wycis HT, Marks M, et al. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349–50.
- Heilbrun MP, Roberts TS, Apuzzo ML, et al. Preliminary experience with Brown-Roberts-Wells (BRW) computerized tomography stereotaxic guidance system. J Neurosurg. 1983;59:217–22.
- Talairach J, Hecaen H, David M, et al. Recherches sur la coagulation thérapeutique des structures sous-corticales chez l'homme. Rev Neurol. 1949;81:4–24.
- al-Rodhan NR, Kelly PJ. Pioneers of stereotactic neurosurgery. Stereotact Funct Neurosurg. 1992;58:60–6.
- Leksell L. A stereotaxic apparatus for intracerebral surgery. Acta Chir Scand. 1950;99:229–33.
- Roberts DW, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545–9.
- Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices. 2012;9:491–500.
- Kubben PL, ter Meulen KJ, Schijns OE, et al. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 2011;12:1062–70.
- Janson CG, Romanova LG, Rudser KD, et al. Improvement in clinical outcomes following optimal targeting of brain ventricular catheters with intraoperative imaging. J Neurosurg. 2014;120:684–96.
- Nimsky C. Intraoperative acquisition of fMRI and DTI. Neurosurg Clin N Am. 2011;22:269–77.
- Campbell PG, Jabbour P, Yadla S, et al. Emerging clinical imaging techniques for cerebral cavernous malformations: a systematic review. Neurosurg Focus. 2010;29:E6.
- Belyaev AS, Peck KK, Brennan NM, et al. Clinical applications of functional MR imaging. Magn Reson Imaging Clin N Am. 2013;21:269–78.